The Characteristics of Cognitive Impairment in ALS Patients Depend on the Lateralization of Motor Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Cognitive Categorization
2.3. Side of Onset Classification
2.4. Statistical Analysis
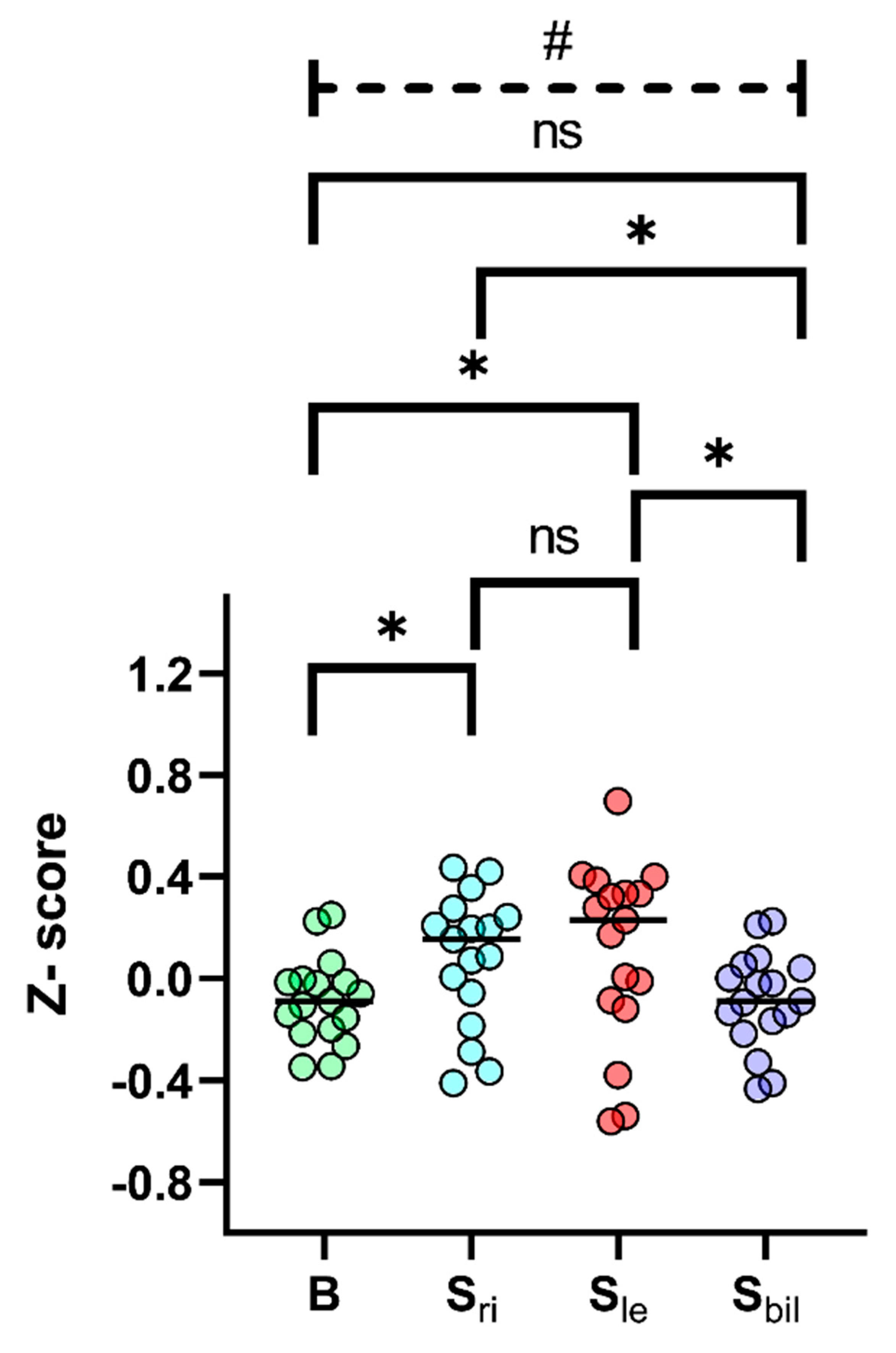
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; Van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Ravits, J.; Paul, P.; Jorg, C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology 2007, 68, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Grad, L.I.; Rouleau, G.A.; Ravits, J.; Cashman, N.R. Clinical Spectrum of Amyotrophic Lateral Sclerosis (ALS). Cold Spring Harb. Perspect. Med. 2017, 7, a024117. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.R.; Wicks, S.; Brownstein, C.A.; Massagli, M.P.; Toronjo, M.; Talbot, K.; Al-Chalabi, A. Concordance between site of onset and limb dominance in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 853–854. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, M.; Adenzato, M.; MacPherson, S.E.; Enrici, I.; Abrahams, S. Evidence of social understanding impairment in patients with Amyotrophic Lateral Sclerosis. PLoS ONE 2011, 6, e25948. [Google Scholar] [CrossRef] [PubMed]
- Phukan, J.; Elamin, M.; Bede, P.; Jordan, N.; Gallagher, L.; Byrne, S.; Lynch, C.; Pender, N.; Hardiman, O. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: A population-based study. J. Neurol. Neurosurg. Psychiatry 2012, 83, 102–108. [Google Scholar] [CrossRef]
- Bersano, E.; Sarnelli, M.F.; Solara, V.; Lazzolino, B.; Peotta, L.; De Marchi, F.; Facchin, A.; Moglia, C.; Canosa, A.; Calvo, A.; et al. Decline of cognitive and behavioral functions in amyotrophic lateral sclerosis: A longitudinal study. Amyotroph. Later. Scler. Frontotemporal Degener. 2020, 1–7. [Google Scholar] [CrossRef]
- Chiò, A.; Moglia, C.; Canosa, A.; Manera, U.; Vasta, R.; Brunetti, M.; Barberis, M.; Corrado, L.; D’Alfonso, S.; Bersano, E.; et al. Cognitive impairment across ALS clinical stages in a population-based cohort. Neurology 2019, 93, e984–e994. [Google Scholar] [CrossRef]
- Chiò, A.; Pagani, M.; Agosta, F.; Calvo, A.; Cistaro, A.; Filippi, M. Neuroimaging in amyotrophic lateral sclerosis: Insights into structural and functional changes. Lancet Neurol. 2014, 13, 1228–1240. [Google Scholar] [CrossRef]
- Chiò, A.; Mora, G.; Moglia, C.; Manera, U.; Canosa, A.S.; Ilardi, A.; Bertuzzo, D.; Bersano, E.; Cugnasco, P.; Grassano, M.; et al. Secular Trends of Amyotrophic Lateral Sclerosis: The Piemonte and Valle d’Aosta Register. JAMA Neurol. 2017, 74, 1097–1104. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; Van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef] [PubMed]
- Strong, M.J.; Abrahams, S.; Goldstein, L.H.; Woolley, S.; Mclaughlin, P.; Snowden, J.; Mioshi, E.; Roberts-South, A.; Benatar, M.; HortobáGyi, T.; et al. Amyotrophic lateral sclerosis: Frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph. Later. Scler. Frontotemporal Degener. 2017, 18, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Iazzolino, B.; Pain, D.; Peotta, L.; Calvo, A.; Moglia, C.; Canosa, A.; Manera, U.; Ilardi, A.; Bombaci, A.; Zucchetti, J.P.; et al. Validation of the revised classification of cognitive and behavioural impairment in ALS. J. Neurol. Neurosurg. Psychiatry 2019, 90, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Calvo, A.; Moglia, C.; Mazzini, L.; Mora, G.; PARALS Study Group. Phenotypic heterogeneity of amyotrophic lateral sclerosis: A population based study. J. Neurol. Neurosurg. Psychiatry 2011, 82, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Eisen, A.; Braak, H.; Del Tredici, K.; Lemon, R.; Ludolph, A.C.; Kiernan, M.C. Cortical influences drive amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Neary, D.; Snowden, J.S.; Mann, D.M. Cognitive change in motor neurone disease/amyotrophic lateral sclerosis (MND/ALS). J. Neurol. Sci. 2000, 180, 15–20. [Google Scholar] [CrossRef]
- Kassubek, J.; Pagani, M. Imaging in amyotrophic lateral sclerosis: MRI and PET. Curr. Opin. Neurol. 2019, 32, 740–746. [Google Scholar] [CrossRef]
- Tsermentseli, S.; Leigh, P.N.; Goldstein, L.H. The anatomy of cognitive impairment in amyotrophic lateral sclerosis: More than frontal lobe dysfunction. Cortex 2012, 48, 166–182. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Van der Hoorn, A.; Burger, H.; Leenders, K.L.; De Jong, B.M. Handedness correlates with the dominant Parkinson side: A systematic review and meta-analysis. Mov. Disord. 2012, 27, 206–210. [Google Scholar] [CrossRef]
- Hammond, G. Correlates of human handedness in primary motor cortex: A review and hypothesis. Neurosci. Biobehav. Rev. 2002, 26, 285–292. [Google Scholar] [CrossRef]
- Priori, A.; Oliviero, A.; Donati, E.; Callea, L.; Bertolasi, L.; Rothwell, J.C. Human handedness and asymmetry of the motor cortical silent period. Exp. Brain Res. 1999, 128, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Devine, M.S.; Kiernan, M.C.; Heggie, S.; McCombe, P.A.; Henderson, R.D. Study of motor asymmetry in ALS indicates an effect of limb dominance on onset and spread of weakness, and an important role for upper motor neurons. Amyotroph. Later. Scler. Frontotemporal Degener. 2014, 15, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.N.; Seignourel, P.; Crucian, G.P.; Okun, M.S.; Rodriguez, R.L.; Skidmore, F.M.; Foster, P.S.; Jacobson, C.E.; Romrell, J.; Bowers, D.; et al. Laterality, region, and type of motor dysfunction correlate with cognitive impairment in Parkinson’s disease. Mov. Disord. 2007, 22, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, R.P.; Espay, A.J.; Morgante, F.; Li, J.-Y.; Teive, H.A.; Dunn, E.; Gallin, E.; Litvan, I. Long-duration Parkinson’s disease: Role of lateralization of motor features. Parkinsonism Relat. Disord. 2013, 19, 77–80. [Google Scholar] [CrossRef][Green Version]
- Abrahams, S.; Leigh, P.N.; Kew, J.J.; Goldstein, L.H.; Lloyd, C.M.; Brooks, D.J. A positron emission tomography study of frontal lobe function (verbal fluency) in amyotrophic lateral sclerosis. J. Neurol. Sci. 1995, 129, 44–46. [Google Scholar] [CrossRef]
- Christidi, F.; Karavasilis, E.; Zalonis, I.; Ferentinos, P.; Giavri, Z.; Wilde, E.A.; Xirou, S.; Rentzos, M.; Zouvelou, V.; Velonakis, G.; et al. Memory-related white matter tract integrity in amyotrophic lateral sclerosis: An advanced neuroimaging and neuropsychological study. Neurobiol. Aging 2017, 49, 69–78. [Google Scholar] [CrossRef]
- Varjacic, A.; Mantini, D.; Demeyere, N.; Gillebert, C.R. Neural signatures of Trail Making Test performance: Evidence from lesion-mapping and neuroimaging studies. Neuropsychologia 2018, 115, 78–87. [Google Scholar] [CrossRef]
- Gerton, B.K.; Brown, T.T.; Meyer-Lindenberg, A.; Kohn, P.; Holt, J.L.; Olsen, R.K.; Berman, K.F. Shared and distinct neurophysiological components of the digits forward and backward tasks as revealed by functional neuroimaging. Neuropsychologia 2004, 42, 1781–1787. [Google Scholar] [CrossRef]
- Aleman, A.; Van’t Wout, M. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex disrupts digit span task performance. Neuropsychobiology 2008, 57, 44–48. [Google Scholar] [CrossRef]
- Karádi, K.; Lucza, T.; Aschermann, Z.; Komoly, S.; Deli, G.; Bosnyák, E. Visuospatial impairment in Parkinson’s disease: The role of laterality. Laterality 2015, 20, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Poletti, M.; Frosini, D.; Pagni, C.; Baldacci, F.; Giuntini, M.; Mazzucchi, S.; Tognoni, G.; Lucetti, C.; Del Dotto, P.; Ceravolo, R.; et al. The relationship between motor symptom lateralization and cognitive performance in newly diagnosed drug-naïve patients with Parkinson’s disease. J. Clin. Exp. Neuropsychol. 2013, 35, 124–131. [Google Scholar] [CrossRef] [PubMed]
| Site/Side of Onset | Bulbar Onset | Right-Side Spinal Onset | Left-Side Spinal Onset | Bilateral Spinal Onset | Total | ||
|---|---|---|---|---|---|---|---|
| n (%r) | n (%r) | n (%r) | n (%r) | n (%r) | |||
| Total | 218 (35.8) | 174 (28.6) | 105 (17.2) | 112 (18.4) | 609 (100.0) | ||
| n (%c) | n (%c) | n (%c) | n (%c) | n (%c) | p # | ||
| Sex | <0.001 # | ||||||
| Male | 90 (41.3) | 115 (66.1) | 63 (60.0) | 78 (69.6) | 346 (56.8) | ||
| Female | 128 (58.7) | 59 (33.9) | 42 (40.0) | 34 (30.4) | 263 (43.2) | ||
| Site of Onset | <0.001 # | ||||||
| Bulbar Onset | 218 (100) | - | - | - | 218 (35.8) | ||
| Upper Limbs Onset | - | 107 (61.5) | 45 (42.9) | 22 (19.6) | 174 (28.6) | ||
| Lower Limbs Onset | - | 67 (38.5) | 60 (57.1) | 90 (80.4) | 217 (35.6) | ||
| Cognitive Classification | <0.001 # | ||||||
| ALS-CN | 102 (46.8) | 108 (62.1) | 68 (64.8) | 61 (54.5) | 339 (55.7) | ||
| ALSci | 15 (6.9) | 23 (13.2) | 11 (10.5) | 11 (9.8) | 60 (9.8) | ||
| ALSbi | 43 (19.7) | 25 (14.4) | 14 (13.3) | 25 (22.3) | 107 (17.6) | ||
| ALScbi | 17 (7.8) | 9 (5.2) | 6 (5.7) | 8 (7.1) | 40 (6.6) | ||
| ALS-FTD | 41 (18.8) | 9 (5.2) | 6 (5.7) | 7 (6.3) | 63 (10.3) | ||
| Median | Median | Median | Median | Median | p | ||
| (IQR) | (IQR) | (IQR) | (IQR) | (IQR) | |||
| Age at Onset (Years) | 70.0 | 67.0 | 66.0 | 70.5 | 69.0 | 0.007 * | |
| (62.7–76.0) | (58.0–74.0) | (59.0–73.0) | (61.2–75.7) | (60.0–74.0) | |||
| Education (Years) | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 0.221 * | |
| (5.0–11.0) | (5.0–11.0) | (5.0–11.0) | (5.0–11.0) | (5.0–12.0) | |||
| Neuropsychological Test | n | Median | Median | Median | Median | Median | p |
| (IQR) | (IQR) | (IQR) | (IQR) | (IQR) | |||
| MMSE | 603 | 27.4 | 28.0 | 28.5 | 27.4 | 27.8 | <0.001 * |
| (25.4-–29.2) | (26.5–30.0) | (27.3–30.0) | (26.0–29.4) | (26.3–30.0) | |||
| Letter Fluency Test | 584 | 25.4 | 31.3 | 31.0 | 27.4 | 28.6 | <0.001 * |
| (19.2-–33.1) | (23.5–38.6) | (23.6–39.1) | (20.9–34.8) | (21.9–35.4) | |||
| Category fluency test | 455 | 17.2 | 19.8 | 19.3 | 18.8 | 19.3 | 0.002 * |
| (14.0–21.0) | (16.5–23.1) | (16.5–22.5) | (15.5–23.9) | (15.5–22.3) | |||
| FAB | 505 | 14.7 | 15.2 | 15.3 | 14.7 | 14.9 | 0.048 * |
| (11.9–16.3) | (13.9–16.7) | (13.5–17.1) | (12.4–16.5) | (13.3–16.5) | |||
| Digit Span FW | 538 | 5.5 | 5.8 | 5.5 | 5.7 | 5.5 | 0.026 * |
| (4.7–6.1) | (5.1–6.5) | (4.9–6.2) | (5.1–6.2) | (4.9–6.3) | |||
| Digit Span BW | 442 | 3.7 | 4.1 | 3.8 | 3.7 | 3.9 | 0.013 * |
| (3.3–4.3) | (3.5–4.7) | (3.5–4.3) | (3.2–4.3) | (3.4–4.4) | |||
| TMT-A | 540 | 43.0 | 39.5 | 35.0 | 37.5 | 39.0 | 0.176 * |
| (28.0–76.0) | (23.3–63.5) | (26.0–52.0) | (25.0–54.0) | (26.0–62.0) | |||
| TMT-B | 540 | 94.0 | 82.0 | 61.0 | 76.5 | 79.0 | 0.024 * |
| (43.0–275.0) | (35.5–144.5) | (38.0–139.0) | (43.3–180.0) | (40.0–179.8) | |||
| TMT-B-A | 540 | 59.5 | 41.0 | 28.0 | 39.0 | 45.0 | 0.013 * |
| (13.3–155.0) | (10.5–90.0) | (7.0–94.5) | (16.0–120.5) | (12.0–121.0) | |||
| RAVLT-ir | 281 | 38.0 | 38.3 | 41.9 | 38.0 | 38.7 | 0.083 * |
| (30.1–44.1) | (31.6–45.5) | (34.3–46.6) | (34.0–43.2 | (32.8–44.5) | |||
| RAVLT-dr | 281 | 7.8 | 7.2 | 8.9 | 7.8 | 7.8 | 0.004 * |
| (4.8–10.3) | (5.1–10.4) | (7.2–11.2) | (5.7–9.9) | (5.6–10.2) | |||
| BSRT-ir | 289 | 5.5 | 5.8 | 6.4 | 5.3 | 5.7 | 0.005 * |
| (4.2–6.6) | (4.7–6.9) | (5.2–7.8) | (4.1–6.8) | (4.5–6.8) | |||
| BSRT-dr | 279 | 6.0 | 6.5 | 7.3 | 6.1 | 6.4 | <0.001 * |
| (4.5–7.2) | (4.8–7.8) | (6.1–8.0) | (4.7–7.4) | (4.9–7.6) | |||
| ROCFT-copy | 426 | 30.8 | 32.4 | 32.0 | 30.8 | 31.5 | 0.005 * |
| (23.1–33.8) | (29.3–35.1) | (29.5–34.4) | (26.3–34.1) | (26.9–34.5) | |||
| ROCFT-dr | 422 | 11.0 | 12.0 | 12.0 | 10.6 | 11.5 | 0.140 * |
| (7.5–15.9) | (8.8–16.6) | (7.4–15.4) | (7.0–14.3) | (7.7–15.8) | |||
| CPM47 | 574 | 27.4 | 29.5 | 29.3 | 27.5 | 28.5 | 0.012 * |
| (23.0–31.5) | (25.2–32.1) | (26.1–31.9) | (21.7–31.5) | (24.3–31.8) | |||
| WCST | 187 | 80.0 | 85.3 | 79.4 | 78.8 | 82.8 | 0.724 * |
| (62.8–92.8) | (58.5–98.7) | (35.1–96.5) | (43.5–94.1) | (52.8–93.9) |
| Neuropsychological Test | Bulbar (B) | Spinal Right-Onset (Sri) | Spinal Left-Onset (Sle) | Spinal Bil-Onset (Sbil) | B vs. Sri vs. Sle vs. Sbil | B vs. Sri | B vs. Sle | B vs. Sbil | Sri vs. Sle | Sri vs. Sbil | Sle vs. Sbil |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | p # | p * | p * | p * | p * | p * | p * | |
| MMSE | −0.02 | 0.19 | 0.40 | −0.02 | <0.001 | 0.009 | <0.001 | 0.999 | 0.999 | 0.179 | 0.003 |
| (−0.78–0.66) | (−0.35–0.96) | (−0.07–0.96) | (−0.55–0.74) | ||||||||
| Letter fluency test | −0.35 | 0.20 | 0.17 | −0.17 | <0.001 | <0.001 | 0.001 | 0.999 | 0.999 | 0.036 | 0.053 |
| (−0.93–0.36) | (−0.53–0.88) | (−0.51–0.93) | (−0.77–0.53) | ||||||||
| Category fluency test | −0.34 | 0.07 | −0.01 | −0.10 | 0.002 | 0.002 | 0.046 | 0.160 | 0.999 | 0.999 | 0.999 |
| (−0.88–0.36) | (−0.47–0.63) | (−0.47–0.53) | (−0.64–0.77) | ||||||||
| FAB | 0.06 | 0.24 | 0.28 | 0.08 | 0.048 | 0.092 | 0.479 | 0.999 | 0.999 | 0.327 | 0.916 |
| (−0.95–0.64) | (−0.23–0.78) | (−0.36–0.91) | (−0.76–0.69) | ||||||||
| Digit Span Forward | −0.11 | 0.15 | −0.09 | 0.05 | 0.026 | 0.035 | 0.999 | 0.187 | 0.999 | 0.999 | 0.999 |
| (−0.95–0.53) | (−0.54–0.87) | (−0.66–0.65) | (−0.54–0.59) | ||||||||
| Digit Span Backward | −0.14 | 0.21 | −0.12 | −0.14 | 0.013 | 0.009 | 0.999 | 0.999 | 0.405 | 0.149 | 0.999 |
| (−0.66–0.38) | (−0.40–0.82) | (−0.41–0.43) | (−0.78–0.45) | ||||||||
| TMT A | 0.22 | 0.29 | 0.38 | 0.33 | 0.176 | 0.579 | 0.310 | 0.982 | 0.999 | 0.999 | 0.999 |
| (−0.47–0.53) | (−0.21–0.62) | (0.03–0.57) | (−0.01–0.59) | ||||||||
| TMT B | 0.26 | 0.36 | 0.54 | 0.41 | 0.024 | 0.215 | 0.029 | 0.999 | 0.999 | 0.999 | 0.734 |
| (−1.24–0.69) | (−0.16–0.75) | (−0.11–0.73) | (−0.45–0.69) | ||||||||
| TMT B-A | 0.20 | 0.41 | 0.56 | 0.43 | 0.013 | 0.083 | 0.023 | 0.999 | 0.999 | 0.999 | 0.531 |
| (−0.91–0.73) | (−0.15–0.76) | (−0.21–0.80) | (−0.51–0.70) | ||||||||
| RAVLT-ir | −0.09 | −0.06 | 0.33 | −0.09 | 0.083 | 0.999 | 0.112 | 0.999 | 0.480 | 0.999 | 0.178 |
| (−0.94–0.57) | (−0.78–0.72) | (−0.50–0.84) | (−0.52–0.47) | ||||||||
| RAVLT-dr | 0.00 | −0.19 | 0.33 | −0.02 | 0.011 | 0.999 | 0.030 | 0.999 | 0.022 | 0.999 | 0.053 |
| (−0.92–0.74) | (−0.83–0.77) | (−0.19–1.03) | (−0.65–0.63) | ||||||||
| BSRT-ir | −0.06 | 0.09 | 0.41 | −0.13 | 0.005 | 0.297 | 0.004 | 0.999 | 0.390 | 0.999 | 0.095 |
| (−0.67–0.50) | (−0.41–0.65) | (−0.18–1.09) | (-0.71–0.59) | ||||||||
| BSRT-dr | −0.01 | 0.27 | 0.70 | 0.04 | <0.001 | 0.790 | <0.001 | 0.999 | 0.033 | 0.999 | 0.006 |
| (−0.80–0.63) | (−0.65–0.96) | (0.08–1.07) | (−0.69–0.76) | ||||||||
| ROCFT-copy | 0.22 | 0.43 | 0.39 | 0.22 | 0.005 | 0.007 | 0.227 | 0.999 | 0.999 | 0.129 | 0.678 |
| (−0.76–0.62) | (0.04–0.80) | (0.06–0.70) | (−0.35–0.66) | ||||||||
| ROCFT-dr | −0.15 | 0.01 | 0.01 | −0.22 | 0.140 | 0.337 | 0.999 | 0.999 | 0.999 | 0.228 | 0.999 |
| (−0.72–0.64) | (−0.52–0.74) | (−0.74–0.55) | (−0.80–0.38) | ||||||||
| CPM47 | −0.02 | 0.35 | 0.32 | 0.00 | 0.012 | 0.050 | 0.062 | 0.999 | 0.999 | 0.340 | 0.335 |
| (−0.79–0.70) | (−0.40–0.81) | (−0.24–0.78) | (−1.03–0.71) | ||||||||
| WCST TOT | 0.25 | 0.42 | 0.23 | 0.21 | 0.724 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
| (−0.31–0.66) | (−0.45–0.85) | (−1.21–0.78) | (−0.94–0.71) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manera, U.; Peotta, L.; Iazzolino, B.; Canosa, A.; Vasta, R.; Palumbo, F.; Torrieri, M.C.; Solero, L.; Daviddi, M.; Grassano, M.; et al. The Characteristics of Cognitive Impairment in ALS Patients Depend on the Lateralization of Motor Damage. Brain Sci. 2020, 10, 650. https://doi.org/10.3390/brainsci10090650
Manera U, Peotta L, Iazzolino B, Canosa A, Vasta R, Palumbo F, Torrieri MC, Solero L, Daviddi M, Grassano M, et al. The Characteristics of Cognitive Impairment in ALS Patients Depend on the Lateralization of Motor Damage. Brain Sciences. 2020; 10(9):650. https://doi.org/10.3390/brainsci10090650
Chicago/Turabian StyleManera, Umberto, Laura Peotta, Barbara Iazzolino, Antonio Canosa, Rosario Vasta, Francesca Palumbo, Maria Claudia Torrieri, Luca Solero, Margherita Daviddi, Maurizio Grassano, and et al. 2020. "The Characteristics of Cognitive Impairment in ALS Patients Depend on the Lateralization of Motor Damage" Brain Sciences 10, no. 9: 650. https://doi.org/10.3390/brainsci10090650
APA StyleManera, U., Peotta, L., Iazzolino, B., Canosa, A., Vasta, R., Palumbo, F., Torrieri, M. C., Solero, L., Daviddi, M., Grassano, M., Moglia, C., Pagani, M., Chiò, A., & Cavallo, M. (2020). The Characteristics of Cognitive Impairment in ALS Patients Depend on the Lateralization of Motor Damage. Brain Sciences, 10(9), 650. https://doi.org/10.3390/brainsci10090650






