An Assessment of the Motor Performance Skills of Children with Autism Spectrum Disorder in the Gulf Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
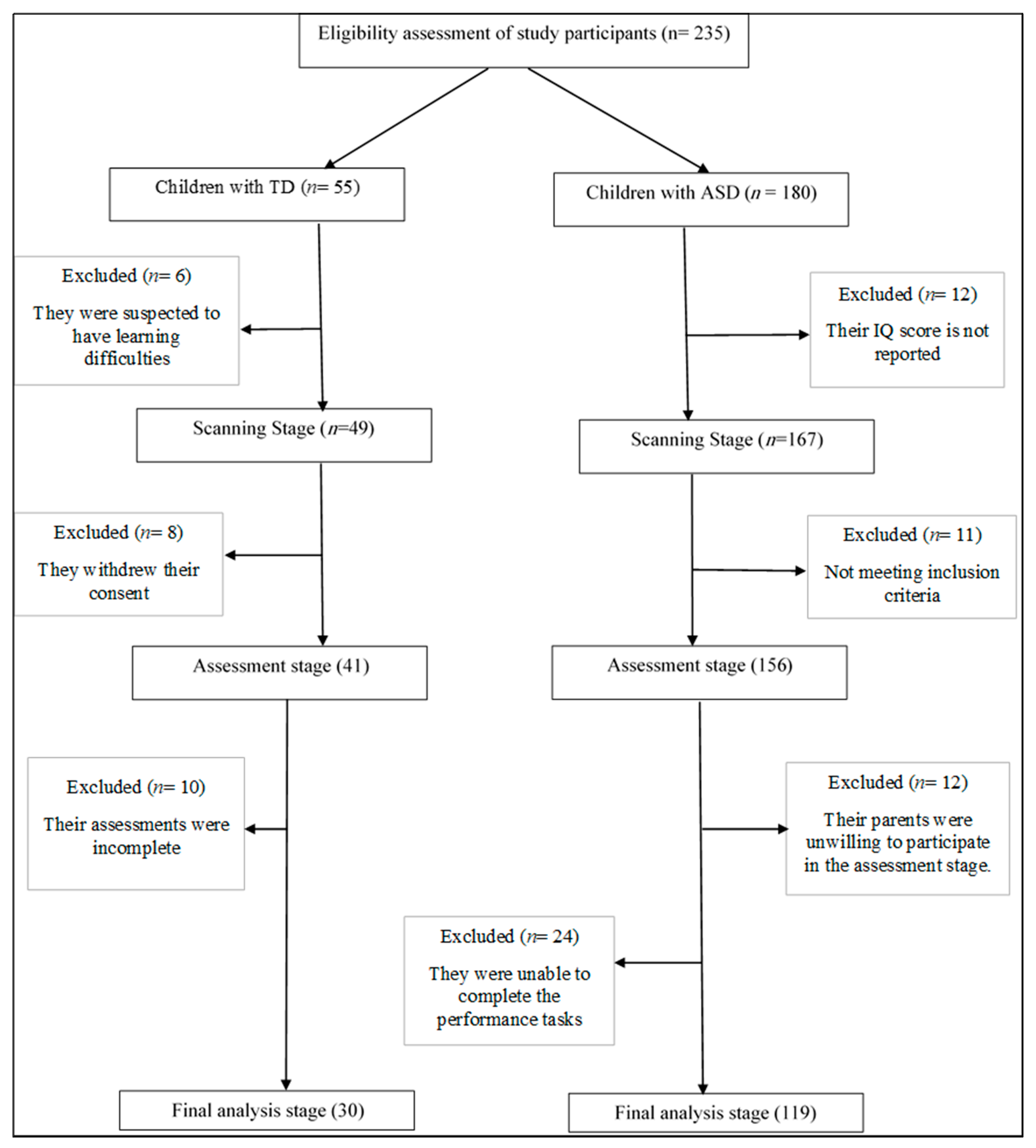
2.2. Participants
2.3. Instruments
2.3.1. Screening Questionnaires
- Gilliam Autism Rating Scale—Third Edition (GARS-3) [51]: After obtaining written permission from the publisher (Pro-Ed), the researcher had previously translated the GARS-3 and made certain cultural adaptations to facilitate its use in the Gulf region. The GARS-3 is a norm-referenced, standardised informant rating scale designed to identify and rate the severity of autism symptomatology in individuals. The GARS-3 items correspond to the diagnostic criteria for ASD set out in the DSM-5 [54]. It consists of 56 Likert-type items that comprise six subtests: restricted/repetitive behaviours (RB; 13 items), social interaction (SI; 14 items), social communication (SC; nine items), emotional responses (ER; eight items), cognitive style (CS; seven items), and maladaptive speech (MS; seven items). The summation of the subscales’ scaled scores yields the composite autism index, which is also reported in terms of the standard score, percentile rank, severity level, and probability of ASD. Two autism indices (four or six) can be formed, depending on whether or not the individual is mute. Higher scaled scores indicate increasingly severe autistic symptoms. Caregivers require approximately 5–10 min to complete the measure.
- Michigan Autism Spectrum Questionnaire (MASQ) [50]: The MASQ is based on the clinical characteristics that may be suggestive of Asperger’s syndrome (AS) or high-functioning ASD (HFA). It includes ten items representing two main functional areas: the quality of the social interaction patterns and the style of both the content and form of communication. The items are rated on a four-point (0–3) scale, with their sum yielding the total score (maximum 30). A cut-off score ≥ 22 is recommended to screen for individuals with HFA or AS. Cut-off scores between 14 and 21 are predictive of ASD or pervasive developmental disorder-not otherwise specified (PDD-NOS), while scores < 14 are predictive of other psychiatric disorders.
- The Clinician-Rated Severity of Autism Spectrum and Social Communication Disorders (CRSASSC) [49]: This two-item scale is used to assess the severity of an individual’s autistic symptoms and his/her level of functioning based on the amount of support required due to challenges associated with the social and communication (SC) domain and the restricted interests and repetitive behaviours (RRB) domain, respectively. Each domain is rated on a four-point Likert scale consistent with the DSM-5 diagnostic criteria: 0 (none), 1 (requiring support), 2 (requiring substantial support), or 3 (requiring very substantial support). The clinical criteria may also help to assign a specific functional level to an individual: mild (level 1), moderate (level 2), or severe (level 3). The level of severity for each item should be independently reported, and a combined overall severity score should not be calculated.
2.3.2. Assessment Measures
2.4. Procedures
2.5. Analysis
3. Results
3.1. The Proportion of Children with ASD Who Exhibit Motor Impairments
3.2. Comparing the ASD and TD Children Based on the BOT-2 Test Raw Scores
3.3. Relationship between Motor Performance and Age
4. Discussion
4.1. Prevalence of Children with ASD Who Exhibited Clinically Significant Motor Abnormalities
4.2. Distinct Nature of Motor Performance Exhibited by Children with ASD and Typically Developing Children
4.3. The Relationship between Age and Motor Performance
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Haibach, P.; Reid, G.; Collier, D. Motor Learning and Development, 2nd ed.; Human Kineticsl: Champaign, IL, USA, 2017. [Google Scholar]
- Piek, J.P.; Hands, B.; Licari, M.K. Assessment of motor functioning in the preschool period. Neuropsychol. Rev. 2012, 22, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Catama Bryan, V.; Calalang Wielm Mae, S.; Cada Renz Karlo, D.; Ballog Angelica, C.; Batton Kaylee, B.; Bigay Ma Lourdes, R.; Borje Denice Jan, J. Motor intervention activities for children with autism spectrum disorders. Int. J. Res. Stud. Psychol. 2017, 6, 27–42. [Google Scholar] [CrossRef]
- Libertus, K.; Hauf, P. Motor skills and their foundational role for perceptual, social, and cognitive development. Front. Psychol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Gowen, E.; Hamilton, A. Motor abilities in autism: A review using a computational context. J. Autism Dev. Disord. 2013, 43, 323–344. [Google Scholar] [CrossRef]
- Abu-Dahab, S.M.N.; Skidmore, E.R.; Holm, M.B.; Rogers, J.C.; Minshew, N.J. Motor and tactile-perceptual skill differences between individuals with high-functioning autism and typically developing individuals ages 5–21. J. Autism Dev. Disord. 2013, 43, 2241–2248. [Google Scholar] [CrossRef]
- Dowd, A.; Rinehart, N.; McGinley, J. Motor function in children with autism: Why is this relevant to psychologists? Clin. Psychol. 2010, 14, 90–96. [Google Scholar] [CrossRef]
- Hilton, C.L.; Zhang, Y.; Whilte, M.R.; Klohr, C.L.; Constantino, J. Motor impairment in sibling pairs concordant and discordant for autism spectrum disorders. Autism 2012, 16, 430–441. [Google Scholar] [CrossRef]
- Van Damme, T.; Simons, J.; Sabbe, B.; Van West, D. Motor abilities of children and adolescents with a psychiatric condition: A systematic literature review. World J. Psychiatry 2015, 5, 315. [Google Scholar] [CrossRef]
- Fournier, K.A.; Hass, C.J.; Naik, S.K.; Lodha, N.; Cauraugh, J.H. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. J. Autism Dev. Disord. 2010, 40, 1227–1240. [Google Scholar] [CrossRef]
- Green, D.; Charman, T.; Pickles, A.; Chandler, S.; Loucas, T.; Simonoff, E.; Baird, G. Impairment in movement skills of children with autistic spectrum disorders. Dev. Med. Child Neurol. 2009, 51, 311–316. [Google Scholar] [CrossRef]
- Liu, T.; Breslin, C.M. Fine and gross motor performance of the MABC-2 by children with autism spectrum disorder and typically developing children. Res. Autism Spectr. Disord. 2013, 7, 1244–1249. [Google Scholar] [CrossRef]
- Moseley, R.L.; Pulvermueller, F. What can autism teach us about the role of sensorimotor systems in higher cognition? New clues from studies on language, action semantics, and abstract emotional concept processing. Cortex 2018, 100, 149–190. [Google Scholar] [CrossRef] [PubMed]
- Whyatt, C.P.; Craig, C.M. Motor skills in children aged 7–10 years, diagnosed with autism spectrum disorder. J. Autism Dev. Disord. 2012, 42, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- McCleery, J.P.; Elliott, N.A.; Sampanis, D.S.; Stefanidou, C.A. Motor development and motor resonance difficulties in autism: Relevance to early intervention for language and communication skills. Front. Integr. Neurosci. 2013, 7, 30. [Google Scholar] [CrossRef]
- Ming, X.; Brimacombe, M.; Wagner, G.C. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 2007, 29, 565–570. [Google Scholar] [CrossRef]
- Dewey, D.; Cantell, M.; Crawford, S.G. Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. J. Int. Neuropsychol. Soc. 2007, 13, 246–256. [Google Scholar] [CrossRef]
- Nobile, M.; Perego, P.; Piccinini, L.; Mani, E.; Rossi, A.; Bellina, M.; Molteni, M. Further evidence of complex motor dysfunction in drug naive children with autism using automatic motion analysis of gait. Autism 2011, 15, 263–283. [Google Scholar] [CrossRef]
- Travers, B.G.; Powell, P.S.; Klinger, L.G.; Klinger, M.R. Motor difficulties in autism spectrum disorder: Linking symptom severity and postural stability. J. Autism Dev. Disord. 2013, 43, 1568–1583. [Google Scholar] [CrossRef]
- Jeste, S.S. The neurology of autism spectrum disorders. Curr. Opin. Neurol. 2011, 24, 132–139. [Google Scholar] [CrossRef]
- Stefanatos, G.A. Autism Spectrum Disorders. In The Neuropsychology of Psychopathology; Noggle, C., Dean, R., Eds.; Springer Publishing Company: New York, NY, USA, 2013; pp. 97–170. [Google Scholar]
- Mostofsky, S.H.; Powell, S.K.; Simmonds, D.J.; Goldberg, M.C.; Caffo, B.; Pekar, J.J. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain 2009, 132, 2413–2425. [Google Scholar] [CrossRef]
- Takarae, Y.; Minshew, N.J.; Luna, B.; Sweeney, J.A. Atypical involvement of frontostriatal systems during sensorimotor control in autism. Psychiatry Res. Neuroimaging 2007, 156, 117–127. [Google Scholar] [CrossRef] [PubMed]
- McAlonan, G.M.; Suckling, J.; Wong, N.; Cheung, V.; Lienenkaemper, N.; Cheung, C.; Chua, S.E. Distinct patterns of grey matter abnormality in high-functioning autism and Asperger’s syndrome. J. Child Psychol. Psychiatry 2008, 49, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, N.J.; Tonge, B.J.; Iansek, R.; McGinley, J.; Brereton, A.V.; Enticott, P.G.; Bradshaw, J.L. Gait function in newly diagnosed children with autism: Cerebellar and basal ganglia related motor disorder. Dev. Med. Child Neurol. 2006, 48, 819–824. [Google Scholar] [CrossRef]
- Cairney, J.; King-Dowling, S. Developmental coordination disorder. In Comorbid Conditions among Children with Autism Spectrum Disorders; Matson, J.L., Ed.; Springer: Berlin, Germany, 2016; pp. 303–322. [Google Scholar]
- Mosconi, M.W.; Sweeney, J.A. Sensorimotor dysfunctions as primary features of autism spectrum disorders. Sci. China Life Sci. 2015, 58, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Pluta, M. Parental perceptions of the effect of child participation in hippotherapy programme on overall improvement of child mental and physical wellbeing. Ann. UMCS Zootech. 2011, 29, 74–84. [Google Scholar] [CrossRef]
- Pan, C.Y. Motor proficiency and physical fitness in adolescent males with and without autism spectrum disorders. Autism 2014, 18, 156–165. [Google Scholar] [CrossRef]
- Travers, B.G.; Kana, R.K.; Klinger, L.G.; Klein, C.L.; Klinger, M.R. Motor learning in individuals with autism spectrum disorder: Activation in superior parietal lobule related to learning and repetitive behaviors. Autism Res. 2015, 8, 38–51. [Google Scholar] [CrossRef]
- Choi, B.; Leech, K.A.; Tager-Flusberg, H.; Nelson, C.A. Development of fine motor skills is associated with expressive language outcomes in infants at high and low risk for autism spectrum disorder. J. Neurodev. Disord. 2018, 10, 14. [Google Scholar] [CrossRef]
- Flanagan, J.E.; Landa, R.; Bhat, A.; Bauman, M. Head lag in infants at risk for autism: A preliminary study. Am. J. Occup. Ther. 2012, 66, 577–585. [Google Scholar] [CrossRef]
- Landa, R.; Garrett-Mayer, E. Development in infants with autism spectrum disorders: A prospective study. J. Child Psychol. Psychiatry 2006, 47, 629–638. [Google Scholar] [CrossRef]
- Lloyd, M.; MacDonald, M.; Lord, C. Motor skills of toddlers with autism spectrum disorders. Autism 2013, 17, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Pasca, S. Motor abnormalities as a putative endophenotype for Autism Spectrum Disorders. Front. Integr. Neurosci. 2013, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Marrus, N.; Eggebrecht, A.T.; Todorov, A.; Elison, J.T.; Wolff, J.J.; Cole, L.; Emerson, R.W. Walking, gross motor development, and brain functional connectivity in infants and toddlers. Cereb. Cortex 2017, 28, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Ozonoff, S.; Young, G.S.; Goldring, S.; Greiss-Hess, L.; Herrera, A.M.; Steele, J.; Rogers, S.J. Gross motor development, movement abnormalities, and early identification of autism. J. Autism Dev. Disord. 2008, 38, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.N.; Galloway, J.C.; Landa, R.J. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behav. Dev. 2012, 35, 838–846. [Google Scholar] [CrossRef]
- Baranek, G.T.; Parham, L.D.; Bodfish, J.W. Sensory and motor features in autism: Assessment and intervention. In Handbook of Autism and Pervasive Developmental Disorders; Volkmar, F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 831–857. [Google Scholar]
- Setoh, P.; Marschik, P.B.; Einspieler, C.; Esposito, G. Autism spectrum disorder and early motor abnormalities: Connected or coincidental companions? Res. Dev. Disabil. 2005, 60, 13–15. [Google Scholar] [CrossRef]
- Williams JH, G.; Whiten, A.; Singh, T. A systematic review of action imitation in autistic spectrum disorder. J. Autism Dev. Disord. 2004, 34, 285–299. [Google Scholar] [CrossRef]
- Chinello, A.; Di Gangi, V.; Valenza, E. Persistent primary reflexes affect motor acts: Potential implications for autism spectrum disorder. Res. Dev. Disabil. 2018, 83, 287–295. [Google Scholar] [CrossRef]
- Piek, J. Infant Motor Development; Human Kinetics: Champaign, IL, USA, 2006. [Google Scholar]
- Darrah, J.; Hodge, M.; Magill-Evans, J.; Kembhavi, G. Stability of serial assessments of motor and communication abilities in typically developing infants—Implications for screening. Early Hum. Dev. 2003, 72, 97–110. [Google Scholar] [CrossRef]
- Pless, M.; Carlsson, M.; Sundelin, C.; Persson, K. Preschool children with developmental coordination disorder: A short-term follow-up of motor status at seven to eight years of age. Acta Paediatr. 2002, 91, 521–528. [Google Scholar] [CrossRef]
- Van Waelvelde, H.; Oostra, A.; Dewitte, G.; Van Den Broeck, C.; Jongmans, M.J. Stability of motor problems in young children with or at risk of autism spectrum disorders, ADHD, and/or developmental coordination disorder. Dev. Med. Child Neurol. 2010, 52, e174–e178. [Google Scholar] [CrossRef] [PubMed]
- Baumer, F.; Sahin, M. Neurological comorbidities in autism spectrum disorder. In Autism Spectrum Disorder; McDougle, C.J., Ed.; Oxford University Press: Oxford, UK, 2016; pp. 99–116. [Google Scholar]
- Bruininks, R.H.; Bruininks, B.D. Bruininks-Oseretsky Test of Motor Proficiency, 2nd ed.; Pearson Assessments: Minneapolis, MN, USA, 2005. [Google Scholar]
- American Psychiatric Association. DSM-5 Task Force. The Clinician-Rated Severity of Autism Spectrum and Social Communication Disorders. 2013. Available online: http://www.psychiatry.org/dsm5 (accessed on 29 February 2020).
- Ghaziuddin, M.; Welch, K. The Michigan Autism Spectrum Questionnaire: A Rating Scale for High-Functioning Autism Spectrum Disorders. Autism Res. Treat. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, J.E. Gilliam Autism Rating Scale, 3rd ed.; Pro-Ed: Austin, TX, USA, 2013. [Google Scholar]
- Oldfield, R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Raven, J.; Raven, J.; Court, J. Manual for Raven’s Progressive Matrices and Vocabulary Scales; Oxford Psychologists Press: Oxford, UK, 1998. [Google Scholar]
- American Psychiatric Association. DSM-5 Task Force, & American Psychiatric Association. In Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Holloway, J.M.; Long, T.M.; Biasini, F. Relationships between gross motor skills and social function in young boys with autism spectrum disorder. Pediatric Phys. Ther. 2018, 30, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Breslin, C.M.; ElGarhy, S. Methods and Procedures for Measuring Comorbid Disorders: Motor Movement and Activity. In Comorbid Conditions among Children with Autism Spectrum Disorders; Matson, J.L., Ed.; Springer: Berlin, Germany, 2015; pp. 91–134. [Google Scholar]
- Deitz, J.C.; Kartin, D.; Kopp, K. Review of the Bruininks-Oseretsky test of motor proficiency, (BOT-2). Phys. Occup. Ther. Pediatr. 2007, 27, 87–102. [Google Scholar] [CrossRef]
- Okuda, P.; Pangelinan, M.; Capellini, S.; Cogo-Moreira, H. Motor skills assessments: Support for a general motor factor for the Movement Assessment Battery for Children-2 and the Bruininks-Oseretsky Test of Motor Proficiency-2. Trends Psychiatry Psychother. 2019, 41, 51–59. [Google Scholar] [CrossRef]
- Jírovec, J.; Musálek, M.; Mess, F. Test of motor proficiency second edition (bot-2): Compatibility of the complete and short form and its usefulness for middle-age school children. Front. Pediatr. 2019, 7, 153. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Hilton, C.L.; Attal, A.; Best, J.R.; Reistetter, T.; Trapani, P.; Collins, D. Exergaming to improve physical and mental fitness in children and adolescents with autism spectrum disorders: Pilot study. Int. J. Sports Exerc. Med. 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Mattard-Labrecque, C.; Amor, L.B.; Couture, M.M. Children with autism and attention difficulties: A pilot study of the association between sensory, motor, and adaptive behaviors. J. Can. Acad. Child Adolesc. Psychiatry 2013, 22, 139. [Google Scholar]
- Olzenak, D.L. Adding Motor Assessment to the Disability Determination Process in School-Aged Children with ASD: Implications for Participation. 2015. Available online: https://ddp.policyresearchinc.org/wp-content/uploads/2015/09/Olzenak_Final-Report.pdf (accessed on 5 December 2019).
- Hilton, C.L.; Cumpata, K.; Klohr, C.; Gaetke, S.; Artner, A.; Johnson, H.; Dobbs, S. Effects of exergaming on executive function and motor skills in children with autism spectrum disorder: A pilot study. Am. J. Occup. Ther. Off. Publ. Am. Occup. Ther. Assoc. 2014, 68, 57–65. [Google Scholar] [CrossRef]
- Mosconi, M.W.; Takarae, Y.; Sweeney, J.A. Motor functioning and dyspraxia in autism spectrum disorders. In Autism Spectrum Disorder; Amaral, D.G., Dawson, G., Geschwind, H.D., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 355–380. [Google Scholar]
- Gaul, D.; Issartel, J. Fine motor skill proficiency in typically developing children: On or off the maturation track? Hum. Mov. Sci. 2016, 46, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Maksoud, G. Fine motor skill proficiency in children with and without down syndrome. J. Phys. Ther. Health Promot. 2016, 4, 43–50. [Google Scholar] [CrossRef]
- Wrotniak, B.H.; Epstein, L.H.; Dorn, J.M.; Jones, K.E.; Kondilis, V.A. The relationship between motor proficiency and physical activity in children. Pediatrics 2006, 118, e1758–e1765. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, B.; Nasser, R. The closeness of the child to the domestic servant and its mediation by negative parenting behaviors in an Arab Gulf country. In Proceedings of the Australasian Conference on Business and Social Sciences, Sydney, Australia, 6 December 2015; pp. 258–269. [Google Scholar]
- Duncan, M.J.; Spence, J.C.; Mummery, W.K. Perceived environment and physical activity: A meta-analysis of selected environmental characteristics. Int. J. Behav. Nutr. Phys. Act. 2005, 2, 11. [Google Scholar] [CrossRef]
- Zeng, N.; Ayyub, M.; Sun, H.; Wen, X.; Xiang, P.; Gao, Z. Effects of physical activity on motor skills and cognitive development in early childhood: A systematic review. Biomed. Res. Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Al-Heizan, M.O.; Al-Abdulwahab, S.S.; Kachanathu, S.J.; Natho, M. Sensory processing dysfunction among Saudi children with and without autism. J. Phys. Ther. Sci. 2015, 27, 1313–1316. [Google Scholar] [CrossRef]
- Alkhalifah, S. Psychometric properties of the Sensory Processing Measure Preschool-Home among Saudi children with autism spectrum disorder: Pilot study. J. Occup. Ther. Sch. Early Interv. 2019, 12, 401–416. [Google Scholar] [CrossRef]
- Must, A.; Phillips, S.; Curtin, C.; Bandini, L.G. Barriers to physical activity in children with autism spectrum disorders: Relationship to physical activity and screen time. J. Phys. Act. Health 2015, 12, 529–534. [Google Scholar] [CrossRef]
- Patz, J.A.; Messina, R.M. Fine motor, oral motor, and self-care development. In Young Children with Special Needs, 5th ed.; Hooper, S.R., Umansky, W., Eds.; Pearson: Upper Saddle River, NJ, USA, 2009; pp. 168–235. [Google Scholar]
- McCarton, C. Assessment and diagnosis of pervasive developmental disorder. In Autism Spectrum Disorders; Hollander, E., Ed.; Dekker: New York, NY, USA, 2003; pp. 101–132. [Google Scholar]
- Coffin, A.B.; Myles, B.S.; Rogers, J.; Szakacs, W. Supporting the Writing Skills of Individuals with Autism Spectrum Disorder through Assistive Technologies. In Technology and the Treatment of Children with Autism Spectrum Disorder; Cardon, T.A., Ed.; Springer: Berlin, Germany, 2016; pp. 59–73. [Google Scholar]
- Abdel Karim, A.E.; Mohammed, A.H. Effectiveness of sensory integration program in motor skills in children with autism. Egypt. J. Med Hum. Genet. 2015, 16, 375–380. [Google Scholar] [CrossRef]
- Betts, D.; Jacobs, D. Everyday Activities to Help Your Young Child with Autism Live Life to the Full: Simple Exercises to Boost Functional Skills, Sensory Processing, Coordination and Self-Care; Jessica Kingsley Publishers: London, UK, 2011. [Google Scholar]
- Semel, E.; Rosner, S. Understanding Williams Syndrome: Behavioral Patterns and Interventions; L. Erlbaum: Mahwah, NJ, USA, 2003. [Google Scholar]
- Hilton, C.; Wente, L.; LaVesser, P.; Ito, M.; Reed, C.; Herzberg, G. Relationship between motor skill impairment and severity in children with Asperger syndrome. Res. Autism Spectr. Disord. 2007, 1, 339–349. [Google Scholar] [CrossRef]
- Staples, K.L.; Reid, G. Fundamental movement skills and autism spectrum disorders. J. Autism Dev. Disord. 2010, 40, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Zikl, P.; Petrů, D.; Daňková, A.; Doležalová, H.; Šafaříková, K. Motor skills of children with autistic spectrum disorder. In SHS Web of Conferences; EDP Sciences: Les Ulis, France, 2016; Volume 26, p. 1076. [Google Scholar]
- Paris, B. Characteristics of autism. In Exploring the Spectrum of Autism and Pervasive Developmental Disorders; Murray-Slutsky, C., Paris, B., Eds.; Therapy Skill Builders (Harcourt Health Sciences): San Antonio, TX, USA, 2000; pp. 7–23. [Google Scholar]
- Dejean, V.M. Vestibular Re Integration of the Autistic Child: Developmental Model for Autism; iUniverse, Inc.: New York, NY, USA, 2008. [Google Scholar]
- Coetzee, D. Strength, running speed, agility and balance profiles of 9-to 10-year-old learners: NW-child study. S. Afr. J. Res. Sport Phys. Educ. Recreat. 2016, 38, 13–30. [Google Scholar]
- Deponio, P.; Macintyre, C. Identifying and Supporting Children with Specific Learning Difficulties: Looking Beyond the Label to Support the Whole Child; Routledge: London, UK, 2003. [Google Scholar]
- Ament, K.; Mejia, A.; Buhlman, R.; Erklin, S.; Caffo, B.; Mostofsky, S.; Wodka, E. Evidence for specificity of motor impairments in catching and balance in children with autism. J. Autism Dev. Disord. 2015, 45, 742–751. [Google Scholar] [CrossRef]
- Molloy, C.A.; Dietrich, K.N.; Bhattacharya, A. Postural stability in children with autism spectrum disorder. J. Autism Dev. Disord. 2003, 33, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Van Sant, A.; Goldberg, C. Normal motor development. In Pediatric Physical Therapy; Tecklin, J.S., Ed.; Lippincott Williams & Wilkins: New York, NY, USA, 1999; pp. 22–25. [Google Scholar]
- DeLong, G.R. The cerebellum in autism. In The Neurology of Autism; Coleman, M., Ed.; Oxford University Press: Oxford, UK, 2005; pp. 75–90. [Google Scholar]
- Pusponegoro, H.D.; Efar, P.; Soebadi, A.; Firmansyah, A.; Chen, H.J.; Hung, K.L. Gross motor profile and its association with socialization skills in children with autism spectrum disorders. Pediatr. Neonatol. 2016, 57, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Noterdaeme, M.; Mildenberger, K.; Minow, F.; Amorosa, H. Evaluation of neuromotor deficits in children with autism and children with a specific speech and language disorder. Eur. Child Adolesc. Psychiatry 2002, 11, 219–225. [Google Scholar] [CrossRef]
- Dixon, G.; Addy, L. Making Inclusion work for Children with Dyspraxia: Practical Strategies for Teachers; Routledge Falmer: London, UK, 2004. [Google Scholar]
- Memari, A.H.; Ghanouni, P.; Shayestehfar, M.; Ghaheri, B. Postural control impairments in individuals with autism spectrum disorder: A critical review of current literature. Asian J. Sports Med. 2014, 5, e22963. [Google Scholar] [CrossRef]
- Bhat, A.N.; Landa, R.J.; Galloway, J.C. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Phys. Ther. 2011, 91, 1116–1129. [Google Scholar] [CrossRef]
- Attwood, T. The Complete Guide to Asperger’s Syndrome; Jessica Kingsley Publishers: London, UK, 2006. [Google Scholar]
- Baetti, S. Argentinian ambulatory integral model to treat autism spectrum disorders. In Autism: The Movement Sensing Perspective; Torres, E.B., Whyatt, C., Eds.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 253–269. [Google Scholar]
- Dirksen, T.; Lussanet, D.; Marc, H.E.; Zentgraf, K.; Slupinski, L.; Wagner, H. Increased Throwing Accuracy Improves Children’s Catching Performance in a Ball-Catching Task from the Movement Assessment Battery (MABC-2). Front. Psychol. 2016, 7, 1122. [Google Scholar] [CrossRef]
- Emck, C.; Bosscher, R.; Beek, P.; Doreleijers, T. Gross motor performance and self-perceived motor competence in children with emotional, behavioural, and pervasive developmental disorders: A review. Dev. Med. Child Neurol. 2009, 51, 501–517. [Google Scholar] [CrossRef]
- Neporent, L. Fitness Walking for Dummies; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Siri, K.; Lyons, T. Cutting-Edge Therapies for Autism; Skyhorse Publishing Inc.: New York, NY, USA, 2014. [Google Scholar]
- Mayall, L.A.; D’Souza, H.; Hill, E.L.; Karmiloff-Smith, A.; Tolmie, A.; Farran, E.K. Motor Abilities and the Motor Profile in Individuals with Williams Syndrome. Adv. Neurodev. Disord. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Wilson, R.B.; Enticott, P.G.; Rinehart, N.J. Motor development and delay: Advances in assessment of motor skills in autism spectrum disorders. Curr. Opin. Neurol. 2018, 31, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Jasmin, E.; Couture, M.; McKinley, P.; Reid, G.; Fombonne, E.; Gisel, E. Sensori-motor and daily living skills of preschool children with autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 231–241. [Google Scholar] [CrossRef]
- MacDonald, M.; Lord, C.; Ulrich, D.A. The relationship of motor skills and social communicative skills in school-aged children with autism spectrum disorder. Adapt. Phys. Act. Q. 2013, 30, 271–282. [Google Scholar] [CrossRef]
- Purpura, G.; Fulceri, F.; Puglisi, V.; Masoni, P.; Contaldo, A. Motor coordination impairment in children with autism spectrum disorder: A pilot study using Movement Assessment Battery for Children-2 Checklist. Minerva Pediatrica 2016, 72. [Google Scholar] [CrossRef] [PubMed]
| Comparison Characteristics | Target Group (N = 119) Children with ASD | Control Group (N = 30) TD Children | t/χ2 | p | ES | |
|---|---|---|---|---|---|---|
| Continuous variables | ||||||
| Age (years) | 8.72 (1.96) * | 9.06 (1.42) * | 1.05 | 0.71 | 0.21 | |
| Non-verbal IQ | 29.76 (1.92) * | 29.80 (2.64) * | 0.69 | 0.060 | 0.17 | |
| Categorical variables | ||||||
| Gender | Male | 95 (79.8%) | 24 (80.0%) | 0.00 | 0.98 | - |
| Female | 24 (20.2%) | 6 (20.0%) | ||||
| Handedness | Right | 104 (87.4%) | 23 (76.7%) | 2.191 | 0.14 | - |
| Lift | 15 (12.6%) | 7(23.3%) | ||||
| Father’s education level | Secondary | 67 (56.3%) | 16 (53.3%) | 0.09 | 0.77 | - |
| College degree | 52 (43.7%) | 14 (46.7%) | ||||
| Mother’s education level | Secondary | 78 (65.5%) | 20 (66.7%) | 0.01 | 0.91 | - |
| College degree | 41(34.5%) | 10 (33.3%) | ||||
| The BOT-2 Subtest | Item | Assessment |
|---|---|---|
| Subtest 1 Fine Motor Precision | Drawing Lines Through Paths | # of Errors |
| Folding Paper | # of Errors | |
| Subtest 2 Fine Motor Integration | Copying a Square | # of Errors |
| Copying a Star | # of Errors | |
| Subtest 3 Manual Dexterity | Transferring Pennies | # of Pennies in 15 s |
| Subtest 4 Bilateral Coordination | Jumping in Place-Same Sides Synchronized | Repetitions |
| Tapping Feet and Fingers-Same Sides Synchronized | Repetitions | |
| Subtest 5 Balance | walking Forward on a Line | Steps |
| Standing on One Leg on a Balance Beam-Eyes Open | Time | |
| Subtest 6 Running Speed Agility | One-Legged Stationary Hop | # of Hops in 15 s |
| Subtest 7 Upper-Limb Coordination | Dropping and Catching a Ball-Both Hands | Catches |
| Dropping a Ball-Alternating Hands | Dribbles | |
| Subtest 8 Strength | Full Push-Ups | # Performed in 30 s |
| Sit-Ups | # Performed in 30 s |
| BOT-2 | ASD Sample (N = 119) | 95% Confidence Interval for the Mean | One-Sample Z-Test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Standard score for the total motor composite * | Min | Max | M | SD | SE | Lower | Upper | Z | p | d |
| 47.0 | 85.0 | 31.90 | 5.03 | 0.46 | 30.98 | 32.81 | −19.74 | <0.001 | 1.81 | |
| The BOT-2 Test Scores | ASD Sample (N = 119) | TDC Group (N = 30) | t-Statistic | p | D | ||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Fine Motor Precision | 5.38 | 2.66 | 10.17 | 2.10 | 9.171 *** | <0.001 *** | 1.99 |
| Fine Motor Integration | 7.43 | 2.17 | 9.53 | 1.36 | 6.623 *** | <0.001 *** | 1.16 |
| Manual Dexterity | 3.28 | 1.62 | 6.33 | 1.56 | 9.322 *** | <0.001 *** | 1.92 |
| Bilateral Coordination | 4.60 | 1.68 | 6.67 | 0.85 | 9.489 *** | <0.001 *** | 1.55 |
| Balance | 5.69 | 1.10 | 7.30 | 0.75 | 7.563 *** | <0.001 *** | 1.71 |
| Running Speed and Agility | 3.08 | 2.03 | 6.97 | 1.40 | 12.267 *** | <0.001 *** | 2.23 |
| Upper-Limb Coordination | 5.13 | 2.80 | 10.20 | 1.73 | 12.472 *** | <0.001 *** | 2.18 |
| Strength | 3.21 | 2.08 | 8.97 | 2.24 | 13.352 *** | <0.001 *** | 2.66 |
| Total Motor Composite | 37.79 | 12.25 | 66.13 | 8.24 | 15.097 *** | <0.001 *** | 2.71 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaedi, R.H. An Assessment of the Motor Performance Skills of Children with Autism Spectrum Disorder in the Gulf Region. Brain Sci. 2020, 10, 607. https://doi.org/10.3390/brainsci10090607
Alsaedi RH. An Assessment of the Motor Performance Skills of Children with Autism Spectrum Disorder in the Gulf Region. Brain Sciences. 2020; 10(9):607. https://doi.org/10.3390/brainsci10090607
Chicago/Turabian StyleAlsaedi, Rehab H. 2020. "An Assessment of the Motor Performance Skills of Children with Autism Spectrum Disorder in the Gulf Region" Brain Sciences 10, no. 9: 607. https://doi.org/10.3390/brainsci10090607
APA StyleAlsaedi, R. H. (2020). An Assessment of the Motor Performance Skills of Children with Autism Spectrum Disorder in the Gulf Region. Brain Sciences, 10(9), 607. https://doi.org/10.3390/brainsci10090607




