Preliminary Evidence of the Role of Medial Prefrontal Cortex in Self-Enhancement: A Transcranial Magnetic Stimulation Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Materials
2.3. Stimuli
2.4. Transcranial Magnetic Stimulation (TMS) Procedure
2.5. Measures of Overclaiming
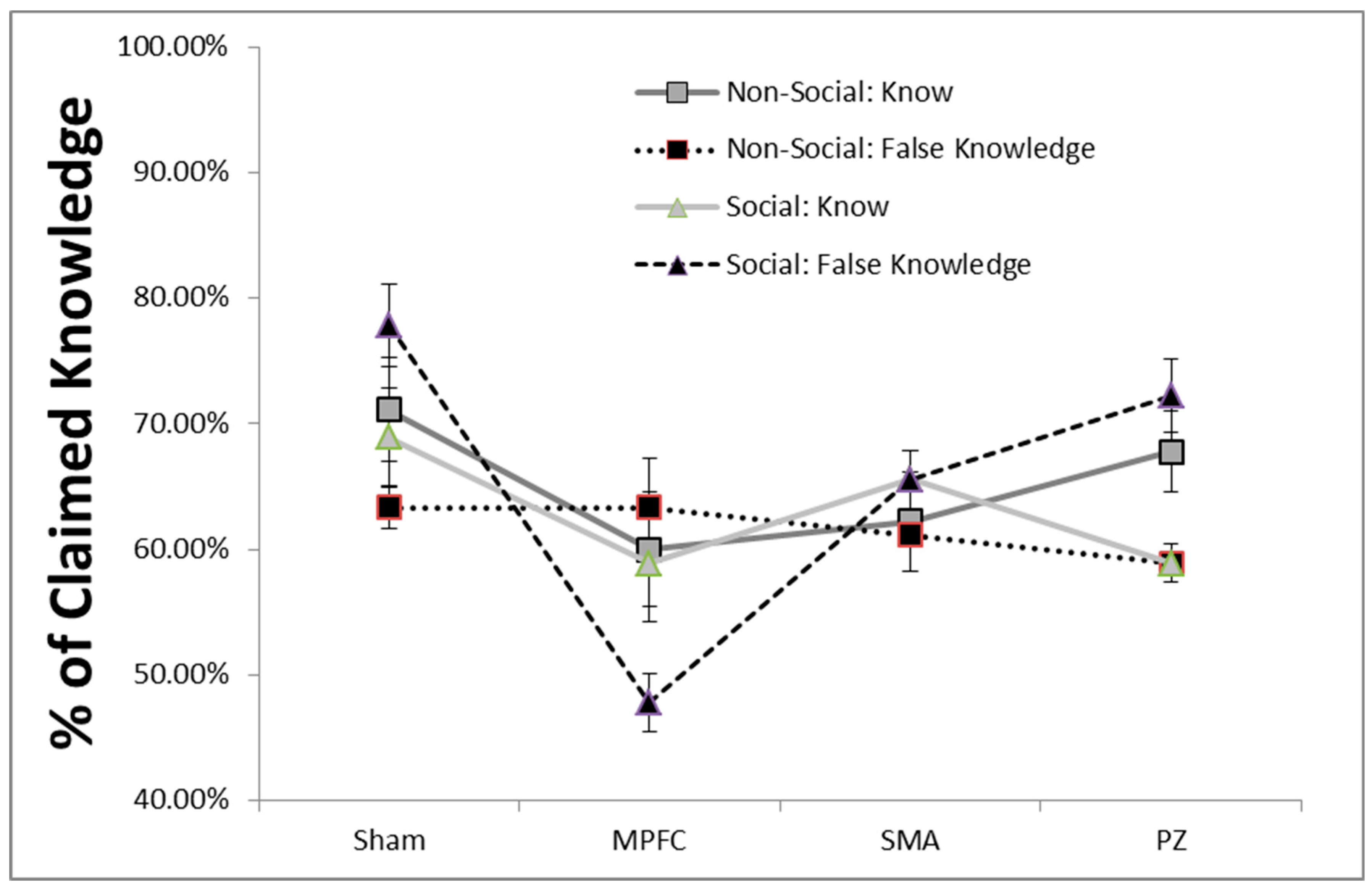
3. Results
4. Discussion
Caveats
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Real Words | Fake Words |
|---|---|
| Lepidoptery | Midfloral |
| Follicle | Vorax |
| Burette | Midternal |
| Lobotomy | Perchery |
| Cognizant | Dex |
| Entomology | Inosmosis |
| Allele | Tribution |
| Amorphous | Prevoid |
| Polymerization | Suffaltible |
| Scalar | Halible |
| Zygote | Formatic |
| Viscosity | Eratifinatic |
| Quark | Ellictitive |
| Artery | Myric |
| Dogmatic | Nampercilious |
| Exocytosis | Precilious |
| Morass | Exultion |
| Opulent | Tibula |
| Tribulation | Saprocy |
| Insipid | Sepititious |
| Supercilious | Oblongular |
| Laud | Rancidious |
| Xenophobia | Geolithic |
| Omnipotent | Trisectomy |
| Unequivocal | Recticious |
| Abdicate | Gruffle |
| Flagellum | Plumistic |
| Extravagant | Berythium |
| Iridescent | Allurium |
| Disgruntled | Florabane |
| Colloquial | Eloquacious |
| Perpetuity | Oblivia |
| Zygomaticus | Prevoid |
| Foment | Catosynthesis |
| Rhizome | Midternal |
| Nazca lines | Bellignorant |
| Intrinsic | Promedic |
| Homo erectus | Bluther |
| Unscrupulous | Lchistamine |
| Soliloquy | Reccentric |
| Justification | Geomorphile |
| The Defenestration of Prague | Paradign |
| Indefinite | Accentrax |
| Clairvoyant | Paradocial |
| Auspicious | Prerecuite |
| Trivial | Morphinil |
| Maleficent | Yusteric |
| Fission | Protrake |
| Inclement | Gauderate |
| Aglet | Quartom |
| Umlaut | Polycerics |
| Latus rectum | Dright |
| Paraben | Hapsate |
| Palm frond | Carcolepsy |
| Turpentine | Jentacular |
| Philtrum | Ternal |
| Uvula | Mimsy |
| Cornichon | Gleek |
| Directrix | Glumium |
| Occipital | Skoch |
| Duodenum | Flubium |
| Clad | Bleeale |
| Dwell | Tongressiant |
| Impel | Lymispre |
| Accede | Guniquated |
| Rebuke | Agnosary |
| Cede | Esagnacious |
| Facile | Nuwmerts |
| Entreat | Craderdon |
| Earnest | Invinceclace |
| Exalt | Logistorgian |
| Ensue | Greepaling |
References
- Andrejevic, M.; Meshi, D.; Bos, W.V.D.; Heekeren, H.R. Individual differences in social desirability are associated with white-matter microstructure of the external capsule. Cogn. Affect. Behav. Neurosci. 2017, 17, 1255–1264. [Google Scholar] [CrossRef]
- Kwan, V.S.Y.; Barrios, V.; Ganis, G.; Gorman, J.L.; Lange, C.; Kumar, M.; Shepard, A.; Keenan, J.P. Assessing the neural correlates of self-enhancement bias: A transcranial magnetic stimulation study. Exp. Brain Res. 2007, 182, 379–385. [Google Scholar] [CrossRef]
- Trower, P.; Gilbert, P.; Sherling, G. Social anxiety, evolution, and self-presentation: An interdisciplinary perspective. In Handbook of Social and Evaluation Anxiety; Leitenberg, H., Ed.; Plenum Press: New York, NY, USA, 1990; pp. 11–45. [Google Scholar]
- Snyder, M. Self-monitoring and expressive behavior. J. Personal. Soc. Psychol. 1974, 30, 526–537. [Google Scholar] [CrossRef]
- Amati, F.; Oh, H.; Kwan, V.S.Y.; Jordan, K.; Keenan, J.P. Overclaiming and the medial prefrontal cortex: A transcranial magnetic stimulation study. Cogn. Neurosci. 2010, 1, 268–276. [Google Scholar] [CrossRef]
- Paulhus, D.L.; Harms, P.D.; Bruce, M.N.; Lysy, D.C. The over-claiming technique: Measuring self-enhancement independent of ability. J. Pers. Soc. Psychol. 2003, 84, 890–904. [Google Scholar] [CrossRef]
- Brown, J.D. Evaluations of Self and Others: Self-Enhancement Biases in Social Judgments. Soc. Cogn. 1986, 4, 353–376. [Google Scholar] [CrossRef]
- Paulhus, D.L.; Harms, P.D. Measuring cognitive ability with the over-claiming technique. Intelligence 2004, 32, 297–314. [Google Scholar] [CrossRef][Green Version]
- Ludeke, S.G.; Makransky, G. Does the Over-Claiming Questionnaire measure overclaiming? Absent convergent validity in a large community sample. Psychol. Assess. 2016, 28, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Kwan, V.S.; Ganis, G.; Gorman, J.L.; Romanowski, J.; Keenan, J.P. Elucidating the neural correlates of egoistic and moralistic self-enhancement. Conscious. Cogn. 2008, 17, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.M.; Paulhus, D.L.; Nathanson, C. The nature of over-claiming: Personality and cognitive factors. In Proceedings of the A Poster Presented to The Annual Meeting of the American Psychological Association, Chicago, IL, USA, 22–25 August 2002. [Google Scholar]
- Okado, Y.; Stark, C.E. Neural processing associated with true and false memory retrieval. Cogn. Affect. Behav. Neurosci. 2003, 3, 323–334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawakami, N.; Miura, E. Can Magic Deception Be Detected at an Unconscious Level? Perception 2016, 46, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Paulhus, D.L.; Reid, D.B. Enhancement and denial in socially desirable responding. J. Personal. Soc. Psychol. 1991, 60, 307–317. [Google Scholar] [CrossRef]
- Bradley, J.V. Overconfidence in ignorant experts. Bull. Psychon. Soc. 1981, 17, 82–84. [Google Scholar] [CrossRef]
- Trivers, R. Deceit and self-deception: The relationship between communication and consciousness. In Man and Beast Revisited; Robinson, M., Tiger, L., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1991; pp. 175–191. [Google Scholar]
- Von Hippel, W.; Trivers, R. The evolution and psychology of self-deception. Behav. Brain Sci. 2011, 34, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Paulhus, D.L.; Williams, K.M. The Dark Triad of personality: Narcissism, Machiavellianism, and psychopathy. J. Res. Pers. 2002, 36, 556–563. [Google Scholar] [CrossRef]
- Denmark, T.; Fish, J.; Jansari, A.; Tailor, J.; Ashkan, K.; Morris, R. Using Virtual Reality to investigate multitasking ability in individuals with frontal lobe lesions. J. Personal. Soc. Psychol. 2019, 74, 63–79. [Google Scholar] [CrossRef]
- Morgan, J.K.; Shaw, D.S.; Forbes, E.E. Fearfulness moderates the link between childhood social withdrawal and adolescent reward response. Soc. Cogn. Affect. Neurosci. 2014, 10, 761–768. [Google Scholar] [CrossRef]
- Sebastian, C.L.; Fontaine, N.M.G.; Bird, G.; Blakemore, S.-J.; A De Brito, S.; McCrory, E.J.P.; Viding, E. Neural processing associated with cognitive and affective Theory of Mind in adolescents and adults. Soc. Cogn. Affect. Neurosci. 2011, 7, 53–63. [Google Scholar] [CrossRef]
- Sebastian, C.L.; Tan, G.C.; Roiser, J.; Viding, E.; Dumontheil, I.; Blakemore, S.-J. Developmental influences on the neural bases of responses to social rejection: Implications of social neuroscience for education. Neuroimage 2011, 57, 686–694. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Beer, J.S.; Robertson, E.R.; Cooper, J.C.; Gabrieli, J.D.; Kihsltrom, J.F.; D’Esposito, M. The neural correlates of direct and reflected self-knowledge. NeuroImage 2005, 28, 797–814. [Google Scholar] [CrossRef]
- Johnson, S.C.; Schmitz, T.W.; Kawahara-Baccus, T.N.; Rowley, H.A.; Alexander, A.L.; Lee, J.; Davidson, R.J. The Cerebral Response during Subjective Choice with and without Self-reference. J. Cogn. Neurosci. 2005, 17, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Tottenham, N. Social scaffolding of human amygdala-mPFCcircuit development. Soc. Neurosci. 2015, 10, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meer, L.; Costafreda, S.; Aleman, A.; David, A.S. Self-reflection and the brain: A theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci. Biobehav. Rev. 2010, 34, 935–946. [Google Scholar] [CrossRef]
- Somerville, L.H.; Kelley, W.M.; Heatherton, T.F. Self-esteem Modulates Medial Prefrontal Cortical Responses to Evaluative Social Feedback. Cereb. Cortex 2010, 20, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, M.A.; Cohn, A.; Ugazio, G.; Ruff, C.C. Increasing honesty in humans with noninvasive brain stimulation. Proc. Natl. Acad. Sci. USA 2017, 114, 4360–4364. [Google Scholar] [CrossRef] [PubMed]
- Farrow, T.; Burgess, J.; Wilkinson, I.D.; Hunter, M. Neural correlates of self-deception and impression-management. Neuropsychologia 2015, 67, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, E.M. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines form the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroenchaphalography Clin. Neurophysiol. 1998, 108, 1–16. [Google Scholar] [CrossRef]
- Rossini, P.; Barker, A.; Berardelli, A.; Caramia, M.; Caruso, G.; Cracco, R.; Dimitrijevic, M.; Hallett, M.; Katayama, Y.; Lücking, C.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 79–92. [Google Scholar] [CrossRef]
- Lou, H.C.; Luber, B.; Crupain, M.; Keenan, J.P.; Nowak, M.; Kjaer, T.W.; Sackeim, H.A.; Lisanby, S.H. Parietal cortex and representation of the mental Self. Proc. Natl. Acad. Sci. USA 2004, 101, 6827–6832. [Google Scholar] [CrossRef]
- Paulhus, D.L. Measurement and control of response bias. In Measures of Personality and Social Psychological Attitudes; Robinson, J.P., Shaver, P.R., Wrightsman, L.S., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 17–59. [Google Scholar]
- Paulhus, D.L.; John, O.P. Egoistic and Moralistic Biases in Self-Perception: The Interplay of Self-Deceptive Styles with Basic Traits and Motives. J. Pers. 1998, 66, 1025–1060. [Google Scholar] [CrossRef]
- Paulhus, D.L. Interpersonal and intrapsychic adaptiveness of trait self-enhancement: A mixed blessing? J. Personal. Soc. Psychol. 1998, 74, 1197–1208. [Google Scholar] [CrossRef]
- Stevens, S.T.; Guise, K.; Christiana, W.; Kumar, M.; Keenan, J.P. Deception, evolution, and the brain. In Evolutionary Cognitive Neuroscience; Platek, S.M., Keenan, J.P., Shackelford, T.K., Eds.; MIT Press: Cambridge, MA, USA, 2007; pp. 518–540. [Google Scholar]
- Park, J.; Sloman, S.A. Causal explanation in the face of contradiction. Mem. Cogn. 2014, 42, 806–820. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.F.; Stuss, D.T. Frontal Lobe Influences on Delusions: A Clinical Perspective. Schizophr. Bull. 1990, 16, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Northoff, G. Personal Identity and Cortical Midline Structure (CMS): Do Temporal Features of CMS Neural Activity Transform Into “Self-Continuity”? Psychol. Inq. 2017, 28, 122–131. [Google Scholar] [CrossRef]
- Qin, P.; Northoff, G. How is our self related to midline regions and the default-mode network? NeuroImage 2011, 57, 1221–1233. [Google Scholar] [CrossRef]
- Izuma, K.; Saito, D.N.; Sadato, N. The roles of the medial prefrontal cortex and striatum in reputation processing. Soc. Neurosci. 2010, 5, 133–147. [Google Scholar] [CrossRef]
- Bastian, B.; Pe, M.L.; Kuppens, P. Perceived social pressure not to experience negative emotion is linked to selective attention for negative information. Cogn. Emot. 2015, 31, 261–268. [Google Scholar] [CrossRef]
- Pennycook, G.; Rand, D.G. Who falls for fake news? The roles of bullshit receptivity, overclaiming, familiarity, and analytic thinking. J. Pers. 2019, 88, 185–200. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor-Lillquist, B.; Kanpa, V.; Crawford, M.; El Filali, M.; Oakes, J.; Jonasz, A.; Disney, A.; Keenan, J.P. Preliminary Evidence of the Role of Medial Prefrontal Cortex in Self-Enhancement: A Transcranial Magnetic Stimulation Study. Brain Sci. 2020, 10, 535. https://doi.org/10.3390/brainsci10080535
Taylor-Lillquist B, Kanpa V, Crawford M, El Filali M, Oakes J, Jonasz A, Disney A, Keenan JP. Preliminary Evidence of the Role of Medial Prefrontal Cortex in Self-Enhancement: A Transcranial Magnetic Stimulation Study. Brain Sciences. 2020; 10(8):535. https://doi.org/10.3390/brainsci10080535
Chicago/Turabian StyleTaylor-Lillquist, Birgitta, Vivek Kanpa, Maya Crawford, Mehdi El Filali, Julia Oakes, Alex Jonasz, Amanda Disney, and Julian Paul Keenan. 2020. "Preliminary Evidence of the Role of Medial Prefrontal Cortex in Self-Enhancement: A Transcranial Magnetic Stimulation Study" Brain Sciences 10, no. 8: 535. https://doi.org/10.3390/brainsci10080535
APA StyleTaylor-Lillquist, B., Kanpa, V., Crawford, M., El Filali, M., Oakes, J., Jonasz, A., Disney, A., & Keenan, J. P. (2020). Preliminary Evidence of the Role of Medial Prefrontal Cortex in Self-Enhancement: A Transcranial Magnetic Stimulation Study. Brain Sciences, 10(8), 535. https://doi.org/10.3390/brainsci10080535




