Hopf Bifurcations in Complex Multiagent Activity: The Signature of Discrete to Rhythmic Behavioral Transitions
Abstract
1. Introduction
2. Dynamical Primitives of Task Actions
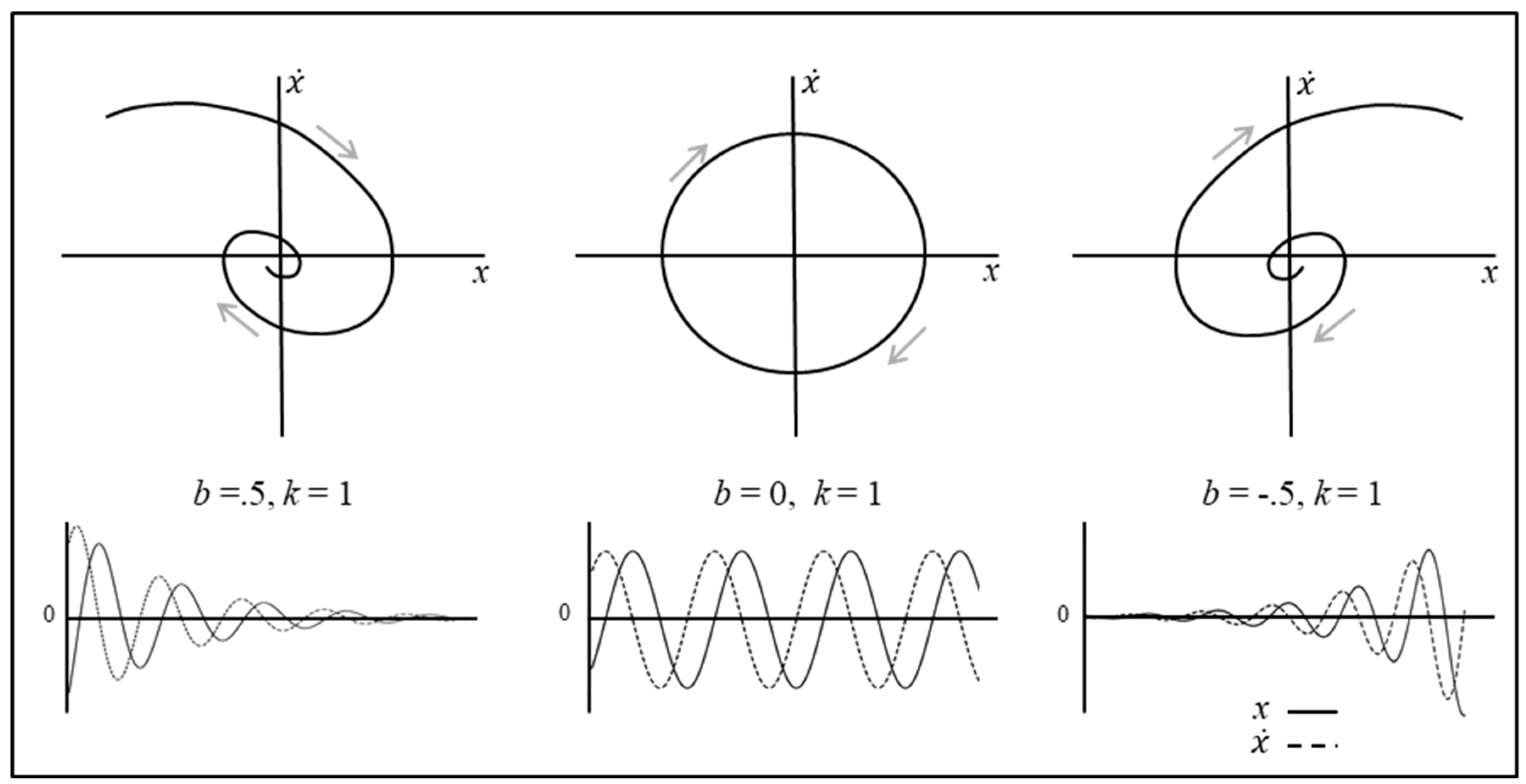
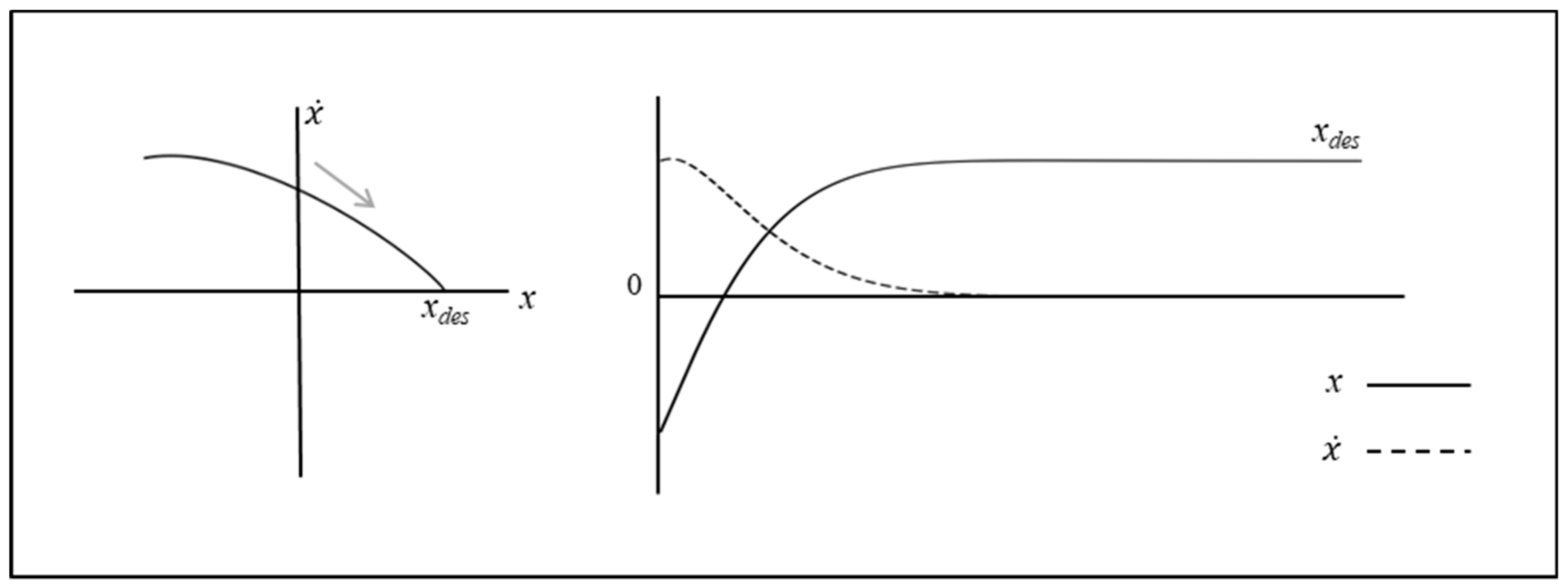
2.1. Fixed-Point Attractor (Damped Mass–Spring) Systems
2.2. Limit Cycle Systems
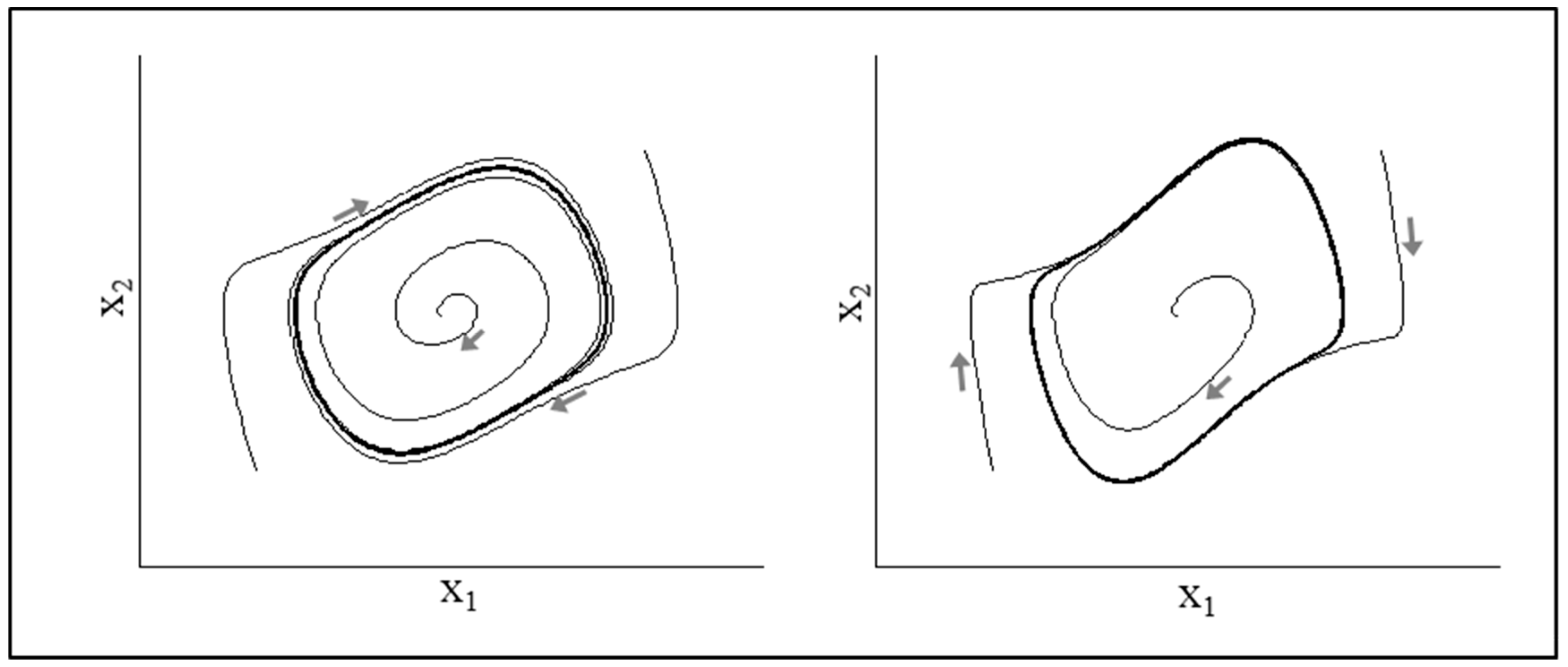
2.3. Hopf Bifurcation: From Discrete to Rhythmic Behavior
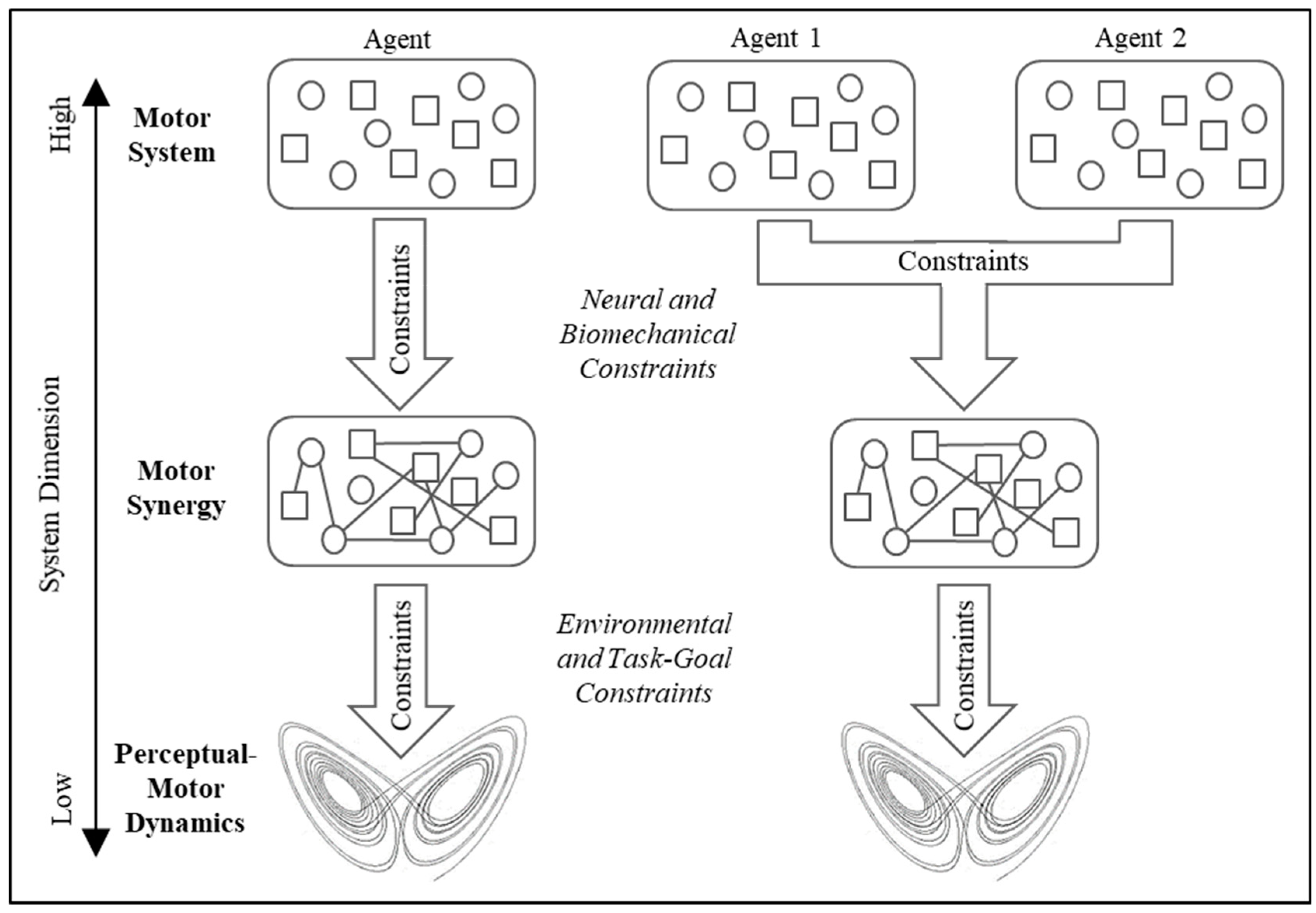
2.4. Dynamical Perceptual-Motor Primitives in Individual Behavior
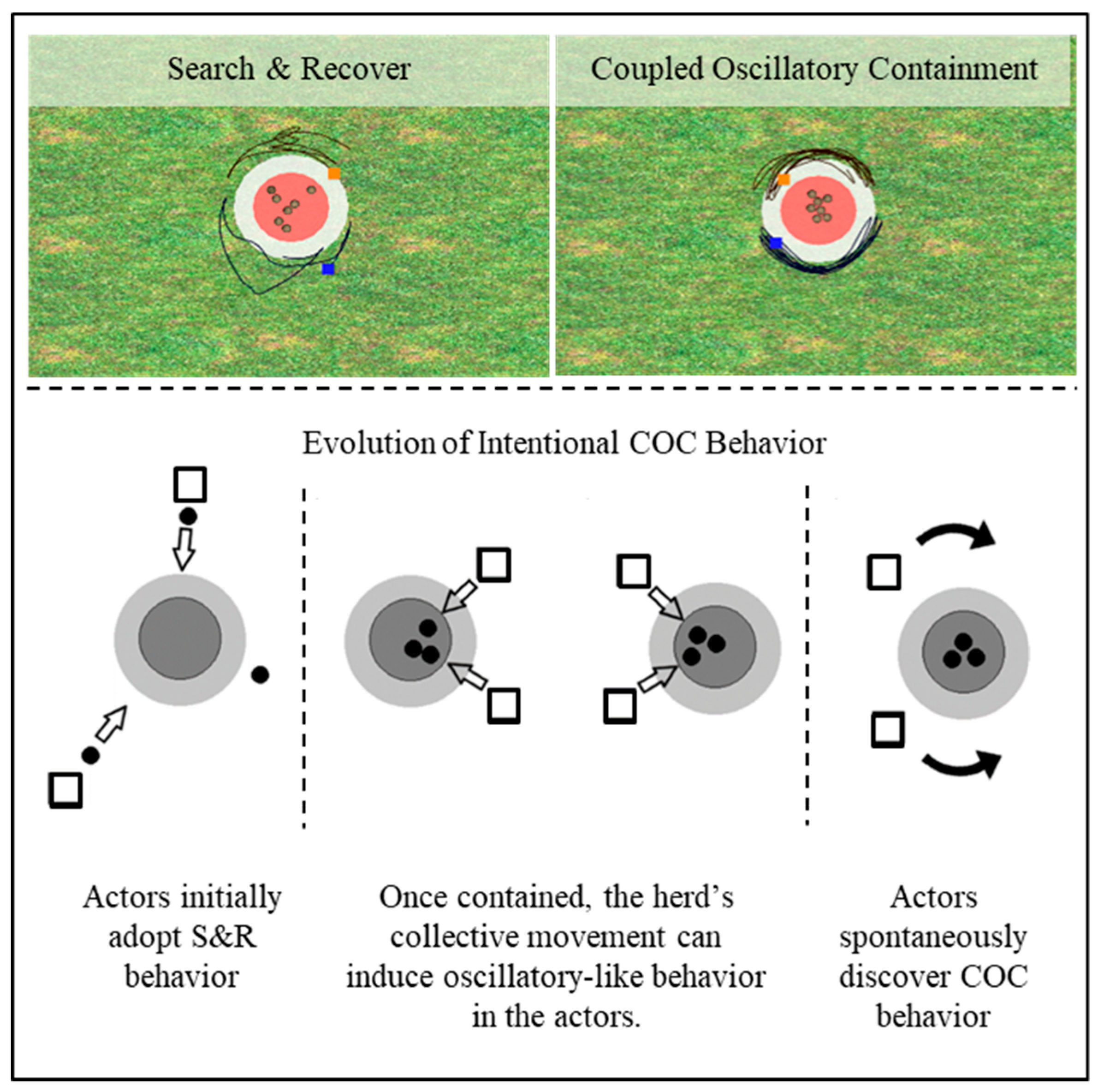
3. Hopf Bifurcation in Multiagent Activity: A Cooperative Shepherding Example
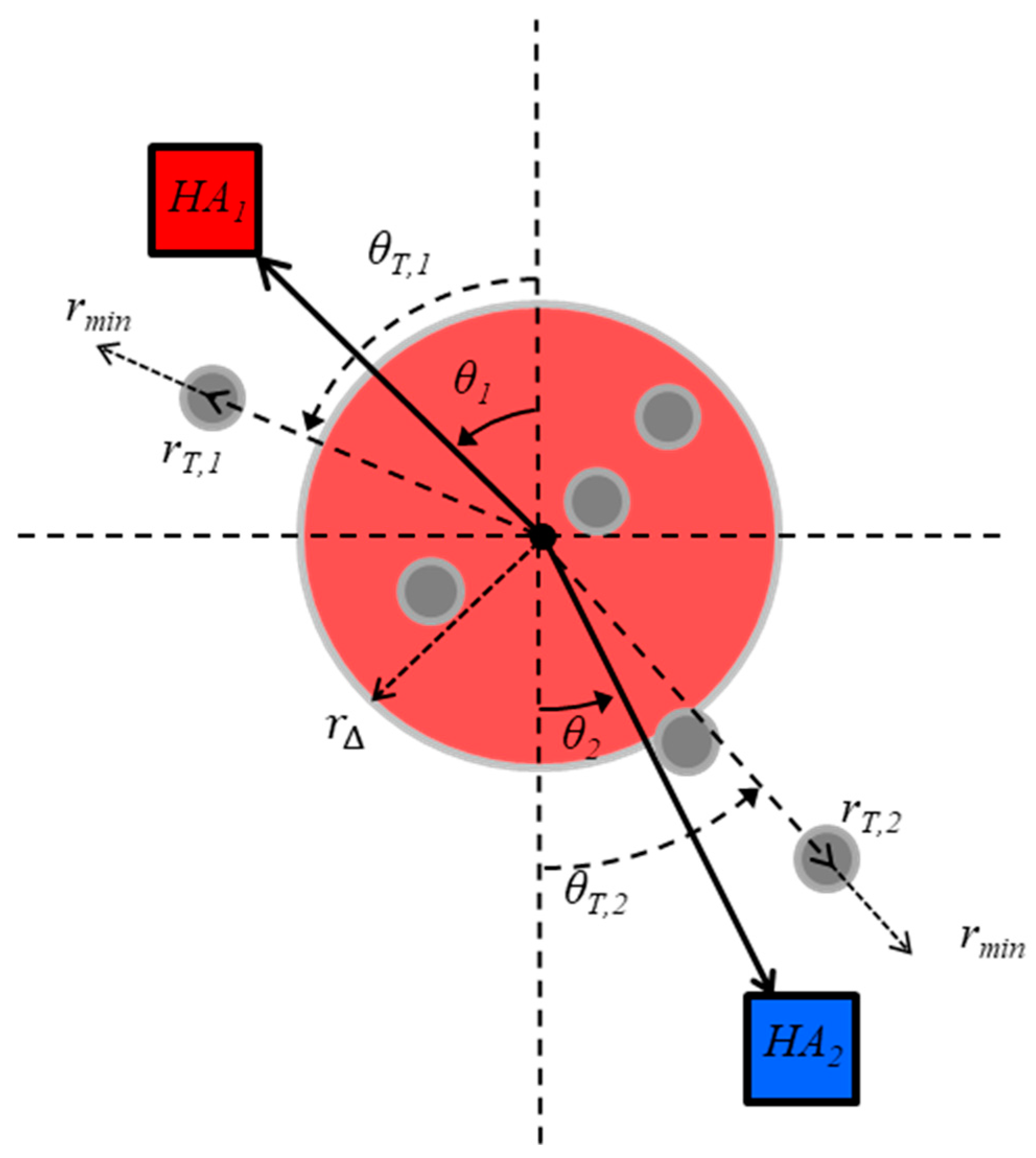
3.1. The Task-Dynamic Model for Multiagent Shepherding
3.2. Hopf Bifurcations as a Signature of Intentional Dynamics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kugler, P.N.; Scott Kelso, J.A.; Turvey, M.T. 1 On the Concept of Coordinative Structures as Dissipative Structures: I. Theoretical Lines of Convergence. Adv. Psychol. 1980, 1, 3–47. [Google Scholar]
- Riley, M.A.; Richardson, M.J.; Shockley, K.; Ramenzoni, V.C. Interpersonal Synergies. Front. Psychol. 2011, 2, 38. [Google Scholar] [CrossRef]
- Latash, M.L. Synergy; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Turvey, M.T. Coordination. Am. Psychol. 1990, 45, 938–953. [Google Scholar] [CrossRef]
- Kay, B.A. The dimensionality of movement trajectories and the degrees of freedom problem: A tutorial. Hum. Mov. Sci. 1988, 7, 343–364. [Google Scholar] [CrossRef]
- Richardson, M.J.; Lopresti-Goodman, S.; Mancini, M.; Kay, B.; Schmidt, R.C. Comparing the attractor strength of intra and interpersonal interlimb coordination using cross-recurrence analysis. Neurosci. Lett. 2008, 438, 340–345. [Google Scholar] [CrossRef]
- Richardson, M.J.; Kallen, R.W. Symmetry-breaking and the contextual emergence of human multiagent coordination and social activity. In Contextuality from Quantum Physics to Psychology; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2015; pp. 229–286. [Google Scholar]
- Warren, W.H. The dynamics of perception and action. Psychol. Rev. 2006, 113, 358–389. [Google Scholar] [CrossRef]
- Saltzman, E.; Kelso, J.A. Skilled actions: A task-dynamic approach. Psychol. Rev. 1987, 94, 84–106. [Google Scholar] [CrossRef]
- Richardson, M.J.; Kallen, R.W.; Nalepka, P.; Harrison, S.J.; Lamb, M.; Chemero, A.; Saltzman, E.; Schmidt, R.C. Modeling embedded interpersonal and multiagent coordination. In Proceedings of the 1st International Conference on Complex Information Systems, Rome, Italy, 1 January 2016; pp. 155–164. [Google Scholar]
- Saltzman, E.; Caplan, D. A graph-dynamic perspective on coordinative structures, the role of affordance-effectivity relations in action selection, and the self-organization of complex activities. Ecol. Psychol. 2015, 27, 300–309. [Google Scholar] [CrossRef]
- Degallier, S.; Ijspeert, A. Modeling discrete and rhythmic movements through motor primitives: A review. Biol. Cybern. 2010, 103, 319–338. [Google Scholar] [CrossRef] [PubMed]
- van Mourik, A.M.; Beek, P.J. Discrete and cyclical movements: Unified dynamics or separate control? Acta Psychol. 2004, 117, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Hogan, N.; Sternad, D. On rhythmic and discrete movements: Reflections, definitions and implications for motor control. Exp. Brain Res. 2007, 181, 13–30. [Google Scholar] [CrossRef]
- Nalepka, P.; Kallen, R.W.; Chemero, A.; Saltzman, E.; Richardson, M.J. Herd Those Sheep: Emergent Multiagent Coordination and Behavioral-Mode Switching. Psychol. Sci. 2017, 28, 630–650. [Google Scholar] [CrossRef] [PubMed]
- Nalepka, P.; Lamb, M.; Kallen, R.W.; Shockly, K.; Chemero, A.; Saltzman, E.; Richardson, M.J. Human social motor solutions for human–machine interaction in dynamical task contexts. Proc. Natl. Acad. Sci. USA 2019, 116, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Strogatz, S. Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry, and Engineering (Studies in Nonlinearity); Westview Press: Boulder, CO, USA, 2001. [Google Scholar]
- Singer, Y.; Tishby, N. Dynamical encoding of cursive handwriting. Biol. Cybern. 1994, 71, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Furuya, S.; Flanders, M.; Soechting, J.F. Hand kinematics of piano playing. J. Neurophysiol. 2011, 106, 2849–2864. [Google Scholar] [CrossRef] [PubMed]
- Ronsse, R.; Sternad, D.; Lefèvre, P. A computational model for rhythmic and discrete movements in uniand bimanual coordination. Neural Comput. 2009, 21, 1335–1370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sternad, D. The primacy of rhythm: How discrete actions merge into a stable rhythmic pattern. J. Neurophysiol. 2019, 121, 574–587. [Google Scholar] [CrossRef]
- Ijspeert, A.J.; Nakanishi, J.; Hoffmann, H.; Pastor, P.; Schaal, S. Dynamical movement primitives: Learning attractor models for motor behaviors. Neural Comput. 2013, 25, 328–373. [Google Scholar] [CrossRef]
- Schaal, S.; Kotosaka, S.; Sternad, D. Nonlinear Dynamical Systems as Movement Primitives. Int. Conf. Hum. Robot. Camb. MA 2001, 38, 117–124. [Google Scholar]
- Sternad, D.; Dean, W.J.; Schaal, S. Interaction of rhythmic and discrete pattern generators in single-joint movements. Hum. Mov. Sci. 2000, 19, 627–664. [Google Scholar] [CrossRef]
- Hogan, N.; Sternad, D. Dynamic primitives of motor behavior. Biol. Cybern. 2012, 106, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Ronsse, R.; Wei, K.; Sternad, D. Optimal Control of a Hybrid Rhythmic-Discrete Task: The Bouncing Ball Revisited. J. Neurophysiol. 2010, 103, 2482–2493. [Google Scholar] [CrossRef] [PubMed]
- Sternad, D.; Marino, H.; Charles, S.K.; Duarte, M.; Dipietro, L.; Hogan, N. Transitions between discrete and rhythmic primitives in a unimanual task. Front. Comput. Neurosci. 2013, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Warren, W.H.; Fajen, B.R. Behavioral Dynamics of Human Locomotion. Ecol. Psychol. 2004, 16, 61–66. [Google Scholar] [CrossRef]
- Lamb, M.; Kallen, R.W.; Harrison, S.J.; Di Bernardo, M.; Minai, A.; Richardson, M.J. To pass or not to pass: Modeling the movement and affordance dynamics of a pick and place task. Front. Psychol. 2017, 8, 1061. [Google Scholar] [CrossRef]
- van der Pol, B. LXXXV. On oscillation hysteresis in a triode generator with two degrees of freedom. Lond. Edinb. Dublin Philos Mag. J. Sci. 1922, 43, 700–719. [Google Scholar] [CrossRef]
- Rayleigh, L. The Theory of Sound; Dover Publications: New York, NY, USA, 1945. [Google Scholar]
- Kay, B.A.; Kelso, J.A.S.; Saltzman, E.L.; Schöner, G. Space-time behavior of single and bimanual rhythmical movements: Data and limit cycle model. J. Exp. Psychol. Hum. Percept. Perform. 1987, 13, 178–192. [Google Scholar] [CrossRef]
- Kelso, J.A.S. The Self-Organization of Brain and Behavior; A Bradford Book; MIT Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Collins, J.J.; Stewart, I.N. Coupled nonlinear oscillators and the symmetries of animal gaits. J. Nonlinear Sci. 1993, 3, 349–392. [Google Scholar] [CrossRef]
- Fink, P.W.; Kelso, J.A.S.; Jirsa, V.K.; De Guzman, G. Recruitment of degrees of freedom stabilizes coordination. J. Exp. Psychol. Hum. Percept. Perform. 2000, 26, 671–692. [Google Scholar] [CrossRef]
- Freyer, F.; Roberts, J.A.; Ritter, P.; Breakspear, M. A Canonical Model of Multistability and Scale-Invariance in Biological Systems. PLoS Comput. Biol. 2012, 8, e1002634. [Google Scholar] [CrossRef]
- Huys, R.; Fernandez, L.; Bootsma, R.J.; Jirsa, V.K. Fitts’ law is not continuous in reciprocal aiming. Proc. Biol. Sci. 2010, 277, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Mottet, D.; Bootsma, R.J. The dynamics of goal-directed rhythmical aiming. Biol. Cybern. 1999, 80, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.J.; Park, J.H.; Shea, C.H. Target width scaling in a repetitive aiming task: Switching between cyclical and discrete units of action. Exp. Brain Res. 2006, 175, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Van Der Wel, R.P.R.D.; Sternad, D.; Rosenbaum, D.A. Moving the arm at different rates: Slow movements are avoided. J. Mot. Behav. 2009, 42, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.J.; Campbell, W.L.; Schmidt, R.C. Movement interference during action observation as emergent coordination. Neurosci. Lett. 2009, 449, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Zaal, F.T.J.M.; Bootsma, R.J.; Van Wieringen, P.C.W. Dynamics of reaching for stationary and moving objects: Data and model. J. Exp. Psychol. Hum. Percept. Perform. 1999, 25, 149–161. [Google Scholar] [CrossRef]
- Sternad, D. Towards a unified theory of rhythmic and discrete movements-behavioral, modeling and imaging results. Underst. Complex. Syst. 2008, 2008, 105–133. [Google Scholar]
- Morice, A.H.P.; Diaz, G.J.; Fajen, B.R.; Basilio, N.; Montagne, G. An Affordance-Based Approach to Visually Guided Overtaking. Ecol. Psychol. 2015, 27, 1–25. [Google Scholar] [CrossRef]
- Harrison, H.S.; Turvey, M.T.; Frank, T.D. Affordance-based perception-action dynamics: A model of visually guided braking. Psychol. Rev. 2016, 123, 305–323. [Google Scholar] [CrossRef]
- Fajen, B.R. Affordance-based control of visually guided action. Ecol. Psychol. 2007, 19, 383–410. [Google Scholar] [CrossRef]
- Warren, W.H.; Fajen, B.R. Behavioral dynamics of visually guided locomotion. Underst. Complex. Syst. 2008, 2008, 45–75. [Google Scholar]
- Beek, P.J.; Turvey, M.T. Temporal Patterning in Cascade Juggling. J. Exp. Psychol. Hum. Percept. Perform. 1992, 18, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.C.; Carello, C.; Turvey, M.T. Phase transitions and critical fluctuations in the visual coordination of rhythmic movements between people. J. Exp. Psychol. Hum. Percept. Perform. 1990, 16, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.C.; O’Brien, B. Evaluating the Dynamics of Unintended Interpersonal Coordination. Ecol. Psychol. 1997, 9, 189–206. [Google Scholar] [CrossRef]
- Richardson, M.J.; Schmidt, R.C.; Kay, B.A. Distinguishing the noise and attractor strength of coordinated limb movements using recurrence analysis. Biol. Cybern. 2007, 96, 59–78. [Google Scholar] [CrossRef]
- Richardson, M.J.; Harrison, S.; Kallen, R.W.; Walton, A.; Eiler, B.A.; Saltzman, E.; Schmidt, R.C. Self-organized complementary joint action: Behavioral dynamics of an interpersonal collision-avoidance task. J. Exp. Psychol. Hum. Percept. Perform. 2015, 41, 665–679. [Google Scholar] [CrossRef]
- Warren, W.H. Collective motion in human crowds. Curr. Dir. Psychol. Sci. 2018, 27, 232–240. [Google Scholar] [CrossRef]
- Nalepka, P.; Kallen, R.W.; Chemero, A.; Saltzman, E.; Richardson, M.J. Practical Applications of Multiagent Shepherding for Human-Machine Interaction. In Proceedings of the PAAMS 2019: Advances in Practical Applications of Survivable Agents and Multi-Agent Systems, Ávila, Spain, 26–28 June 2019; pp. 168–179. [Google Scholar]
- Rigoli, L.M.; Nalepka, P.; Douglas, H.; Kallen, R.W.; Hosking, S.; Best, C.; Saltzman, E.; Richardson, M.J. Employing Models of Human Social Motor Behavior for Artificial Agent Trainers. In Proceedings of the 19th International Conference on Autonomous Agents and Multiagent Systems (AAMAS 2020), Auckland, New Zealand, 9–13 May 2020; p. 9. [Google Scholar]
- Strömbom, D.; Mann, R.P.; Wilson, A.M.; Hales, S.; Morton, A.J.; Sumpter, D.J.T.; King, A.J. Solving the shepherding problem: Heuristics for herding autonomous, interacting agents. J. R. Soc. Interface 2014, 11. [Google Scholar] [CrossRef]
- Haken, H.; Kelso, J.A.S.; Bunz, H. A theoretical model of phase transitions in human hand movements. Biol. Cybern. 1985, 51, 347–356. [Google Scholar] [CrossRef]
- Schmidt, R.C.; Richardson, M.J. Dynamics of Interpersonal Coordination. In Coordination: Neural, Behavioral and Social Dynamics; Fuchs, A., Jirsa, V.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 281–308. [Google Scholar]
- Stephen, D.G.; Dixon, J.A.; Isenhower, R.W. Dynamics of Representational Change: Entropy, Action, and Cognition. J. Exp. Psychol. Hum. Percept. Perform. 2009, 35, 1811–1832. [Google Scholar] [CrossRef]
- Dumas, G.; de Guzman, G.C.; Tognoli, E.; Kelso, J.A.S. The human dynamic clamp as a paradigm for social interaction. Proc. Natl. Acad. Sci. USA 2014, 111, E3726–E3734. [Google Scholar] [CrossRef] [PubMed]
- Nalepka, P.; Silva, P.L.; Kallen, R.W.; Shockley, K.; Chemero, A.; Saltzman, E.; Richardson, M.J. Similitude in Task-Dynamic Graph Structure during Collaborative Activity in Different Contexts. submitted.
- Kugler, P.N.; Shaw, R.E.; Vincente, K.J.; Kinsella-Shaw, J. Inquiry into intentional systems I: Issues in ecological physics. Psychol. Res. 1990, 52, 98–121. [Google Scholar] [CrossRef]
- Shaw, R.E.; Kadar, E.; Sim, M.; Repperger, D.W. The intentional spring: A strategy for modeling systems that learn to perform intentional acts. J. Mot. Behav. 1992, 24, 3–28. [Google Scholar] [CrossRef]
- Kugler, P.N.; Turvey, M.T. Information, Natural Law, and the Self-Assembly of Rhythmic Movement; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 1987. [Google Scholar]
- Saltzman, E.L.; Munhall, K.G. Skill Acquisition and Development: The Roles of State-, Parameter-, and Graph-Dynamics. J. Mot. Behav. 1992, 24, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Muro, C.; Escobedo, R.; Spector, L.; Coppinger, R.P. Wolf-pack (Canis lupus) hunting strategies emerge from simple rules in computational simulations. Behav. Processes 2011, 88, 192–197. [Google Scholar] [CrossRef] [PubMed]
- D’Vincent, C.G.; Nilson, R.M.; Hanna, R.E. Vocalization and coordinated feeding behavior of the humpback whale in Southeastern Alaska. Sci. Rep. Whales Res. Inst. 1985, 36, 41–47. [Google Scholar]
- Özdemir, A.; Gauci, M.; Gross, R. Shepherding with robots that do not compute. In Proceedings of the 14th European Conference on Artificial Life ECAL 2017, Lyon, France, 4–8 September 2017; pp. 332–339. [Google Scholar]
- Beek, P.J.; Peper, C.E.; Stegeman, D.F. Dynamical models of movement coordination. Hum. Mov. Sci. 1995, 14, 573–608. [Google Scholar] [CrossRef]
- Amazeen, P.G. From physics to social interactions: Scientific unification via dynamics. Cogn. Syst. Res. 2018, 52, 640–657. [Google Scholar] [CrossRef]
- Słowiński, P.; Alderisio, F.; Zhai, C.; Shen, Y.; Tino, P.; Bortolon, C.; Capdevielle, D.; Cohen, L.; Khoramshahi, M.; Billard, A.; et al. Unravelling socio-motor biomarkers in schizophrenia. Npj Schizophr. 2017, 3, 8. [Google Scholar] [CrossRef]
- Sutton, R.; Barto, A. Reinforcement Learning; The MIT Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Haith, A.M.; Krakauer, J.W. Model-based and model-free mechanisms of human motor learning. Adv. Exp. Med. Biol. 2013, 782, 1–21. [Google Scholar]
- Mukovskiy, A.; Taubert, N.; Endres, D.; Vassallo, C.; Naveau, M.; Stasse, O.; Soueres, P.; Giese, M.A. Modeling of coordinated human body motion by learning of structured dynamic representations. In Springer Tracts in Advanced Robotics; Springer: Cham, Switzerland, 2017; Volume 117, pp. 237–267. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, G.; Nalepka, P.; Kallen, R.W.; Richardson, M.J. Hopf Bifurcations in Complex Multiagent Activity: The Signature of Discrete to Rhythmic Behavioral Transitions. Brain Sci. 2020, 10, 536. https://doi.org/10.3390/brainsci10080536
Patil G, Nalepka P, Kallen RW, Richardson MJ. Hopf Bifurcations in Complex Multiagent Activity: The Signature of Discrete to Rhythmic Behavioral Transitions. Brain Sciences. 2020; 10(8):536. https://doi.org/10.3390/brainsci10080536
Chicago/Turabian StylePatil, Gaurav, Patrick Nalepka, Rachel W. Kallen, and Michael J. Richardson. 2020. "Hopf Bifurcations in Complex Multiagent Activity: The Signature of Discrete to Rhythmic Behavioral Transitions" Brain Sciences 10, no. 8: 536. https://doi.org/10.3390/brainsci10080536
APA StylePatil, G., Nalepka, P., Kallen, R. W., & Richardson, M. J. (2020). Hopf Bifurcations in Complex Multiagent Activity: The Signature of Discrete to Rhythmic Behavioral Transitions. Brain Sciences, 10(8), 536. https://doi.org/10.3390/brainsci10080536




