Functional Network Mapping Reveals State-Dependent Response to IGF1 Treatment in Rett Syndrome
Abstract
1. Introduction
1.1. Rett Syndrome
1.2. Network Pathology
1.3. IGF1 Treatment
1.4. Targeting Networks in Rett Syndrome
2. Materials and Methods
2.1. Subjects
2.2. IGF1 Administration
2.3. Data Collection
2.3.1. Clinical Characterisation
2.3.2. Electrophysiological Recordings
2.4. EEG Feature Extraction
2.4.1. Spectral Power
2.4.2. Profiles
2.4.3. Network Measures
2.5. Statistical Analyses
2.5.1. Effect of Treatment
2.5.2. Predicting Treatment Response
2.5.3. Model Training
2.5.4. Validation
2.5.5. Sequential Elimination
3. Results
3.1. Demographics
3.2. Treatment Effect
3.3. Responder Status
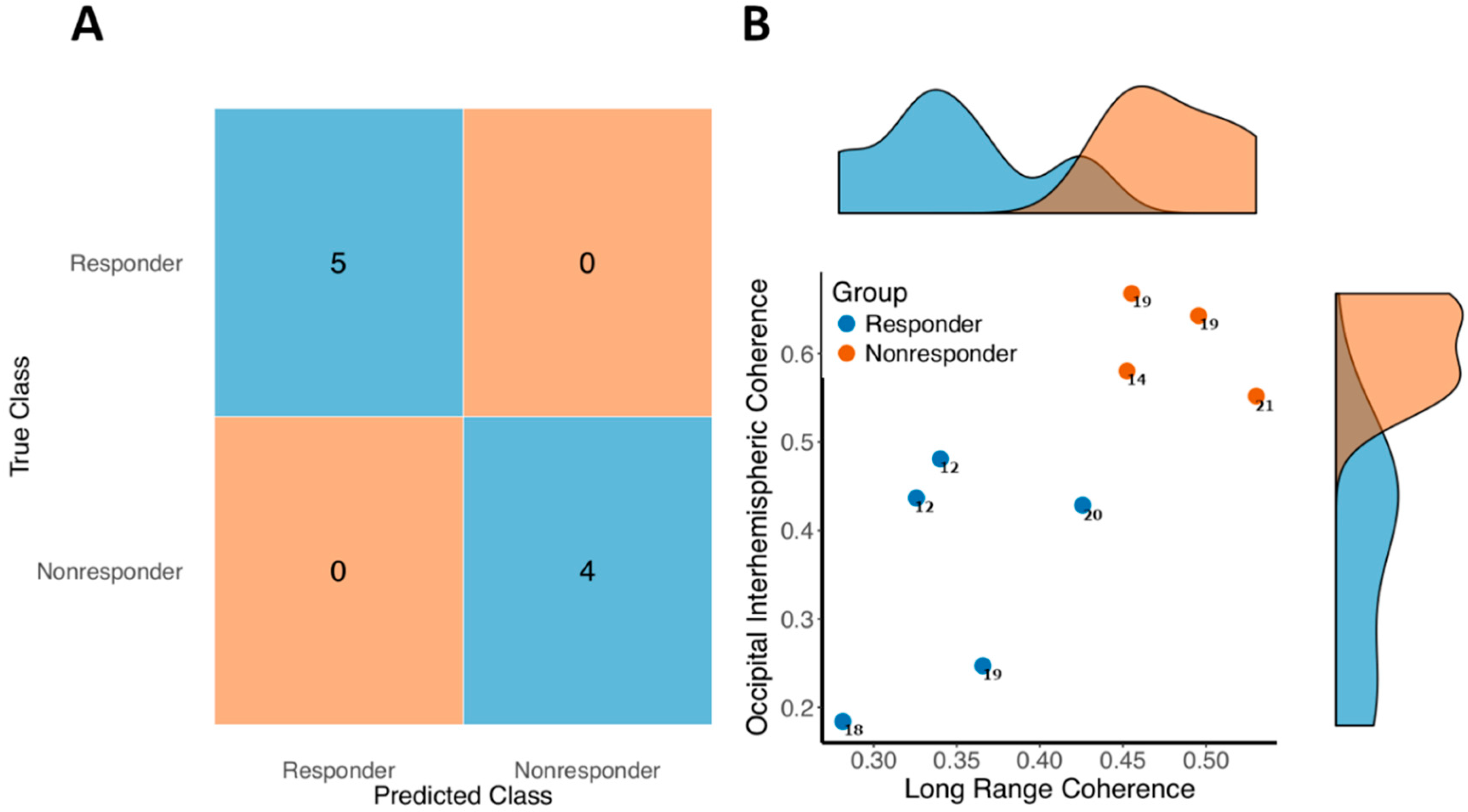
3.4. Predicting Response
4. Discussion
4.1. IGF1 Treatment
4.2. Treatment-Induced Network Effects
4.3. Network Variability and State-Dependency
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. International Severity Score
- Growth and Development
- Muscular-Skeletal Appearance
- Movement
- Mental-Cortical
- Brainstem-Autonomic
- 0:
- no abnormality
- 1:
- mild abnormality
- 2:
- severe abnormality
Appendix B. EEG Recording
References
- Chahrour, M.; Zoghbi, H.Y. The story of Rett syndrome: From clinic to neurobiology. Neuron 2007, 56, 422–437. [Google Scholar] [CrossRef]
- Neul, J.L.; Zoghbi, H.Y. Rett syndrome: A prototypical neurodevelopmental disorder. Neuroscience 2004, 10, 118–128. [Google Scholar] [CrossRef]
- Neul, J.L.; Kaufmann, W.E.; Glaze, D.G.; Christodoulou, J.; Clarke, A.J.; Bahi-Buisson, N.; Leonard, H.; Bailey, M.E.S.; Schanen, N.C.; Zappella, M.; et al. Rett syndrome: Revised diagnostic criteria and nomenclature. Ann. Neurol. 2010, 68, 944–950. [Google Scholar] [CrossRef]
- Kozinetz, C.A.; Skender, M.L.; MacNaughton, N.; Almes, M.J.; Schultz, R.J.; Percy, A.K.; Glaze, D.G. Epidemiology of Rett syndrome: A population-based registry. Pediatrics 1993, 91, 445–450. [Google Scholar]
- Pini, G.; Bigoni, S.; Congiu, L.; Romanelli, A.M.; Scusa, M.F.; Di Marco, P.; Benincasa, A.; Morescalchi, P.; Ferlini, A.; Bianchi, F.; et al. Rett syndrome: A wide clinical and autonomic picture. Orphanet J. Rare Dis. 2016, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, B. Clinical manifestations and stages of Rett syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; Stallworth, J.L.; Everman, D.B.; Skinner, S.A. Neurobiologically-based treatments in Rett syndrome: Opportunities and challenges. Expert Opin. Orphan Drugs 2016, 4, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Leonard, H.; Cobb, S.; Downs, J. Clinical and biological progress over 50 years in Rett syndrome. Nat. Rev. Neurol. 2017, 13, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; Johnston, M.V.; Blue, M.E. MeCP2 expression and function during brain development: Implications for Rett syndrome’s pathogenesis and clinical evolution. Brain Dev. 2005, 27 (Suppl. 1), S77–S87. [Google Scholar] [CrossRef]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, Y.-C.; Yu, J.; Miao, S.; Zheng, J.; Xu, L.; Zhou, Y.; Li, D.; Zhang, C.; Tao, J.; et al. CDKL5, a protein associated with Rett syndrome, regulates neuronal morphogenesis via rac1 signaling. J. Neurosci. 2010, 30, 12777–12786. [Google Scholar] [CrossRef] [PubMed]
- Pini, G.; Bigoni, S.; Engerström, I.W.; Calabrese, O.; Felloni, B.; Scusa, M.F.; Di Marco, P.; Borelli, P.; Bonuccelli, U.; Julu, P.O.O.; et al. Variant of Rett syndrome and CDKL5 gene: Clinical and autonomic description of 10 cases. Neuropediatrics 2012, 43, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-L.; Qiu, Z. MeCP2: Multifaceted roles in gene regulation and neural development. Neurosci. Bull. 2014, 30, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, Y.; Tidei, J.J.; Shen, M.; Hoang, J.T.; Wagner, D.F.; Zhao, X. Loss of MeCP2 in immature neurons leads to impaired network integration. Hum. Mol. Genet. 2019, 28, 245–257. [Google Scholar] [CrossRef]
- Van den Heuvel, M.P.; Sporns, O. A cross-disorder connectome landscape of brain dysconnectivity. Nat. Rev. Neurosci. 2019, 20, 435–446. [Google Scholar] [CrossRef]
- Fox, M.D. Mapping symptoms to brain Networks with the human connectome. N. Engl. J. Med. 2018, 379, 2237–2245. [Google Scholar] [CrossRef]
- McMackin, R.; Muthuraman, M.; Groppa, S.; Babiloni, C.; Taylor, J.; Kiernan, M.; Nasseroleslami, B.; Hardiman, O. Measuring network disruption in neurodegenerative diseases: New approaches using signal analysis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1011–1020. [Google Scholar] [CrossRef]
- Dukic, S.; McMackin, R.; Buxo, T.; Fasano, A.; Chipika, R.; Pinto-Grau, M.; Costello, E.; Schuster, C.; Hammond, M.; Heverin, M.; et al. Patterned functional network disruption in amyotrophic lateral sclerosis. Hum. Brain Mapp. 2019, 40, 4827–4842. [Google Scholar] [CrossRef]
- Mithani, K.; Mikhail, M.; Morgan, B.R.; Wong, S.; Weil, A.G.; Deschenes, S.; Wang, S.; Bernal, B.; Guillen, M.R.; Ochi, A.; et al. Connectomic profiling Identifies responders to vagus nerve stimulation. Ann. Neurol. 2019, 86, 743–753. [Google Scholar] [CrossRef]
- Saby, J.N.; Peters, S.U.; Roberts, T.P.L.; Nelson, C.A.; Marsh, E.D. Evoked potentials and EEG analysis in Rett syndrome and related developmental encephalopathies: Towards a biomarker for translational research. Front. Integr. Neurosci. 2020, 14, 30. [Google Scholar] [CrossRef]
- Roche, K.J.; LeBlanc, J.J.; Levin, A.R.; O’Leary, H.M.; Baczewski, L.M.; Nelson, C.A. Electroencephalographic spectral power as a marker of cortical function and disease severity in girls with Rett syndrome. J. Neurodev. Disord. 2019, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, H.M.; Kaufmann, W.E.; Barnes, K.V.; Rakesh, K.; Kapur, K.; Tarquinio, D.C.; Cantwell, N.G.; Roche, K.J.; Rose, S.A.; Walco, A.C.; et al. Placebo-controlled crossover assessment of mecasermin for the treatment of Rett syndrome. Ann. Clin. Transl. Neurol. 2018, 5, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Keogh, C.; Pini, G.; Dyer, A.H.; Bigoni, S.; Dimarco, P.; Gemo, I.; Reilly, R.; Tropea, D. Clinical and genetic Rett syndrome variants are defined by stable electrophysiological profiles. BMC Pediatr. 2018, 18. [Google Scholar] [CrossRef]
- Chang, Q.; Khare, G.; Dani, V.; Nelson, S.; Jaenisch, R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron 2006, 49, 341–348. [Google Scholar] [CrossRef]
- Zhou, Z.; Hong, E.J.; Cohen, S.; Zhao, W.; Ho, H.H.; Schmidt, L.; Chen, W.G.; Lin, Y.; Savner, E.; Griffith, E.C.; et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent bdnf transcription, dendritic growth, and spine maturation. Neuron 2006, 52, 255–269. [Google Scholar] [CrossRef]
- Wu, D.; Pardridge, W.M. Neuroprotection with noninvasive neurotrophin delivery to the brain. Proc. Natl. Acad. Sci. USA 1999, 96, 254–259. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Torres-Alemán, I. The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 2012, 13, 225–239. [Google Scholar] [CrossRef]
- Khwaja, O.S.; Sahin, M. Translational research. Curr. Opin. Pediatr. 2011, 23, 633–639. [Google Scholar] [CrossRef]
- Pini, G.; Scusa, M.F.; Congiu, L.; Benincasa, A.; Morescalchi, P.; Bottiglioni, I.; Di Marco, P.; Borelli, P.; Bonuccelli, U.; Della-Chiesa, A.; et al. IGF1 as a potential treatment for Rett syndrome: Safety assessment in six Rett patients. Autism Res. Treat. 2012, 2012, 679801. [Google Scholar] [CrossRef]
- Guy, J.; Gan, J.; Selfridge, J.; Cobb, S.; Bird, A. Reversal of neurological defects in a mouse model of Rett syndrome. Science 2007, 315, 1143–1147. [Google Scholar] [CrossRef]
- Robinson, L.; Guy, J.; McKay, L.; Brockett, E.; Spike, R.C.; Selfridge, J.; De Sousa, D.; Merusi, C.; Riedel, G.; Bird, A.; et al. Morphological and functional reversal of phenotypes in a mouse model of Rett syndrome. Brain 2012, 135, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Mathai, S.; Liang, H.; Gunn, A.J. Insulin-like growth factor-1 and its derivatives: Potential pharmaceutical application for treating neurological conditions. Recent Pat. CNS Drug Discov. 2013, 8, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Tropea, D.; Giacometti, E.; Wilson, N.R.; Beard, C.; McCurry, C.; Fu, D.D.; Flannery, R.; Jaenisch, R.; Sur, M. Partial reversal of Rett syndrome-like symptoms in MeCP2 mutant mice. Proc. Natl. Acad. Sci. USA 2009, 106, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Garcia, R.I.; Kwok, S.; Banerjee, A.; Petravicz, J.; Woodson, J.; Mellios, N.; Tropea, D.; Sur, M. Functional recovery with recombinant human IGF1 treatment in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA. 2014, 111, 9941–9946. [Google Scholar] [CrossRef] [PubMed]
- Landi, S.; Putignano, E.; Boggio, E.M.; Giustetto, M.; Pizzorusso, T.; Ratto, G.M. The short-time structural plasticity of dendritic spines is altered in a model of Rett syndrome. Sci. Rep. 2011, 1, 1–7. [Google Scholar] [CrossRef]
- Glaze, D.G.; Neul, J.L.; Kaufmann, W.E.; Berry-Kravis, E.; Condon, S.; Stoms, G.; Oosterholt, S.; Della Pasqua, O.; Glass, L.; Jones, N.E.; et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology 2019, 92, e1912–e1925. [Google Scholar] [CrossRef]
- Pini, G.; Congiu, L.; Benincasa, A.; Dimarco, P.; Bigoni, S.; Dyer, A.H.; Mortimer, N.; Della-chiesa, A.; Leary, S.O.; Mcnamara, R.; et al. Illness severity, social and cognitive ability, and EEG analysis of ten patients with Rett syndrome treated with mecasermin (recombinant human IGF1). Autism Res. Treat. 2016, 2016, 5073078. [Google Scholar] [CrossRef]
- Khwaja, O.S.; Ho, E.; Barnes, K.V.; Leary, H.M.O.; Pereira, L.M.; Finkelstein, Y. Safety, pharmacokinetics, and preliminary assessment of efficacy of mecasermin (recombinant human IGF1) for the treatment of Rett syndrome. Proc. Natl. Acad. Sci. USA 2014, 111, 4596–4601. [Google Scholar] [CrossRef]
- Guzzetta, A.; Baldini, S.; Bancale, A.; Baroncelli, L.; Ciucci, F.; Ghirri, P.; Putignano, E.; Sale, A.; Viegi, A.; Berardi, N.; et al. Massage accelerates brain development and the maturation of visual function. J. Neurosci. 2009, 29, 6042–6051. [Google Scholar] [CrossRef]
- Kerr, A.M.; Nomura, Y.; Armstrong, D.; Anvret, M.; Belichenko, P.V.; Budden, S.; Cass, H.; Christodoulou, J.; Clarke, A.; Ellaway, C.; et al. Guidelines for reporting clinical features in cases with MECP2 mutations. Brain Dev. 2001, 23, 208–211. [Google Scholar] [CrossRef]
- Kagan, J.; Snidman, N. Early childhood predictors of adult anxiety disorders. Biol. Psychiatry 1999, 46, 1536–1541. [Google Scholar] [CrossRef]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG; Oxford University Press: Oxford, UK, 2006; ISBN 019505038X. [Google Scholar]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I. Principal component analysis. In International Encyclopedia of Statistical Science; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1094–1096. [Google Scholar]
- Keogh, C.; Pini, G.; Gemo, I.; Tropea, D. Statistical modelling of cortical Connectivity using non-Invasive electroencephalograms. J. Vis. Exp. 2019. [Google Scholar] [CrossRef] [PubMed]
- Schölkopf, B.; Smola, A.J. Learning with Kernels: Support Vector Machines, Regularization, Optimization, and Beyond; MIT Press: Cambridge, MA, USA, 2002; ISBN 0262194759. [Google Scholar]
- Ross, K.A.; Jensen, C.S.; Snodgrass, R.; Dyreson, C.E.; Jensen, C.S.; Snodgrass, R.; Skiadopoulos, S.; Sirangelo, C.; Larsgaard, M.L.; Grahne, G.; et al. Cross-validation. In Encyclopedia of Database Systems; Springer: Boston, MA, USA, 2009; pp. 532–538. [Google Scholar]
- Linker, S.B.; Mendes, A.P.D.; Marchetto, M.C. IGF1 treatment causes unique transcriptional response in neurons from individuals with idiopathic autism. Mol. Autism 2020, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Pini, G.; Scusa, M.F.; Benincasa, A.; Bottiglioni, I.; Congiu, L.; Vadhatpour, C.; Romanelli, A.M.; Gemo, I.; Puccetti, C.; McNamara, R.; et al. Repeated insulin-like growth factor 1 treatment in a patient with Rett syndrome: A single case study. Front. Pediatr. 2014, 2, 52. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Miller, M.T.; Li, K.; Sur, M.; Kaufmann, W.E. Towards a better diagnosis and treatment of Rett syndrome: A model synaptic disorder. Brain 2019, 142, 239–248. [Google Scholar] [CrossRef]
- Krishnan, K.; Wang, B.-S.; Lu, J.; Wang, L.; Maffei, A.; Cang, J.; Huang, Z.J. MeCP2 regulates the timing of critical period plasticity that shapes functional connectivity in primary visual cortex. Proc. Natl. Acad. Sci. USA 2015, 112, E4782–E4791. [Google Scholar] [CrossRef]
- Boggio, E.M.; Pancrazi, L.; Gennaro, M.; Lo Rizzo, C.; Mari, F.; Meloni, I.; Ariani, F.; Panighini, A.; Novelli, E.; Biagioni, M.; et al. Visual impairment in FOXG1-mutated individuals and mice. Neuroscience 2016, 324, 496–508. [Google Scholar] [CrossRef]
- Mazziotti, R.; Lupori, L.; Sagona, G.; Gennaro, M.; Della Sala, G.; Putignano, E.; Pizzorusso, T. Searching for biomarkers of CDKL5 disorder: Early-onset visual impairment in CDKL5 mutant mice. Hum. Mol. Genet. 2017, 26, 2290–2298. [Google Scholar] [CrossRef]
- LeBlanc, J.J.; DeGregorio, G.; Centofante, E.; Vogel-Farley, V.K.; Barnes, K.; Kaufmann, W.E.; Fagiolini, M.; Nelson, C.A. Visual evoked potentials detect cortical processing deficits in Rett syndrome. Ann. Neurol. 2015, 78, 775–786. [Google Scholar] [CrossRef]
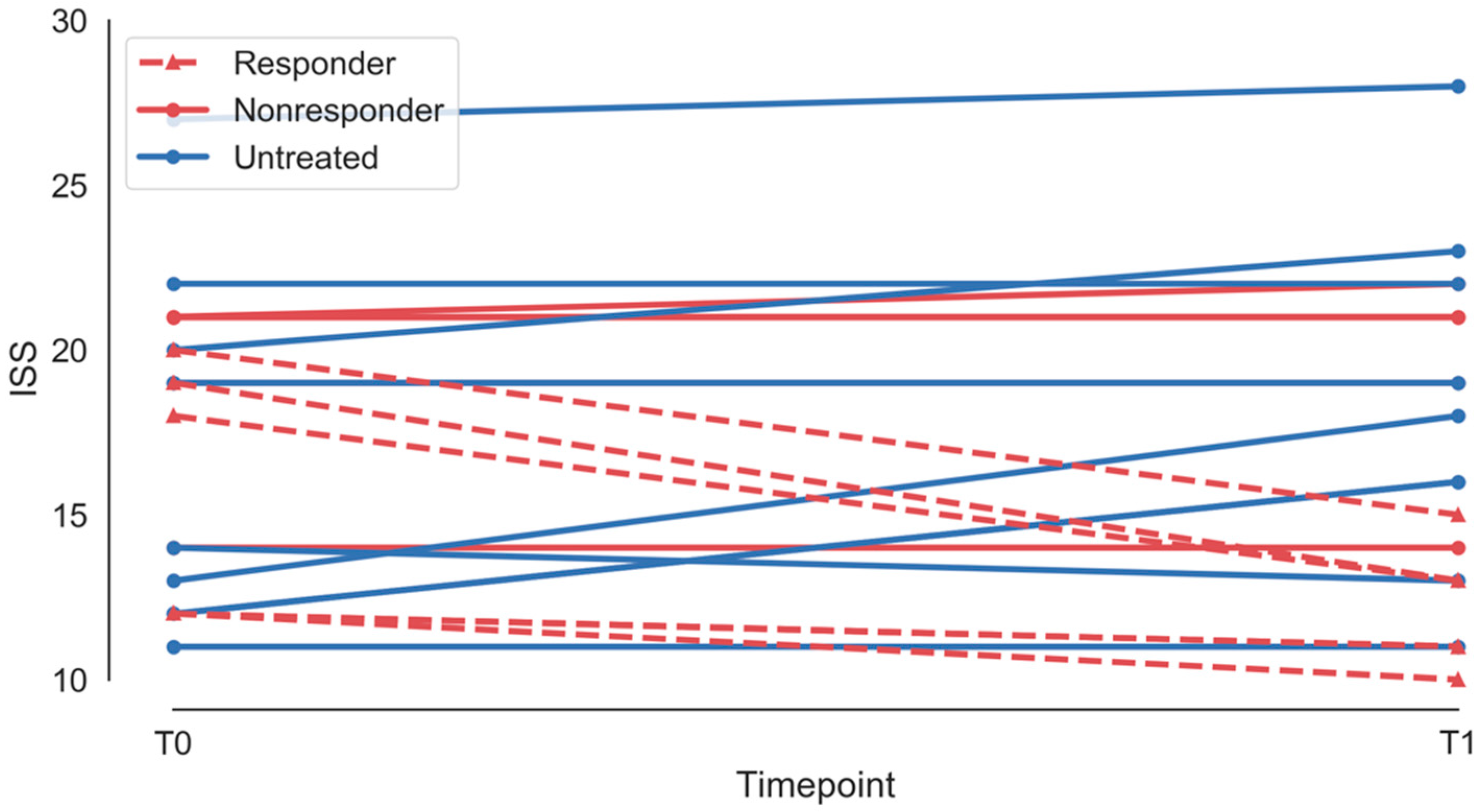

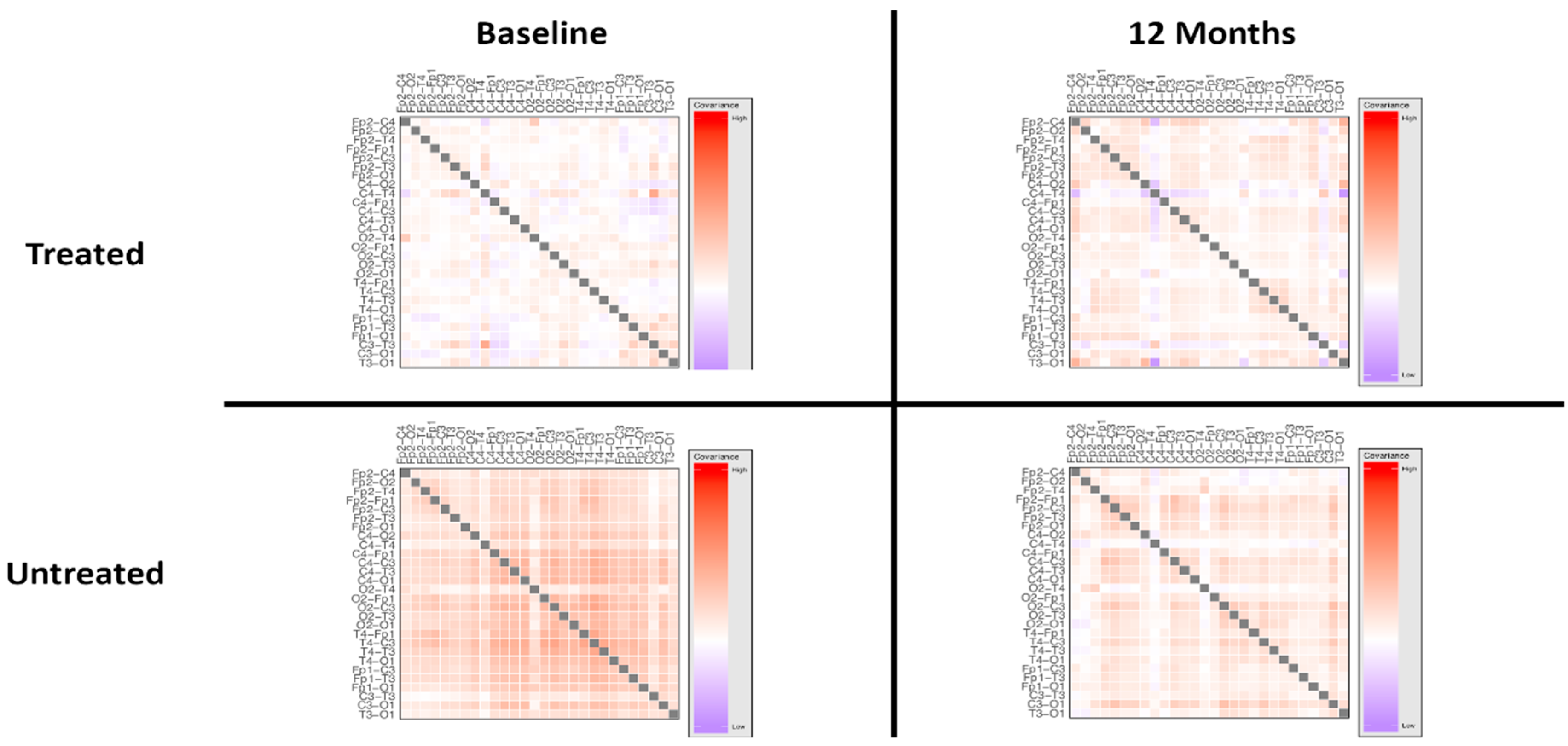

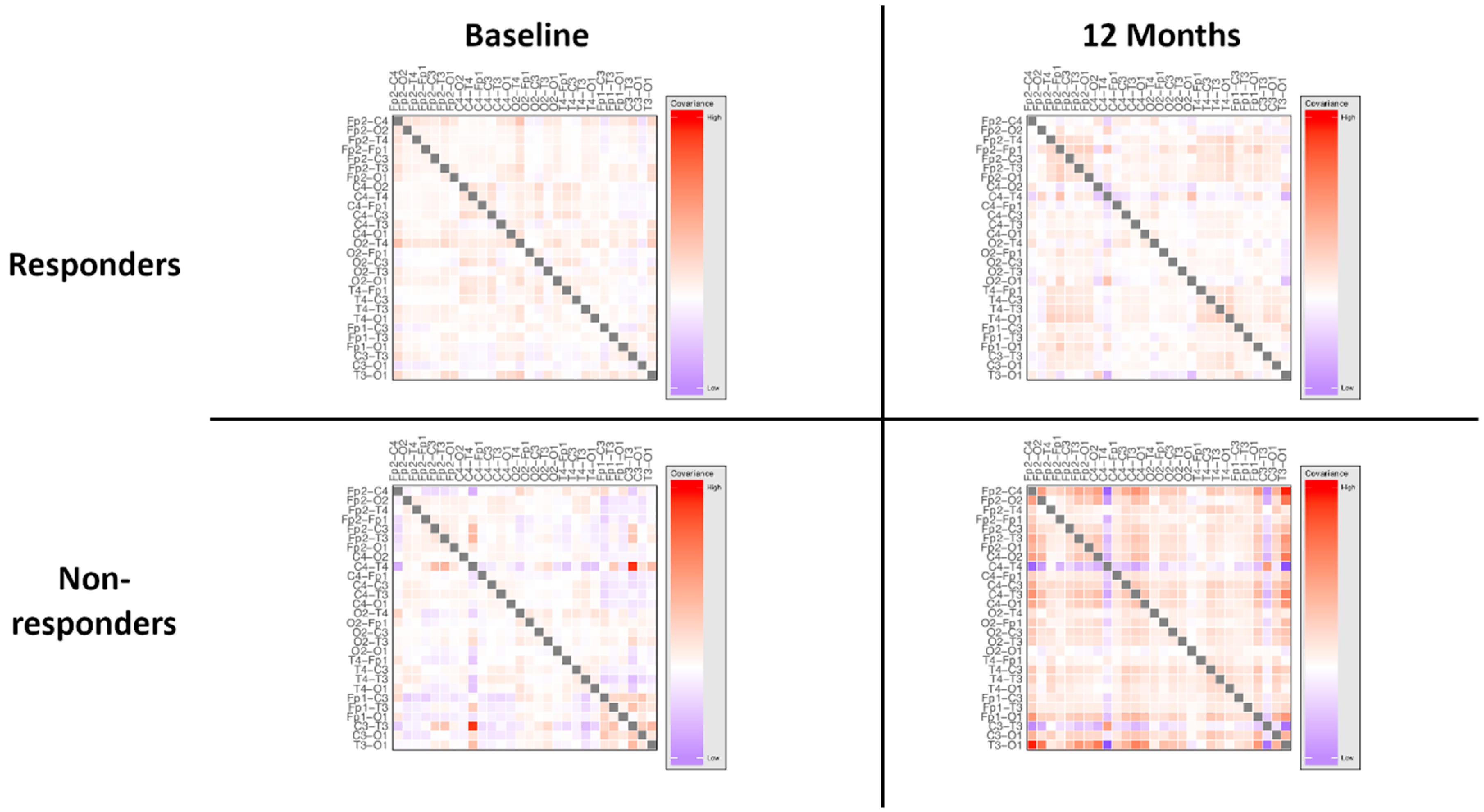
| Patient ID | Treatment | Age | Follow-Up | ISS, T1 | ISS, T2 | Responder |
|---|---|---|---|---|---|---|
| 1 | Treated | 10 | 14 | 12 | 11 | Responder |
| 2 | Treated | 4 | 13 | 18 | 13 | Responder |
| 3 | Treated | 3 | 11 | 21 | 21 | Nonresponder |
| 4 | Treated | 4 | 7 | 21 | 22 | Nonresponder |
| 5 | Treated | 3 | 12 | 12 | 10 | Responder |
| 6 | Treated | 10 | 7 | 19 | 13 | Responder |
| 7 | Treated | 9 | 15 | 19 | 19 | Nonresponder |
| 8 | Treated | 4 | 13 | 14 | 14 | Nonresponder |
| 9 | Treated | 6 | 14 | 20 | 15 | Responder |
| 10 | Untreated | 2 | 4 | 12 | 16 | N/A |
| 11 | Untreated | 2 | 11 | 13 | 18 | N/A |
| 12 | Untreated | 8 | 13 | 20 | 19 | N/A |
| 13 | Untreated | 14 | 16 | 14 | 13 | N/A |
| 14 | Untreated | 6 | 18 | 19 | 19 | N/A |
| 15 | Untreated | 10 | 12 | 27 | 28 | N/A |
| 16 | Untreated | 12 | 12 | 20 | 23 | N/A |
| 17 | Untreated | 12 | 12 | 22 | 22 | N/A |
| 18 | Untreated | 5 | 12 | 11 | 11 | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keogh, C.; Pini, G.; Gemo, I.; Kaufmann, W.E.; Tropea, D. Functional Network Mapping Reveals State-Dependent Response to IGF1 Treatment in Rett Syndrome. Brain Sci. 2020, 10, 515. https://doi.org/10.3390/brainsci10080515
Keogh C, Pini G, Gemo I, Kaufmann WE, Tropea D. Functional Network Mapping Reveals State-Dependent Response to IGF1 Treatment in Rett Syndrome. Brain Sciences. 2020; 10(8):515. https://doi.org/10.3390/brainsci10080515
Chicago/Turabian StyleKeogh, Conor, Giorgio Pini, Ilaria Gemo, Walter E. Kaufmann, and Daniela Tropea. 2020. "Functional Network Mapping Reveals State-Dependent Response to IGF1 Treatment in Rett Syndrome" Brain Sciences 10, no. 8: 515. https://doi.org/10.3390/brainsci10080515
APA StyleKeogh, C., Pini, G., Gemo, I., Kaufmann, W. E., & Tropea, D. (2020). Functional Network Mapping Reveals State-Dependent Response to IGF1 Treatment in Rett Syndrome. Brain Sciences, 10(8), 515. https://doi.org/10.3390/brainsci10080515






