Cerebral Substrates for Controlling Rhythmic Movements
Abstract
1. Introduction
2. Brain Regions Responsible for Rhythmic Movements
2.1. Patient Studies
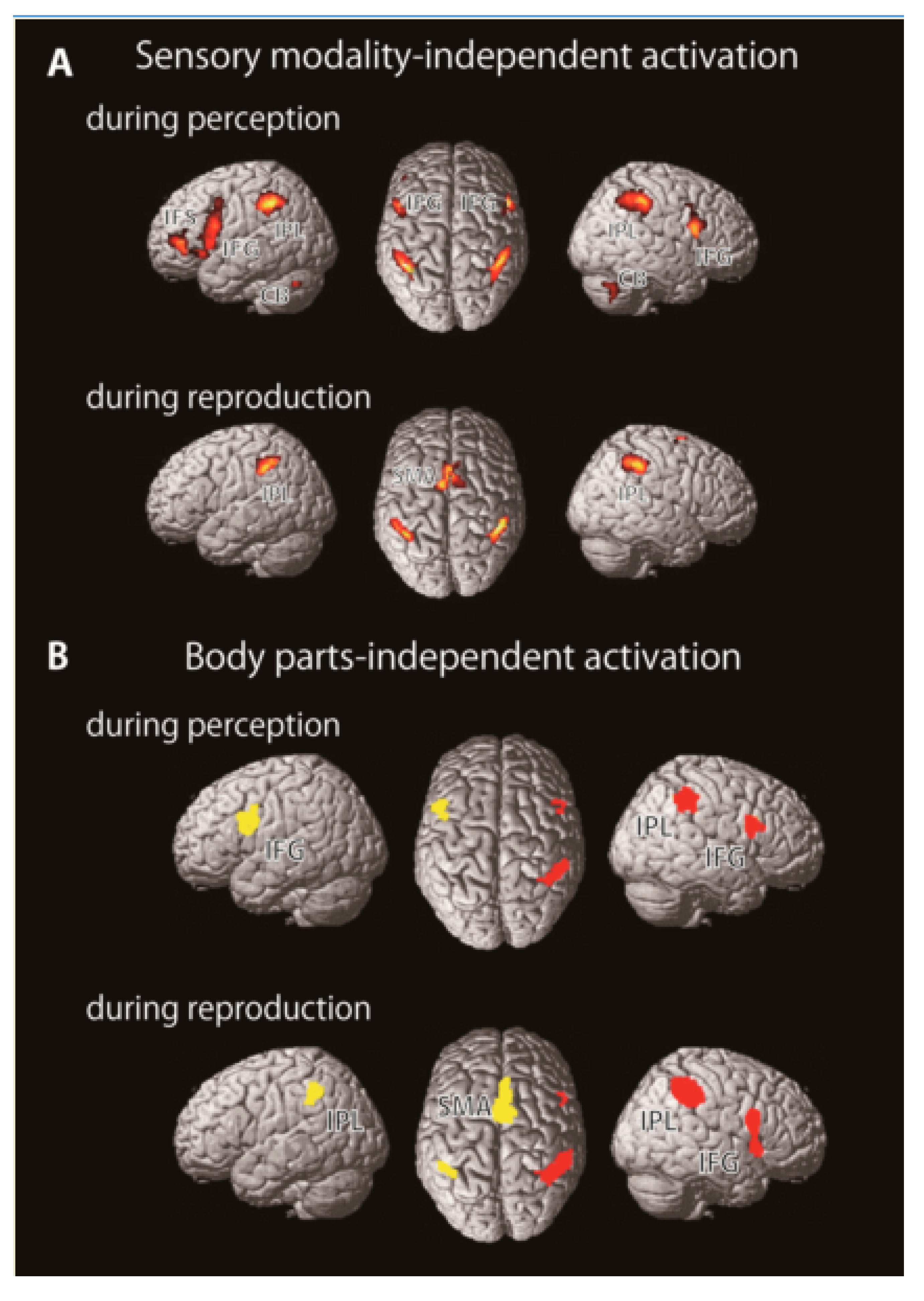
2.2. Brain Imaging Studies
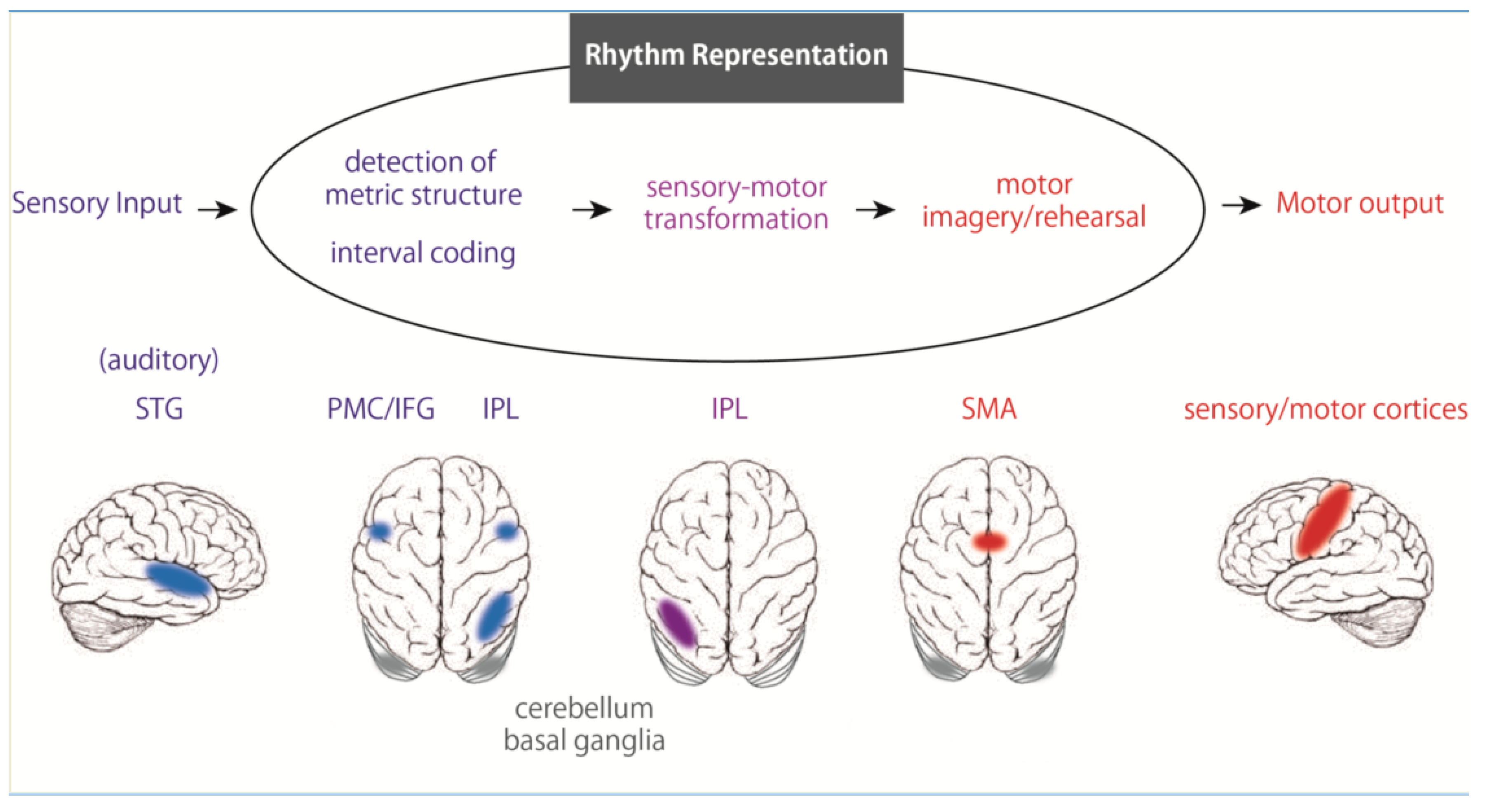
3. Rhythm Representation in the Brain
4. Temporal Information Processing
5. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fraisse, P. Perception and estimation of time. Annu. Rev. Psychol. 1984, 35, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.W.; Meyer, L.B. The Rhythmic Structure of Music; University of Chicago Press: Chicago, IL, USA, 1960. [Google Scholar]
- Bednark, J.G.; Campbell, M.E.; Cunnington, R. Basal ganglia and cortical networks for sequential ordering and rhythm of complex movements. Front. Hum. Neurosci. 2015, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.L.; Ehrsson, H.H.; Forssberg, H.; Ullén, F. Effector-independent voluntary timing: Behavioural and neuroimaging evidence. Eur. J. Neurosci. 2005, 22, 3255–3265. [Google Scholar] [CrossRef] [PubMed]
- De Manzano, Ö.; Ullén, F. Activation and connectivity patterns of the presupplementary and dorsal premotor areas during free improvisation of melodies and rhythms. Neuroimage 2012, 63, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Konoike, N.; Kotozaki, Y.; Jeong, H.; Miyazaki, A.; Sakaki, K.; Shinada, T.; Sugiura, M.; Kawashima, R.; Nakamura, K. Temporal and Motor Representation of Rhythm in Fronto-Parietal Cortical Areas: An fMRI Study. PLoS ONE 2015, 10, e0130120. [Google Scholar] [CrossRef][Green Version]
- Konoike, N.; Kotozaki, Y.; Miyachi, S.; Miyauchi, C.M.; Yomogida, Y.; Akimoto, Y.; Kuraoka, K.; Sugiura, M.; Kawashima, R.; Nakamura, K. Rhythm information represented in the fronto-parieto-cerebellar motor system. Neuroimage 2012, 63, 328–338. [Google Scholar] [CrossRef]
- Lewis, P.A.; Miall, R.C. Distinct systems for automatic and cognitively controlled time measurement: Evidence from neuroimaging. Curr. Opin. Neurobiol. 2003, 13, 250–255. [Google Scholar] [CrossRef]
- Ramnani, N.; Passingham, R.E. Changes in the human brain during rhythm learning. J. Cogn. Neurosci. 2001, 13, 952–966. [Google Scholar] [CrossRef]
- Rao, S.M.; Harrington, D.L.; Haaland, K.Y.; Bobholz, J.A.; Cox, R.W.; Binder, J.R. Distributed neural systems underlying the timing of movements. J. Neurosci. 1997, 17, 5528–5535. [Google Scholar] [CrossRef]
- Schwartze, M.; Rothermich, K.; Kotz, S.A. Functional dissociation of pre-SMA and SMA-proper in temporal processing. Neuroimage 2012, 60, 290–298. [Google Scholar] [CrossRef]
- Xu, D.; Liu, T.; Ashe, J.; Bushara, K.O. Role of the olivo-cerebellar system in timing. J. Neurosci. 2006, 26, 5990–5995. [Google Scholar] [CrossRef] [PubMed]
- Jerde, T.A.; Childs, S.K.; Handy, S.T.; Nagode, J.C.; Pardo, J.V. Dissociable systems of working memory for rhythm and melody. Neuroimage 2011, 57, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Karabanov, A.; Blom, O.; Forsman, L.; Ullén, F. The dorsal auditory pathway is involved in performance of both visual and auditory rhythms. Neuroimage 2009, 44, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Witt, S.T.; Laird, A.R.; Meyerand, M.E. Functional neuroimaging correlates of finger-tapping task variations: An ALE meta-analysis. Neuroimage 2008, 42, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Halsband, U.; Ito, N.; Tanji, J.; Freund, H.-J. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain 1993, 116, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Picton, T.W.; Stuss, N.T.; Shallice, T.; Alexander, M.P.; Gillingham, S. Keeping time: Effects of focal frontal lesions. Neuropsychologia 2006, 44, 1195–1209. [Google Scholar] [CrossRef]
- Frischer, M. Voluntary vs. autonomous control of repetitive finger tapping in a patient with Parkinson’s disease. Neuropsychologia 1989, 27, 1261–1266. [Google Scholar] [CrossRef]
- Freeman, J.S.; Cody, F.W.; Schady, W. The influence of external timing cues upon the rhythm of voluntary movements in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1993, 56, 1078–1084. [Google Scholar] [CrossRef]
- Harrington, D.L.; Haaland, K.Y.; Knight, R.T. Cortical Networks Underlying Mechanisms of Time Perception. J. Neurosci. 1998, 18, 1085–1095. [Google Scholar] [CrossRef]
- Ivry, R.B. Force and timing components of the motor program. J. Mot. Behav. 1986, 18, 449–474. [Google Scholar] [CrossRef]
- Ivry, R.B.; Keele, S.W. Timing functions of the cerebellum. J. Cogn. Neurosci. 1989, 1, 136–152. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, D.J.; Freeman, J.S.; Cody, F.W. The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson’s disease. Brain 1996, 119, 51–70. [Google Scholar] [CrossRef]
- Pastor, M.A.; Jahanshahi, M.; Artieda, J.; Obeso, J.A. Performance of repetitive wrist movements in parkinson’s disease. Brain 1992, 115, 875–891. [Google Scholar] [CrossRef] [PubMed]
- Wing, A.M.; Keele, S.; Margolin, D.I. Motor Disorder and the Timing of Repetitive Movements. Ann. N. Y. Acad. Sci. 1984, 423, 183–192. [Google Scholar] [CrossRef]
- Wing, A.M.; Miller, E. Basal ganglia lesions and psychological analyses of the control of voluntary movement. Ciba Found. Symp. 1984, 107, 242–257. [Google Scholar] [PubMed]
- Schwartze, M.; Keller, P.E.; Patel, A.D.; Kotz, S.A. The impact of basal ganglia lesions on sensorimotor synchronization, spontaneous motor tempo, and the detection of tempo changes. Behav. Brain Res. 2011, 216, 685–691. [Google Scholar] [CrossRef]
- Spencer, R.M.; Ivry, R.B. Comparison of patients with Parkinson’s disease or cerebellar lesions in the production of periodic movements involving event-based or emergent timing. Brain Cogn. 2005, 58, 84–93. [Google Scholar] [CrossRef]
- Aparicio, P.; Diedrichsen, J.; Ivry, R.B. Effects of focal basal ganglia lesions on timing and force control. Brain Cogn. 2005, 58, 62–74. [Google Scholar] [CrossRef]
- Teki, S.; Grube, M.; Kumar, S.; Griffiths, T. Distinct neural substrates of duration-based and beat-based auditory timing. J. Neurosci. 2011, 31, 3805–3812. [Google Scholar] [CrossRef]
- Grahn, J.A.; Rowe, J.B. Feeling the beat: Premotor and striatal interactions in musicians and nonmusicians during beat perception. J. Neurosci. 2009, 29, 7540–7548. [Google Scholar] [CrossRef]
- Grahn, J.A. The role of the basal ganglia in beat perception: Neuroimaging and neuropsychological investigations. Ann. N. Y. Acad. Sci. 2009, 1169, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Bo, J.; Block, H.J.; Clark, J.E.; Bastian, A.J. A cerebellar deficit in sensorimotor prediction explains movement timing variability. J. Neurophysiol. 2008, 100, 2825–2832. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ivry, R.B.; Keele, S.W.; Diener, H.C. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp. Brain Res. 1988, 73, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Schlerf, J.E.; Spencer, R.M.C.; Zelaznik, H.N.; Ivry, R.B. Timing of rhythmic movements in patients with cerebellar degeneration. Cerebellum 2007, 6, 221–231. [Google Scholar] [CrossRef]
- Schwartze, M.; Keller, P.E.; Kotz, S.A. Spontaneous, synchronized, and corrective timing behavior in cerebellar lesion patients. Behav. Brain Res. 2016, 312, 285–293. [Google Scholar] [CrossRef]
- Spencer, R.M.; Bjedov, I.; Tenaillon, O.; Gérard, B.; Souza, V.; Denamur, E.; Radman, M.; Taddei, F.; Matic, I. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science 2003, 300, 1437–1439. [Google Scholar] [CrossRef]
- Grube, M.; Cooper, F.E.; Chinnery, P.F.; Griffiths, T.D. Dissociation of duration-based and beat-based auditory timing in cerebellar degeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 11597–11601. [Google Scholar] [CrossRef]
- Grube, M.; Lee, K.-H.; Griffiths, T.D.; Barker, A.T.; Woodruff, P.W. Transcranial Magnetic Theta-Burst Stimulation of the Human Cerebellum Distinguishes Absolute, Duration-Based from Relative, Beat-Based Perception of Subsecond Time Intervals. Front. Psychol. 2010, 1, 171. [Google Scholar] [CrossRef]
- Bengtsson, S.L.; Ehrsson, H.H.; Forssberg, H.; Ullén, F. Dissociating brain regions controlling the temporal and ordinal structure of learned movement sequences. Eur. J. Neurosci. 2004, 19, 2591–2602. [Google Scholar] [CrossRef]
- Belin, P.; McAdams, S.; Thivard, L.; Smith, B.; Savel, S.; Zilbovicius, M.; Samson, S.; Samson, Y. The neuroanatomical substrate of sound duration discrimination. Neuropsychologia 2002, 40, 1956–1964. [Google Scholar] [CrossRef]
- Rao, S.M.; Mayer, A.R.; Harrington, D.L. The evolution of brain activation during temporal processing. Nat. Neurosci. 2001, 4, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Mathiak, K.; Hertrich, I.; Grodd, W.; Ackermann, H. Discrimination of temporal information at the cerebellum: Functional magnetic resonance imaging of nonverbal auditory memory. Neuroimage 2004, 21, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, T.D.; Johnsrude, I.; Dean, J.L.; Green, G.G.R. A common neural substrate for the analysis of pitch and duration pattern in segmented sound? Neuroreport 1999, 10, 3825–3830. [Google Scholar] [CrossRef] [PubMed]
- Ivry, R.B.; Spencer, R.M.C.; Zelaznik, H.N.; Diedrichsen, J. The cerebellum and event timing. Ann. N. Y. Acad. Sci. 2002, 978, 302–317. [Google Scholar] [CrossRef]
- Penhune, V.B.; Zatorre, R.J.; Evans, A.C. Cerebellar contributions to motor timing: A PET study of auditory and visual rhythm reproduction. J. Cogn. Neurosci. 1998, 10, 752–765. [Google Scholar] [CrossRef]
- Sakai, K.; Hikosaka, O.; Miyauchi, S.; Takino, R.; Tamada, T.; Iwata, N.K.; Nielsen, M. Neural representation of a rhythm depends on its interval ratio. J. Neurosci. 1999, 19, 10074–10081. [Google Scholar] [CrossRef]
- Schubotz, R.I.; Friederici, A.D.; Von Cramon, D.Y. Time perception and motor timing: A common cortical and subcortical basis revealed by fMRI. Neuroimage 2000, 11, 1–12. [Google Scholar] [CrossRef]
- Thaut, M.H.; Stephan, K.M.; Wunderlich, G.; Schicks, W.; Tellmann, L.; Herzog, H.; McIntosh, G.C.; Seitz, R.J.; Hömberg, V. Distinct cortico-cerebellar activations in rhythmic auditory motor synchronization. Cortex 2009, 45, 44–53. [Google Scholar] [CrossRef]
- Chen, J.L.; Zatorre, R.J.; Penhune, V.B. Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. Neuroimage 2006, 32, 1771–1781. [Google Scholar] [CrossRef]
- Chen, J.L.; Penhune, V.B.; Zatorre, R.J. Listening to musical rhythms recruits motor regions of the brain. Cereb. Cortex 2008, 18, 2844–2854. [Google Scholar] [CrossRef]
- Bengtsson, S.L.; Ullén, F.; Ehrsson, H.H.; Hashimoto, T.; Kito, T.; Naito, E.; Forssberg, H.; Sadato, N. Listening to rhythms activates motor and premotor cortices. Cortex 2009, 45, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Koelsch, S.; Siebel, W.A. Towards a neural basis of music perception. Trends Cogn. Sci. 2005, 9, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Maess, B.; Koelsch, S.; Gunter, T.C.; Friederici, A.D. Musical syntax is processed in Broca’s area: An MEG study. Nat. Neurosci. 2001, 4, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Uddén, J.; Bahlmann, J. A rostro-caudal gradient of structured sequence processing in the left inferior frontal gyrus. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2023–2032. [Google Scholar] [CrossRef]
- Friederici, A.D. Syntactic, prosodic, and semantic processes in the brain: Evidence from event-related neuroimaging. J. Psycholinguist. Res. 2001, 30, 237–250. [Google Scholar] [CrossRef]
- Coull, J.T.; Vidal, F.; Nazarian, B.; Macar, F. Functional anatomy of the attentional modulation of time estimation. Science 2004, 303, 1506–1508. [Google Scholar] [CrossRef]
- Harrington, D.L.; Zimbelman, J.L.; Hinton, S.C.; Rao, S.M. Neural modulation of temporal encoding, maintenance, and decision processes. Cereb. Cortex 2009, 20, 1274–1285. [Google Scholar] [CrossRef]
- Coull, J.T.; Davranche, K.; Nazarian, B.; Vidal, F. Functional anatomy of timing differs for production versus prediction of time intervals. Neuropsychologia 2013, 51, 309–319. [Google Scholar] [CrossRef]
- Battelli, L.; Cavanagh, P.; Martini, P.; Barton, J.J.S. Bilateral deficits of transient visual attention in right parietal patients. Brain 2003, 126, 2164–2174. [Google Scholar] [CrossRef]
- Battelli, L.; Walsh, V.; Pascual-Leone, A.; Cavanagh, P. The ‘when’ parietal pathway explored by lesion studies. Curr. Opin. Neurobiol. 2008, 18, 120–126. [Google Scholar] [CrossRef]
- Oliveri, M.; Koch, G.; Salerno, S.; Torriero, S.; Gerfo, E.L.; Caltagirone, C. Representation of time intervals in the right posterior parietal cortex: Implications for a mental time line. Neuroimage 2009, 46, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Grahn, J.A.; Rowe, J.B. Finding and feeling the musical beat: Striatal dissociations between detection and prediction of regularity. Cereb. Cortex 2012, 23, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, A.J.; Särkämö, T.; Rodríguez-Fornells, A.; Ripollés, P.; Münte, T.F.; Soinila, S. Neural architectures of music—Insights from acquired amusia. Neurosci. Biobehav. Rev. 2019, 107, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Ivry, R.B.; Schlerf, J.E. Dedicated and intrinsic models of time perception. Trends Cogn. Sci. 2008, 12, 273–280. [Google Scholar] [CrossRef]
- Mauk, M.D.; Buonomano, D.V. The neural basis of temporal processing. Annu. Rev. Neurosci. 2004, 27, 307–340. [Google Scholar] [CrossRef]
- Buhusi, C.V.; Meck, W.H. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 2005, 6, 755–765. [Google Scholar] [CrossRef]
- Gibbon, J.; Church, R.M.; Meck, W.H. Scalar timing in memory. Ann. N. Y. Acad. Sci. 1984, 423, 52–77. [Google Scholar] [CrossRef]
- Matell, M.S.; Meck, W.H. Cortico-striatal circuits and interval timing: Coincidence detection of oscillatory processes. Cogn. Brain Res. 2004, 21, 139–170. [Google Scholar] [CrossRef]
- Karmarkar, U.R.; Buonomano, D.V. Timing in the absence of clocks: Encoding time in neural network states. Neuron 2007, 53, 427–438. [Google Scholar] [CrossRef]
- Merchant, H.; Harrington, D.L.; Meck, W.H. Neural basis of the perception and estimation of time. Annu. Rev. Neurosci. 2013, 36, 313–336. [Google Scholar] [CrossRef]
- Merchant, H.; Zarco, W.; Prado, L. Do we have a common mechanism for measuring time in the hundreds of millisecond range? Evidence from multiple-interval timing tasks. J. Neurophysiol. 2008, 99, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Konoike, N.; Mikami, A.; Miyachi, S. The influence of tempo upon the rhythmic motor control in macaque monkeys. Neurosci. Res. 2012, 74, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Tomonaga, M.; Matsuzawa, T. Spontaneous synchronized tapping to an auditory rhythm in a chimpanzee. Sci. Rep. 2013, 3, 1566. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.D.; Iversen, J.R.; Bregman, M.R.; Schulz, I. Experimental evidence for synchronization to a musical beat in a nonhuman animal. Curr. Biol. 2009, 19, 827–830. [Google Scholar] [CrossRef]
- Rouse, A.A.; Cook, P.F.; Large, E.W.; Reichmuth, C. Beat Keeping in a Sea Lion As Coupled Oscillation: Implications for Comparative Understanding of Human Rhythm. Front. Neurosci. 2016, 10, 257. [Google Scholar] [CrossRef]
- Zarco, W.; Merchant-Nancy, H.; Prado, L.; Méndez, J.C. Subsecond timing in primates: Comparison of interval production between human subjects and rhesus monkeys. J. Neurophysiol. 2009, 102, 3191–3202. [Google Scholar] [CrossRef]
- Merchant-Nancy, H.; Pérez, O.; Bartolo, R.; Méndez, J.C.; Mendoza, G.; Gámez, J.; Yc, K.; Prado, L. Sensorimotor neural dynamics during isochronous tapping in the medial premotor cortex of the macaque. Eur. J. Neurosci. 2015, 41, 586–602. [Google Scholar] [CrossRef]
- Merchant, H.; Pérez, O.; Zarco, W.; Gámez, J. Interval tuning in the primate medial premotor cortex as a general timing mechanism. J. Neurosci. 2013, 33, 9082–9096. [Google Scholar] [CrossRef]
- Merchant, H.; Zarco, W.; Pérez, O.; Prado, L.; Bartolo, R. Measuring time with different neural chronometers during a synchronization-continuation task. Proc. Natl. Acad. Sci. USA 2011, 108, 19784–19789. [Google Scholar] [CrossRef]
- Taut, M. Rhythm, Music, and the Brain: Scientific Foundations and Clinical Applications (Studies on New Music Research); Routledge: Abingdon-on-Thames, UK, 2006. [Google Scholar]
- Nombela, C.; Hughes, L.E.; Owen, A.M.; Grahn, J.A. Into the groove: Can rhythm influence Parkinson’s disease? Neurosci. Biobehav. Rev. 2013, 37, 2564–2570. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konoike, N.; Nakamura, K. Cerebral Substrates for Controlling Rhythmic Movements. Brain Sci. 2020, 10, 514. https://doi.org/10.3390/brainsci10080514
Konoike N, Nakamura K. Cerebral Substrates for Controlling Rhythmic Movements. Brain Sciences. 2020; 10(8):514. https://doi.org/10.3390/brainsci10080514
Chicago/Turabian StyleKonoike, Naho, and Katsuki Nakamura. 2020. "Cerebral Substrates for Controlling Rhythmic Movements" Brain Sciences 10, no. 8: 514. https://doi.org/10.3390/brainsci10080514
APA StyleKonoike, N., & Nakamura, K. (2020). Cerebral Substrates for Controlling Rhythmic Movements. Brain Sciences, 10(8), 514. https://doi.org/10.3390/brainsci10080514




