Actigraph-Measured Movement Correlates of Attention-Deficit/Hyperactivity Disorder (ADHD) Symptoms in Young People with Tuberous Sclerosis Complex (TSC) with and without Intellectual Disability and Autism Spectrum Disorder (ASD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessments
2.2.1. Behaviour and Intellectual Ability
2.2.2. Features of TSC
2.2.3. Actigraphs
2.3. Statistical Analysis
3. Results
3.1. Sample Characteristics
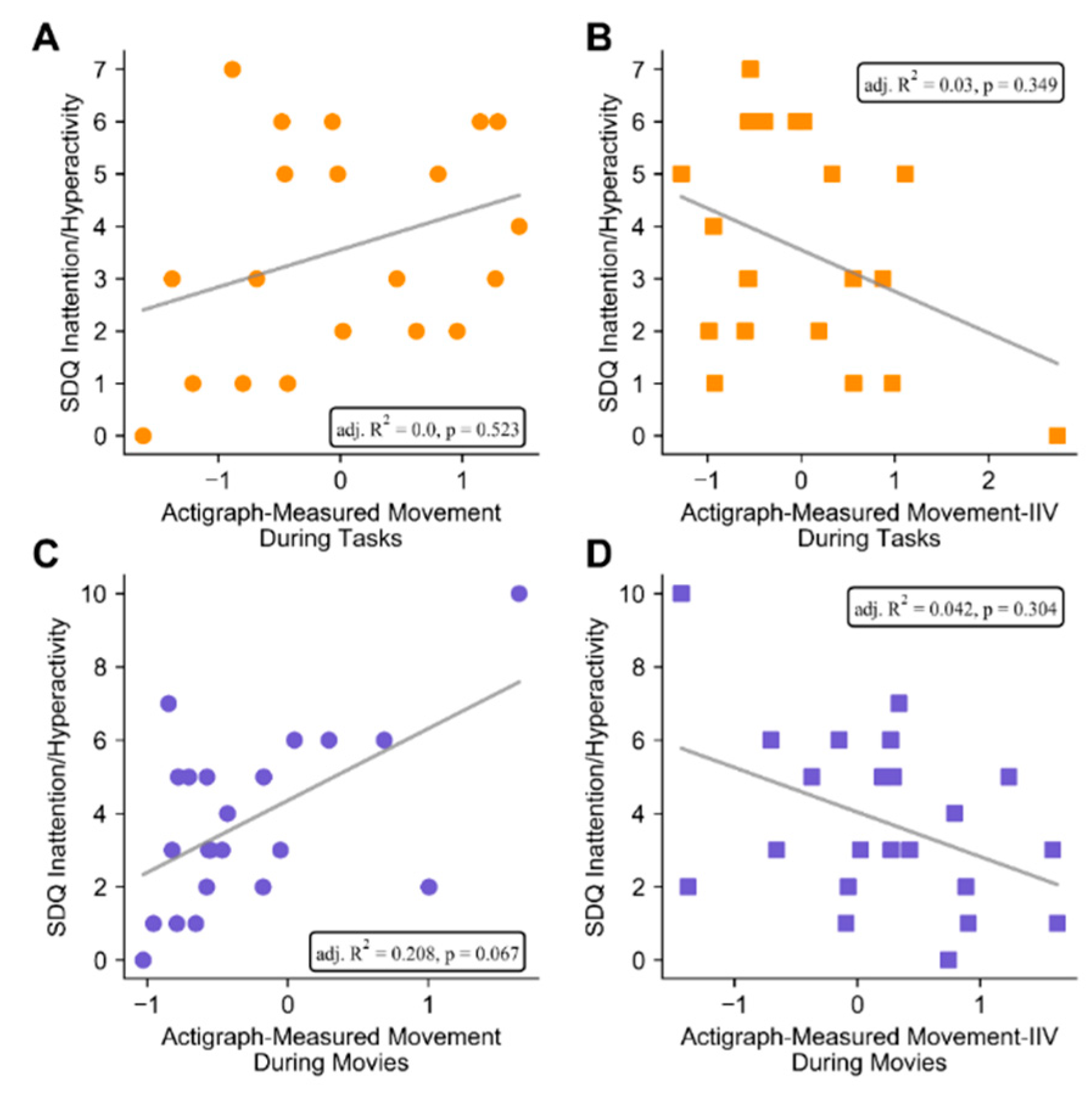
3.2. Associations between Actigraphy and Parent-Rated ADHD Symptoms
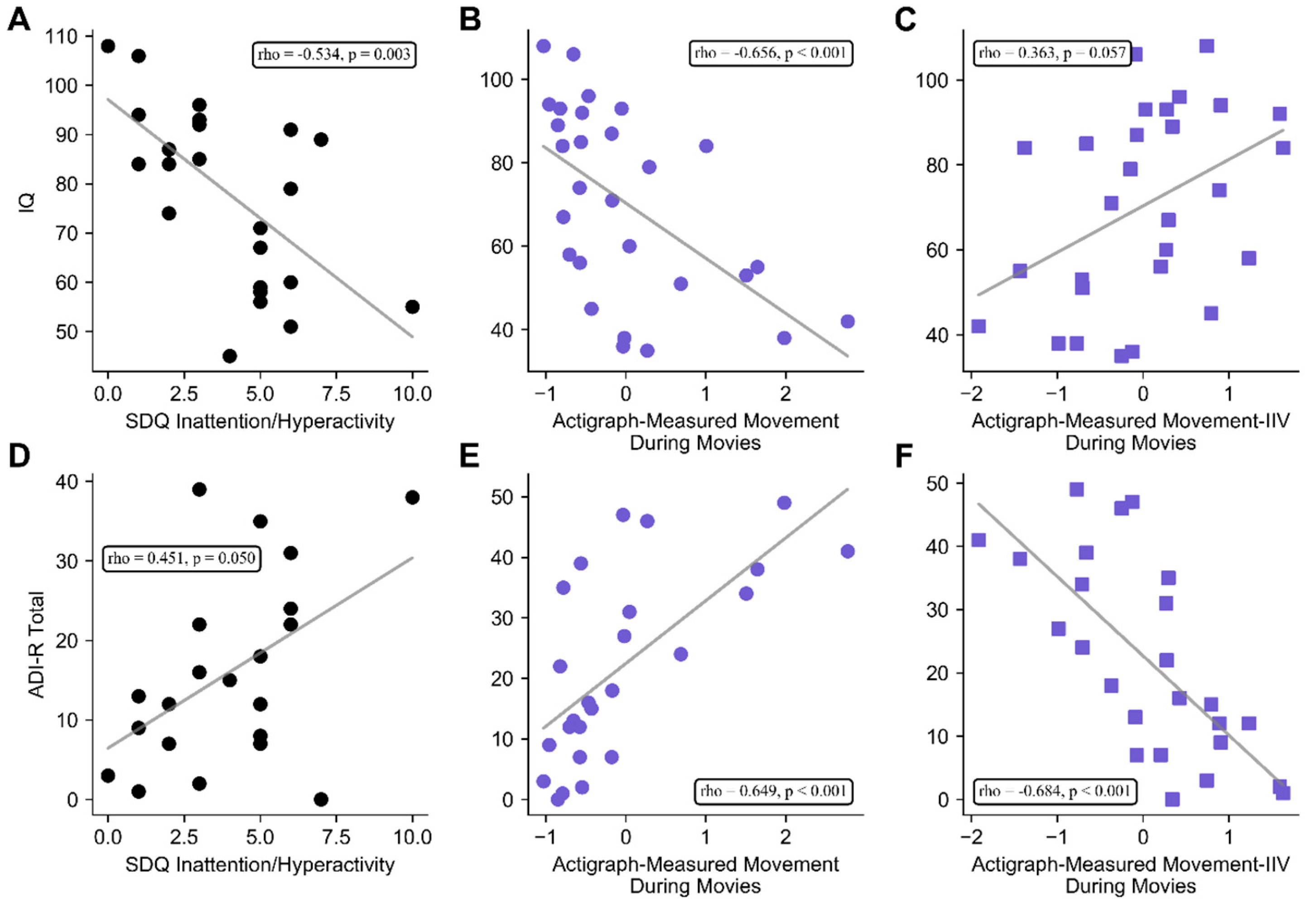
3.3. Associations between Clinical Features of TSC, Parent-Rated ADHD Symptoms, and Actigraph Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Health Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Einziger, T.; Levi, L.; Zilberman-Hayun, Y.; Auerbach, J.G.; Atzaba-Poria, N.; Arbelle, S.; Berger, A. Predicting ADHD Symptoms in Adolescence from Early Childhood Temperament Traits. J. Abnorm. Child Psychol. 2017, 46, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Shephard, E.; Bedford, R.; Milosavljevic, B.; Gliga, T.; Jones, E.J.H.; Pickles, A.; Johnson, M.H.; Charman, T. The BASIS Team Early developmental pathways to childhood symptoms of attention-deficit hyperactivity disorder, anxiety and autism spectrum disorder. J. Child Psychol. Psychiatry 2018, 60, 963–974. [Google Scholar] [CrossRef] [PubMed]
- De Crescenzo, F.; Licchelli, S.; Ciabattini, M.; Menghini, D.; Armando, M.; Alfieri, P.; Mazzone, L.; Pontrelli, G.; Livadiotti, S.; Foti, F.; et al. The use of actigraphy in the monitoring of sleep and activity in ADHD: A meta-analysis. Sleep Med. Rev. 2016, 26, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Murillo, L.G.; Cortese, S.; Anderson, D.; Di Martino, A.; Castellanos, F.X. Locomotor activity measures in the diagnosis of attention deficit hyperactivity disorder: Meta-analyses and new findings. J. Neurosci. Methods 2015, 252, 14–26. [Google Scholar] [CrossRef]
- Brandt, V.; Patalay, P.; Koerner, J.K.A. Predicting ADHD symptoms and diagnosis at age 14 from objective activity levels at age 7 in a large UK cohort. Eur. Child Adolesc. Psychiatry 2020, 1–8. [Google Scholar] [CrossRef]
- Cheung, C.H.M.; Rijsdijk, F.; McLoughlin, G.; Brandeis, D.; Banaschewski, T.; Asherson, P.; Kuntsi, J. Cognitive and neurophysiological markers of ADHD persistence and remission. Br. J. Psychiatry 2016, 208, 548–555. [Google Scholar] [CrossRef]
- De Crescenzo, F.; Armando, M.; Mazzone, L.; Ciliberto, M.; Sciannamea, M.; Figueroa, C.; Janiri, L.; Quested, D.; Vicari, S. The use of actigraphy in the monitoring of methylphenidate versus placebo in ADHD: A meta-analysis. Atten. Deficit Hyperact. Disord. 2013, 6, 49–58. [Google Scholar] [CrossRef]
- Hall, C.; Valentine, A.Z.; Groom, M.J.; Walker, G.; Sayal, K.; Daley, D.; Hollis, C. The clinical utility of the continuous performance test and objective measures of activity for diagnosing and monitoring ADHD in children: A systematic review. Eur. Child Adolesc. Psychiatry 2015, 25, 677–699. [Google Scholar] [CrossRef]
- Chu, K.-C.; Lu, H.-K.; Huang, M.-C.; Lin, S.-J.; Liu, W.-I.; Huang, Y.-S.; Hsu, J.-F.; Wang, C.-H. Using Mobile Electroencephalography and Actigraphy to Diagnose Attention-Deficit/Hyperactivity Disorder: Case-Control Comparison Study. JMIR Ment. Health 2020, 7, e12158. [Google Scholar] [CrossRef]
- Pellegrini, S.; Murphy, M.; Lovett, E. The QbTest for ADHD assessment: Impact and implementation in Child and Adolescent Mental Health Services. Child. Youth Serv. Rev. 2020, 114, 105032. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Y.; Huang, W.; Zhang, T.; Gao, C.; Xing, Y.; Zheng, Y. WeDA: Designing and Evaluating A Scale-driven Wearable Diagnostic Assessment System for Children with ADHD. In Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems, Honolulu, HI, USA, 25–30 April 2020. [Google Scholar]
- Veenman, B.; Luman, M.; Oosterlaan, J. Further Insight into the Effectiveness of a Behavioral Teacher Program Targeting ADHD Symptoms Using Actigraphy, Classroom Observations and Peer Ratings. Front. Psychol. 2017, 8, 1157. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, P.; Bombardieri, R.; Jozwiak, S. Tuberous sclerosis. Lancet 2008, 372, 657–668. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Monteggia, L.M. mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat. Neurosci. 2013, 16, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Prather, P.; De Vries, P.J. Behavioral and Cognitive Aspects of Tuberous Sclerosis Complex. J. Child Neurol. 2004, 19, 666–674. [Google Scholar] [CrossRef] [PubMed]
- De Vries, P.J.; Hunt, A.; Bolton, P.F. The psychopathologies of children and adolescents with tuberous sclerosis complex (TSC). Eur. Child Adolesc. Psychiatry 2007, 16, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Gillberg, I.C.; Gillberg, C.; Ahlsén, G. Autistic behaviour and attention deficits in tuberous sclerosis: A population-based study. Dev. Med. Child Neurol. 1994, 36, 50–56. [Google Scholar] [CrossRef]
- Hunt, A. Development, behaviour and seizures in 300 cases of tuberous sclerosis. J. Intellect. Disabil. Res. 2008, 37, 41–51. [Google Scholar] [CrossRef]
- Muzykewicz, D.A.; Newberry, P.; Danforth, N.; Halpern, E.F.; Thiele, E.A. Psychiatric comorbid conditions in a clinic population of 241 patients with tuberous sclerosis complex. Epilepsy Behav. 2007, 11, 506–513. [Google Scholar] [CrossRef]
- Polanczyk, G.V.; Willcutt, E.G.; Salum, G.A.; Kieling, C.; Rohde, L.A. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int. J. Epidemiol. 2014, 43, 434–442. [Google Scholar] [CrossRef]
- Reilly, C.; Holland, N. Symptoms of attention defict hyperactivity disorder in children and adults with intellectual disability: A review. J. Appl. Res. Intellect. Disabil. 2011, 24, 291–309. [Google Scholar] [CrossRef]
- Tye, C.; Runicles, A.K.; Whitehouse, A.J.O.; Alvares, G.A. Characterizing the Interplay Between Autism Spectrum Disorder and Comorbid Medical Conditions: An Integrative Review. Front. Psychol. 2019, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Bolton, P.F. Neuroepileptic correlates of autistic symptomatology in tuberous sclerosis. Ment. Retard. Dev. Disabil. Res. Rev. 2004, 10, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Bolton, P.F.; Park, R.J.; Higgins, J.N.P.; Griffiths, P.D.; Pickles, A. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain 2002, 125, 1247–1255. [Google Scholar] [CrossRef]
- D’Agati, E.; Moavero, R.; Cerminara, C.; Curatolo, P. Attention-Deficit Hyperactivity Disorder (ADHD) and Tuberous Sclerosis Complex. J. Child Neurol. 2009, 24, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Kothare, S.; Singh, K.; Hochman, T.; Chalifoux, J.R.; Staley, B.A.; Weiner, H.L.; Menzer, K.; Devinsky, O. Genotype/phenotype in tuberous sclerosis complex: Associations with clinical and radiologic manifestations. Epilepsia 2014, 55, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Shehata, H.S.; Abdelghaffar, H.M.; Nasreldin, M.; Elmazny, A.; Abdelalim, A.; Sabbah, A.; Shalaby, N.M. Clinical patterns and outcomes of status epilepticus in patients with tuberous sclerosis complex. Ther. Clin. Risk Manag. 2017, 13, 779–785. [Google Scholar] [CrossRef]
- Kofler, M.J.; Rapport, M.D.; Sarver, D.E.; Raiker, J.S.; Orban, S.A.; Friedman, L.M.; Kolomeyer, E.G. Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clin. Psychol. Rev. 2013, 33, 795–811. [Google Scholar] [CrossRef]
- Adamo, N.; Hodsoll, J.; Asherson, P.; Buitelaar, J.K.; Kuntsi, J. Ex-Gaussian, Frequency and Reward Analyses Reveal Specificity of Reaction Time Fluctuations to ADDH and Not Autism Traits. J. Abnorm. Child Psychol. 2018, 47, 557–567. [Google Scholar] [CrossRef]
- Castellanos, F.; Sonuga-Barke, E.J.; Milham, M.P.; Tannock, R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends Cogn. Sci. 2006, 10, 117–123. [Google Scholar] [CrossRef]
- Smits-Engelsman, B.; Hill, E. The Relationship Between Motor Coordination and Intelligence Across the IQ Range. Pediatrics 2012, 130, e950–e956. [Google Scholar] [CrossRef]
- Kam, H.J.; Lee, K.; Cho, S.-M.; Shin, Y.M.; Park, R.W. High-Resolution Actigraphic Analysis of ADHD: A Wide Range of Movement Variability Observation in Three School Courses—A Pilot Study. Healthc. Inform. Res. 2011, 17, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.C.; Asherson, P.; Rijsdijk, F.; Kuntsi, J. Is Overactivity a Core Feature in ADHD? Familial and Receiver Operating Characteristic Curve Analysis of Mechanically Assessed Activity Level. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 1023–1030. [Google Scholar] [CrossRef]
- Lin, L.-C.; Ouyang, C.-S.; Chiang, C.-T.; Wu, R.-C.; Yang, R.-C. Quantitative Analysis of Movements in Children with Attention-Deficit Hyperactivity Disorder Using a Smart Watch at School. Appl. Sci. 2020, 10, 4116. [Google Scholar] [CrossRef]
- Dueck, A.; Reis, O.; Bastian, M.; Van Treeck, L.; Weirich, S.; Haessler, F.; Fiedler, A.; Koelch, M.; Berger, C. Feasibility of a Complex Setting for Assessing Sleep and Circadian Rhythmicity in a Fragile X Cohort. Front. Psychol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R. The Strengths and Difficulties Questionnaire: A research note. J. Child Psychol. Psychiatry 1997, 38, 581–586. [Google Scholar] [CrossRef]
- Emerson, E. Use of the Strengths and Difficulties Questionnaire to assess the mental health needs of children and adolescents with intellectual disabilities. J. Intellect. Dev. Disabil. 2005, 30, 14–23. [Google Scholar] [CrossRef]
- Kaptein, S.; Jansen, D.E.M.C.; Vogels, A.G.C.; Reijneveld, S.A. Mental health problems in children with intellectual disability: Use of the Strengths and Difficulties Questionnaire. J. Intellect. Disabil. Res. 2007, 52, 125–131. [Google Scholar] [CrossRef]
- Yates, J.R.; MacLean, C.; Higgins, J.N.P.; Humphrey, A.; Clifford, M.; Carcani-Rathwell, I.; Sampson, J.R.; Bolton, P.F.; Le Maréchal, K. The Tuberous Sclerosis 2000 Study: Presentation, initial assessments and implications for diagnosis and management. Arch. Dis. Child. 2011, 96, 1020–1025. [Google Scholar] [CrossRef]
- Wechsler, D. WASI-II: Wechsler Abbreviated Scale of Intelligence; Psychological Corporation: New York, NY, USA, 2011. [Google Scholar]
- Sparrow, S.S.; Balla, D.A.; Cicchetti, D.V. Vineland Adaptive Behavior Scales, 2nd ed.; Vineland-II; Pearson Assessments: Bloomington, IN, USA, 2005. [Google Scholar]
- Bolton, P.F.; Clifford, M.; Tye, C.; Maclean, C.; Humphrey, A.; Le Marechal, K.; Higgins, J.N.; Neville, B.G.; Rijsdjik, F.; Tuberous Sclerosis 2000 Study Group. Intellectual abilities in tuberous sclerosis complex: Risk factors and correlates from the Tuberous Sclerosis 2000 Study. Psychol. Med. 2015, 45, 2321–2331. [Google Scholar] [CrossRef]
- Tye, C.; Thomas, L.E.; Sampson, J.R.; Lewis, J.; O’Callaghan, F.; Yates, I.J.R.; Bolton, P.F. Secular changes in severity of intellectual disability in tuberous sclerosis complex: A reflection of improved identification and treatment of epileptic spasms? Epilepsia Open 2018, 3, 276–280. [Google Scholar] [CrossRef]
- Le Couteur, A.; Lord, A.; Rutter, M. The Autism Diagnostic Interview-Revised (ADI-R); Western Psychological Services: Los Angeles, CA, USA, 2003. [Google Scholar]
- Humphrey, A.; Ploubidis, G.B.; Yates, I.J.R.; Steinberg, T.; Bolton, P.F. The Early Childhood Epilepsy Severity Scale (E-Chess). Epilepsy Res. 2008, 79, 139–145. [Google Scholar] [CrossRef]
- Bishop, D.V.M. Test for Reception of Grammar: TROG-2; Harcourt Assessment: London, UK, 2003. [Google Scholar]
- The Jamovi Project. About—Jamovi. Available online: https://www.jamovi.org/about.html (accessed on 27 July 2020).
- De Vries, P.J.; Gardiner, J.; Bolton, P.F. Neuropsychological attention deficits in tuberous sclerosis complex (TSC). Am. J. Med Genet. Part A 2009, 149, 387–395. [Google Scholar] [CrossRef]
- Conners, C.K. Conners, 3rd ed.; Multi-Health Systems Inc.: North Tonawanda, NY, USA, 2008. [Google Scholar]
- Barkley, R.A. Issues in the diagnosis of attention-deficit/hyperactivity disorder in children. Brain Dev. 2003, 25, 77–83. [Google Scholar] [CrossRef]
- Kofler, M.J.; Raiker, J.S.; Sarver, D.E.; Wells, E.L.; Soto, E.F. Is hyperactivity ubiquitous in ADHD or dependent on environmental demands? Evidence from meta-analysis. Clin. Psychol. Rev. 2016, 46, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, M.; Healey, D.M.; Halperin, J.M. One-week temporal stability of hyperactivity in preschoolers with ADHD during psychometric assessment. Psychiatry Clin. Neurosci. 2013, 68, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, N.; Okada, A.; Kaku, R.; Kuriki, N.; Hanada, K.; Shirakawa, O. Differentiation between attention-deficit/hyperactivity disorder and pervasive developmental disorders with hyperactivity on objective activity levels using actigraphs. Psychiatry Clin. Neurosci. 2009, 63, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Irwin, L.N.; Groves, N.B.; Soto, E.F.; Kofler, M.J. Is There a Functional Relation Between Set Shifting and Hyperactivity in Children with Attention-Deficit/Hyperactivity Disorder (ADHD)? J. Int. Neuropsychol. Soc. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kofler, M.J.; Sarver, D.E.; Wells, E.L. Working Memory and Increased Activity Level (Hyperactivity) in ADHD: Experimental Evidence for a Functional Relation. J. Atten. Disord. 2015, 24, 1330–1344. [Google Scholar] [CrossRef]
- Dekkers, T.J.; Rapport, M.D.; Calub, C.A.; Eckrich, S.J.; Irurita, C. ADHD and hyperactivity: The influence of cognitive processing demands on gross motor activity level in children. Child Neuropsychol. 2020, 1–20. [Google Scholar] [CrossRef]
- Bellato, A.; Arora, I.; Hollis, C.; Groom, M.J. Is autonomic nervous system function atypical in attention deficit hyperactivity disorder (ADHD)? A systematic review of the evidence. Neurosci. Biobehav. Rev. 2020, 108, 182–206. [Google Scholar] [CrossRef]
- Geisler, J.; Romanos, M.; Hegerl, U.; Hensch, T. Hyperactivity and sensation seeking as autoregulatory attempts to stabilize brain arousal in ADHD and mania? Atten. Deficit Hyperact. Disord. 2014, 6, 159–173. [Google Scholar] [CrossRef]
- Mayes, S.D.; Calhoun, S.L.; Mayes, R.D.; Molitoris, S. Autism and ADHD: Overlapping and discriminating symptoms. Res. Autism Spectr. Disord. 2012, 6, 277–285. [Google Scholar] [CrossRef]
- Pan, C.-Y.; Tsai, C.-L.; Chu, C.-H. Fundamental Movement Skills in Children Diagnosed with Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder. J. Autism Dev. Disord. 2009, 39, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Sinzig, J.; Walter, D.; Doepfner, M. Attention Deficit/Hyperactivity Disorder in Children and Adolescents With Autism Spectrum Disorder. J. Atten. Disord. 2009, 13, 117–126. [Google Scholar] [CrossRef] [PubMed]
- McGarty, A.; Penpraze, V.; Melville, C. Accelerometer use during field-based physical activity research in children and adolescents with intellectual disabilities: A systematic review. Res. Dev. Disabil. 2014, 35, 973–981. [Google Scholar] [CrossRef]
- Ward, D.S.; Evenson, K.R.; Vaughn, A.; Rodgers, A.B.; Troiano, R.P. Accelerometer Use in Physical Activity: Best Practices and Research Recommendations. Med. Sci. Sports Exerc. 2005, 37, S582–S588. [Google Scholar] [CrossRef]
- Imeraj, L.; Sonuga-Barke, E.; Antrop, I.; Roeyers, H.; Wiersema, R.; Bal, S.; Deboutte, D. Altered circadian profiles in attention-deficit/hyperactivity disorder: An integrative review and theoretical framework for future studies. Neurosci. Biobehav. Rev. 2012, 36, 1897–1919. [Google Scholar] [CrossRef]
- Antrop, I.; Roeyers, H.; De Baecke, L. Effects of Time of Day on Classroom Behaviour in Children with ADHD. Sch. Psychol. Int. 2005, 26, 29–43. [Google Scholar] [CrossRef]
- Dane, A.V.; Schachar, R.; Tannock, R. Does Actigraphy Differentiate ADHD Subtypes in a Clinical Research Setting? J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 752–760. [Google Scholar] [CrossRef]
| Measure | Mean (Standard Deviation) | Participants (% of Total) |
|---|---|---|
| Age | 15.3 (3.4) | 30 (100) |
| Gender | - | 30 (100) |
| male | - | 10 (33.3) |
| female | - | 20 (66.7) |
| Mutation | - | 26 (86.7) |
| TSC1 | - | 3 (11.5) |
| TSC2 | - | 18 (69.2) |
| NMI | - | 5 (19.2) |
| not tested | - | 4 (13.3) |
| Testing Site | - | 30 (100) |
| lab | - | 16 (53.3) |
| home | - | 14 (46.7) |
| Actigraphy | - | 30 (100) |
| cognitive test movement | 0 (0.957) | 20 (66.7) |
| cognitive test movement IIV | 0 (0.953) | 20 (66.7) |
| movie movement | 0 (0.972) | 28 (93.3) |
| movie movement IIV | 0 (0.880) | 28 (93.3) |
| IQ (WASI-II or VABS-II) | 70.6 (22.4) | 30 (100) |
| ADHD Symptoms (SDQ Hyperactivity/Inattention) | 3.9 (2.3) | 25 (83.3) |
| Autistic Symptom Severity (ADI-R) | 21.4 (15.4) | 27 (90.0) |
| Brain Lesions | - | 28 (93.3) |
| total tubers | 19.3 (14.8) | - |
| frontal tubers | 10.2 (8.2) | - |
| parietal tubers | 3.2 (2.4) | - |
| temporal tubers | 2.9 (3.1) | - |
| occipital tubers | 3.9 (4.0) | - |
| cerebellum tubers | 0.0 (0.0) | - |
| SENs * | 4.0 (3.7) | - |
| Epilepsy Symptoms (E-Chess) | 6.0 (4.9) | 26 (86.7) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Earnest, T.; Shephard, E.; Tye, C.; McEwen, F.; Woodhouse, E.; Liang, H.; Sheerin, F.; Bolton, P.F. Actigraph-Measured Movement Correlates of Attention-Deficit/Hyperactivity Disorder (ADHD) Symptoms in Young People with Tuberous Sclerosis Complex (TSC) with and without Intellectual Disability and Autism Spectrum Disorder (ASD). Brain Sci. 2020, 10, 491. https://doi.org/10.3390/brainsci10080491
Earnest T, Shephard E, Tye C, McEwen F, Woodhouse E, Liang H, Sheerin F, Bolton PF. Actigraph-Measured Movement Correlates of Attention-Deficit/Hyperactivity Disorder (ADHD) Symptoms in Young People with Tuberous Sclerosis Complex (TSC) with and without Intellectual Disability and Autism Spectrum Disorder (ASD). Brain Sciences. 2020; 10(8):491. https://doi.org/10.3390/brainsci10080491
Chicago/Turabian StyleEarnest, Tom, Elizabeth Shephard, Charlotte Tye, Fiona McEwen, Emma Woodhouse, Holan Liang, Fintan Sheerin, and Patrick F. Bolton. 2020. "Actigraph-Measured Movement Correlates of Attention-Deficit/Hyperactivity Disorder (ADHD) Symptoms in Young People with Tuberous Sclerosis Complex (TSC) with and without Intellectual Disability and Autism Spectrum Disorder (ASD)" Brain Sciences 10, no. 8: 491. https://doi.org/10.3390/brainsci10080491
APA StyleEarnest, T., Shephard, E., Tye, C., McEwen, F., Woodhouse, E., Liang, H., Sheerin, F., & Bolton, P. F. (2020). Actigraph-Measured Movement Correlates of Attention-Deficit/Hyperactivity Disorder (ADHD) Symptoms in Young People with Tuberous Sclerosis Complex (TSC) with and without Intellectual Disability and Autism Spectrum Disorder (ASD). Brain Sciences, 10(8), 491. https://doi.org/10.3390/brainsci10080491





