Adolescent Intermittent Ethanol Exposure Effects on Kappa Opioid Receptor Mediated Dopamine Transmission: Sex and Age of Exposure Matter
Abstract
1. Introduction
2. Methods
2.1. Experimental Subjects
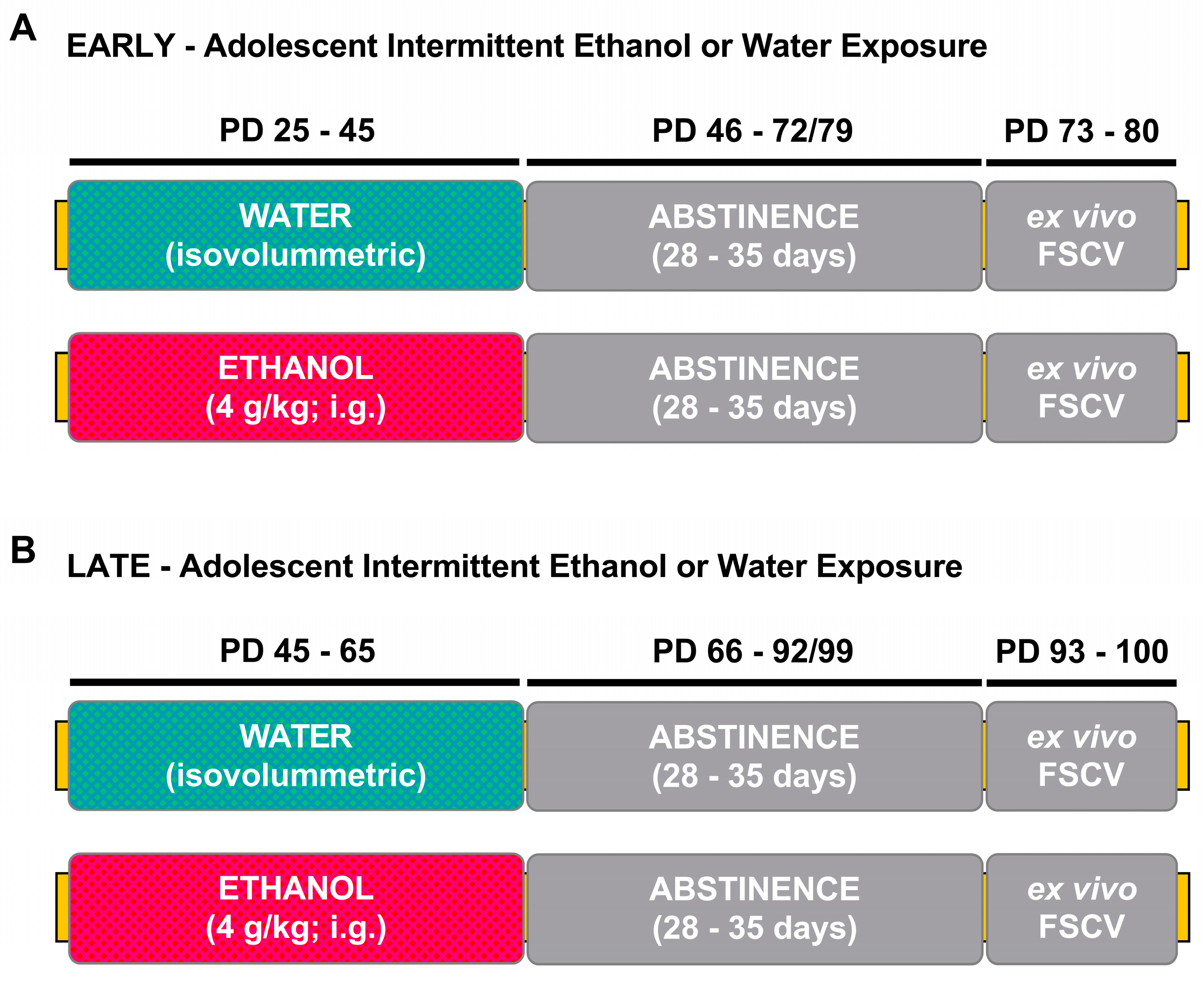
2.2. Adolescent Intermittent Ethanol Exposure
2.3. Statistical Analysis
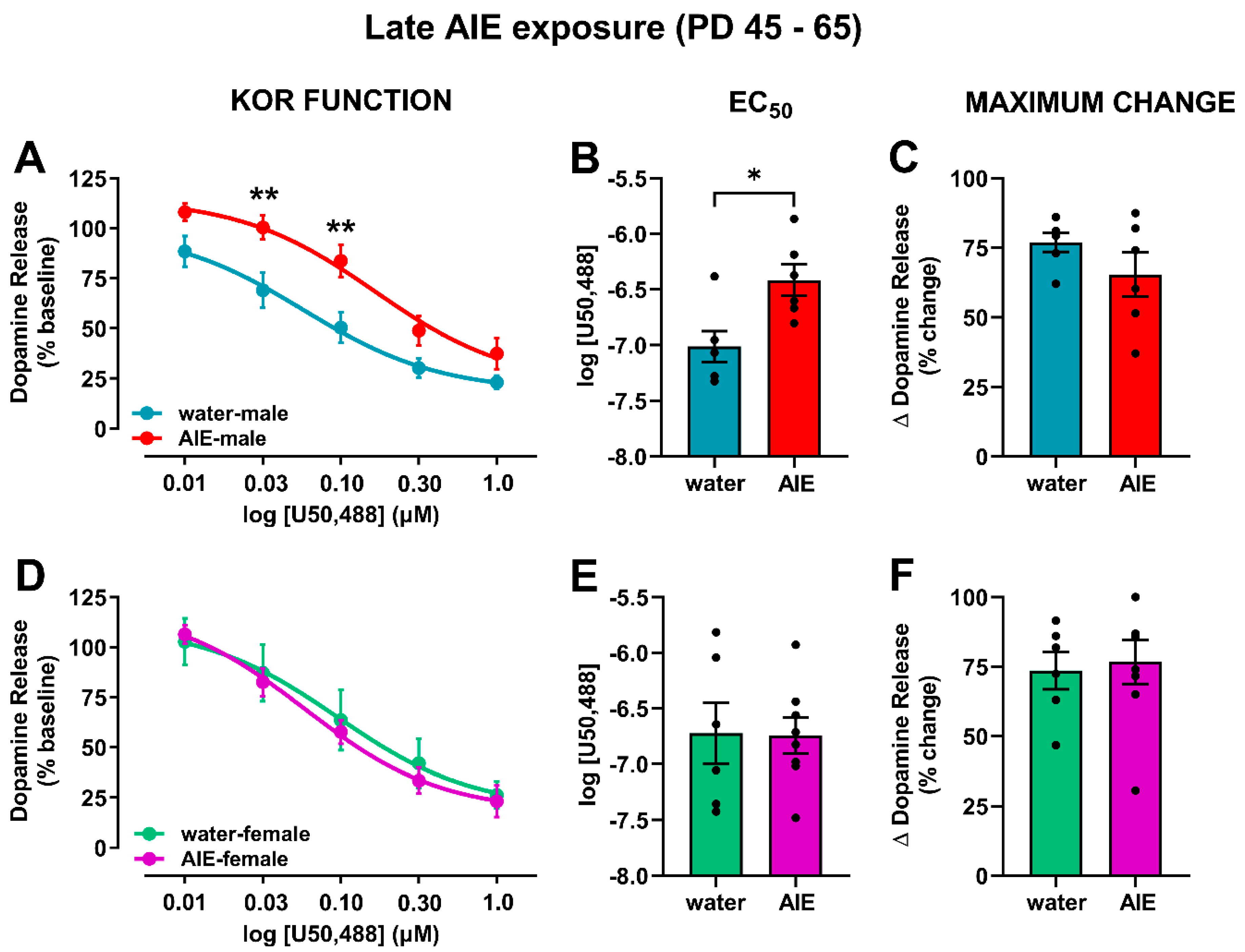
3. Results
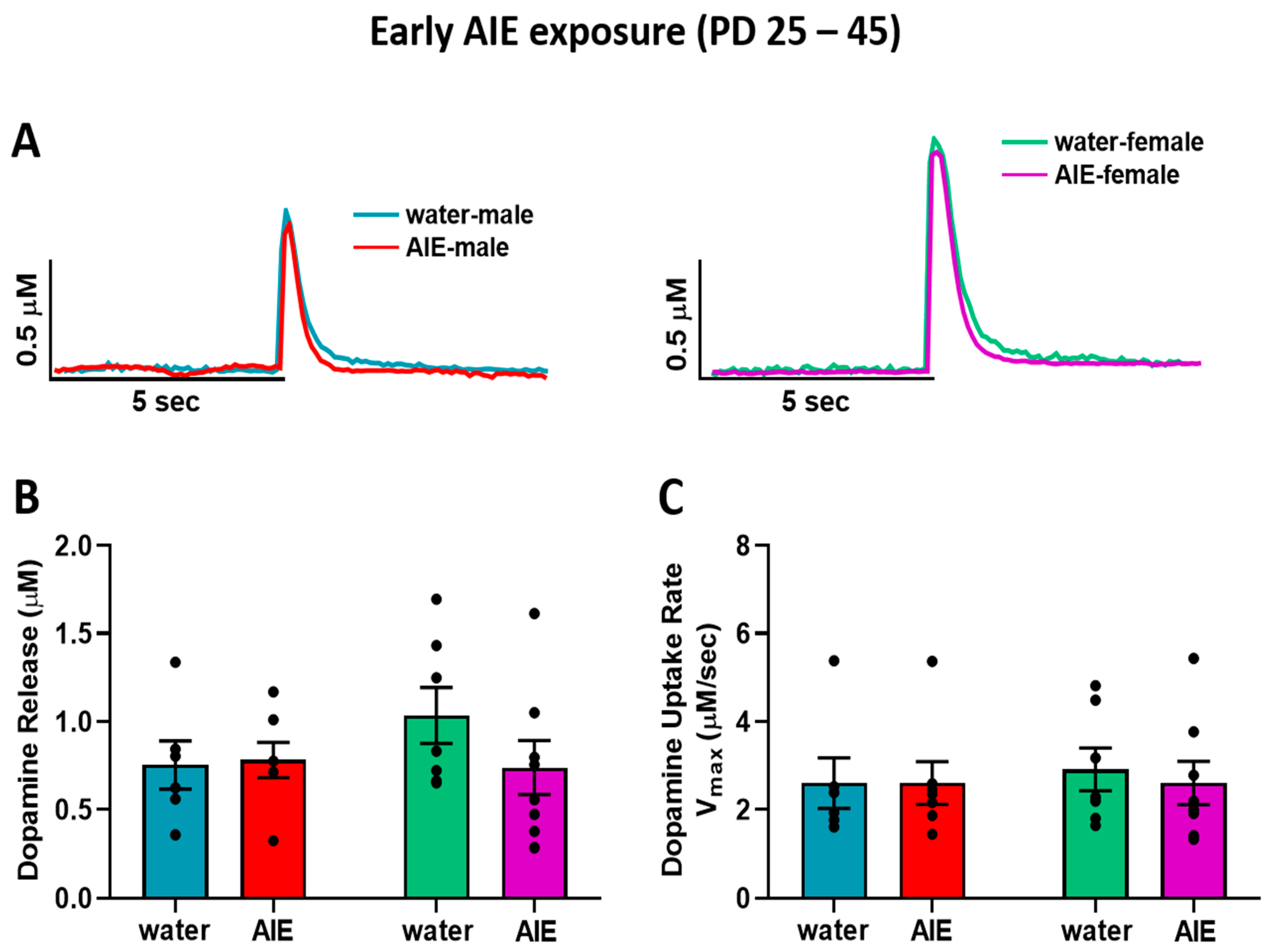
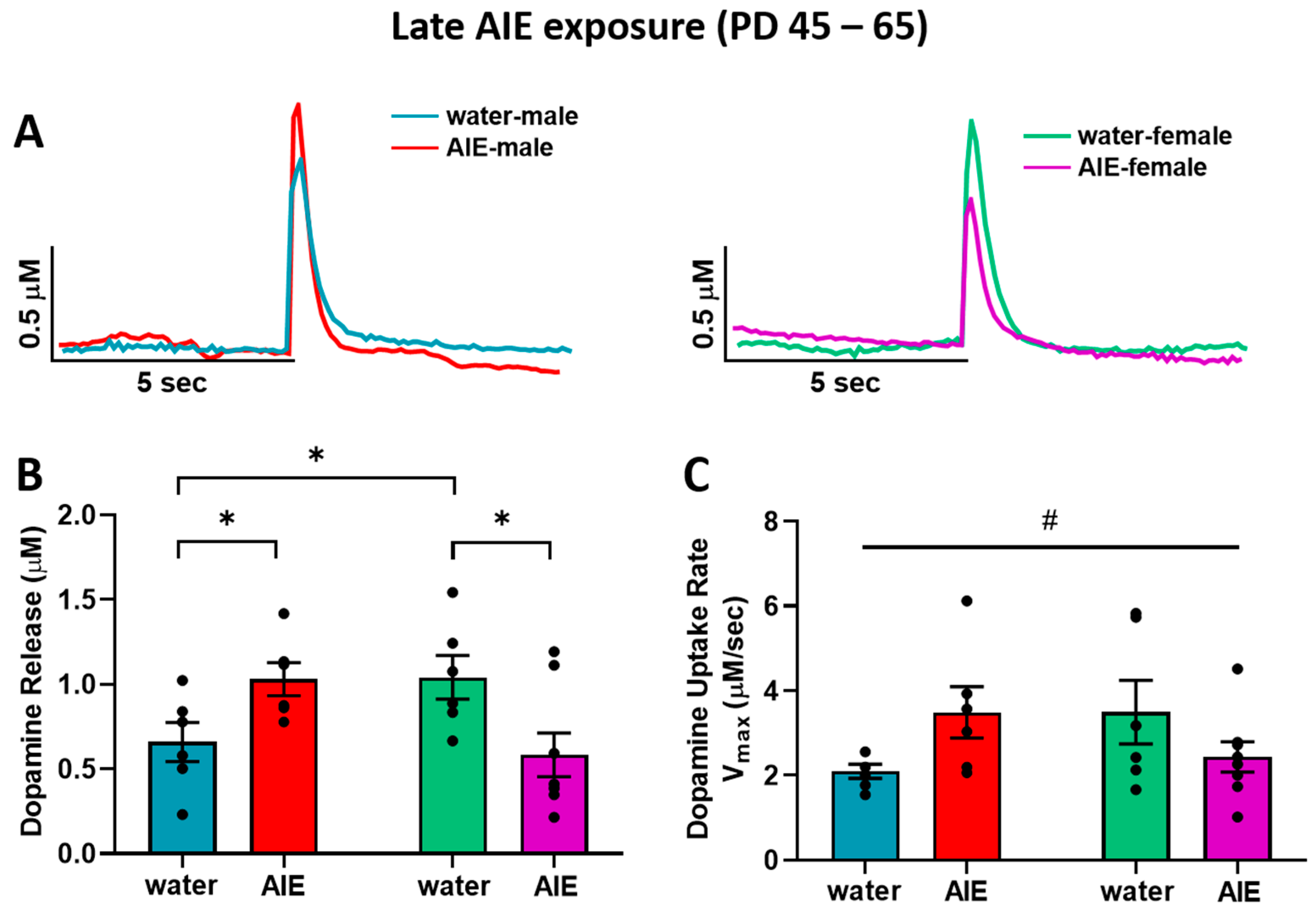
3.1. Late-AIE Exposure Disrupts Dopamine Release in Male and Female Rats
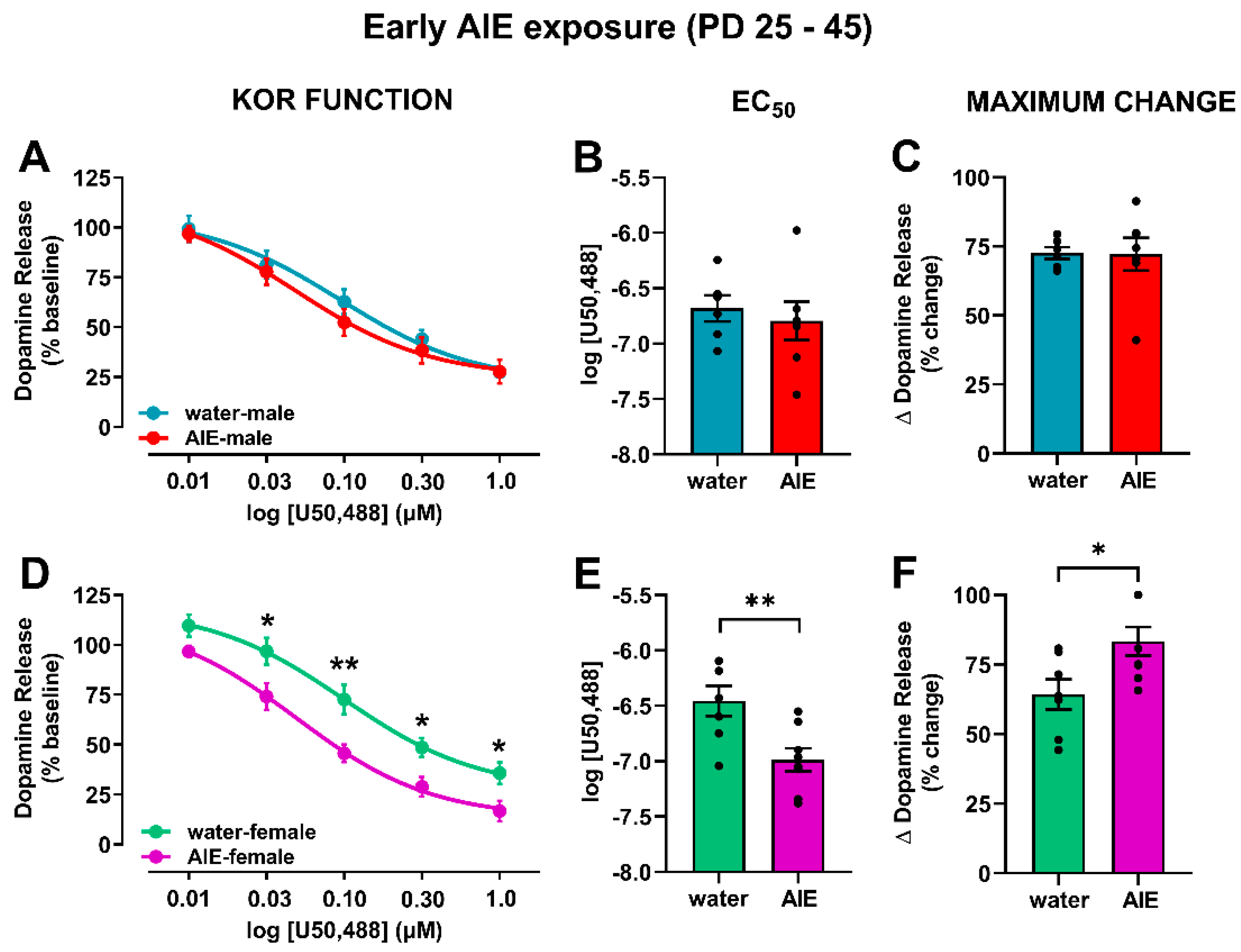
3.2. AIE Exposure Affects Accumbal KOR Function in an Age- and Sex-Dependent Manner
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Geels, L.M.; Bartels, M.; van Beijsterveldt, T.C.E.M.; Willemsen, G.; van der Aa, N.; Boomsma, D.I.; Vink, J.M. Trends in adolescent alcohol use: Effects of age, sex and cohort on prevalence and heritability. Addiction 2012, 107, 518–527. [Google Scholar] [CrossRef]
- Merikangas, K.R.; McClair, V.L. Epidemiology of substance use disorders. Hum. Genet. 2012, 131, 779–789. [Google Scholar] [CrossRef]
- Lipari, R.N. Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health; Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2018.
- Grant, B.F.; Dawson, D.A. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. J. Subst. Abuse 1997, 9, 103–110. [Google Scholar] [CrossRef]
- Dawson, D.A.; Goldstein, R.B.; Chou, S.P.; Ruan, W.J.; Grant, B.F. Age at First Drink and the First Incidence of Adult-Onset DSM-IV Alcohol Use Disorders. Alcohol. Clin. Exp. Res. 2008, 32, 2149–2160. [Google Scholar] [CrossRef]
- Ehlers, C.L.; Slutske, W.S.; Gilder, D.A.; Lau, P.; Wilhelmsen, K.C. Age at First Intoxication and Alcohol Use Disorders in Southwest California Indians. Alcohol. Clin. Exp. Res. 2006, 30, 1856–1865. [Google Scholar] [CrossRef]
- DeWit, D.J.; Adlaf, E.M.; Offord, D.R.; Ogborne, A.C. Age at First Alcohol Use: A Risk Factor for the Development of Alcohol Disorders. Am. J. Psychiatry 2000, 157, 745–750. [Google Scholar] [CrossRef]
- Kann, L.; McManus, T.; Harris, W.A.; Shanklin, S.L.; Flint, K.H.; Queen, B.; Lowry, R.; Chyen, D.; Whittle, L.; Thornton, J.; et al. Youth Risk Behavior Surveillance—United States, 2017. MMWR Surveill. Summ. 2018, 67, 1–114. [Google Scholar] [CrossRef]
- Adger, H.; Saha, S. Alcohol Use Disorders in Adolescents. Pediatr. Rev. 2013, 34, 103–114. [Google Scholar] [CrossRef][Green Version]
- Spear, L.P. Effects of adolescent alcohol consumption on the brain and behaviour. Nat. Rev. Neurosci. 2018, 19, 197–214. [Google Scholar] [CrossRef]
- Van Skike, C.E.; Diaz-Granados, J.L.; Matthews, D.B. Chronic intermittent ethanol exposure produces persistent anxiety in adolescent and adult rats. Alcohol. Clin. Exp. Res. 2015, 39, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Varlinskaya, E.I.; Hosová, D.; Towner, T.; Werner, D.F.; Spear, L.P. Effects of chronic intermittent ethanol exposure during early and late adolescence on anxiety-like behaviors and behavioral flexibility in adulthood. Behav. Brain Res. 2020, 378, 112292. [Google Scholar] [CrossRef] [PubMed]
- Varlinskaya, E.I.; Truxell, E.; Spear, L.P. Chronic intermittent ethanol exposure during adolescence: Effects on social behavior and ethanol sensitivity in adulthood. Alcohol 2014, 48, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, L.A.; Grosenick, L.; Finkelstein, J.C.; Kauvar, I.V.; Fenno, L.E.; Adhikari, A.; Lammelet, S.; Mirzabekov, J.J.; Airan, R.D.; Zalocusky, K.A.; et al. Natural neural projection dynamics underlying social behavior. Cell 2014, 157, 1535–1551. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Heien, M.L.A.V.; Wightman, R.M. Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J. Neurosci. 2002, 22, 10477–10486. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, J.E.; Conrad, K.L.; Carr, S.B.; Ford, K.A.; McGehee, D.S.; Marinelli, M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J. Neurophysiol. 2012, 108, 1620–1630. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, M.; McCutcheon, J.E. Heterogeneity of dopamine neuron activity across traits and states. Neuroscience 2014, 282, 176–197. [Google Scholar] [CrossRef]
- Gessa, G.L.; Muntoni, F.; Collu, M.; Vargiu, L.; Mereu, G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985, 348, 201–203. [Google Scholar] [CrossRef]
- Brodie, M.S.; Shefner, S.A.; Dunwiddie, T.V. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990, 508, 65–69. [Google Scholar] [CrossRef]
- Imperato, A.; Di Chiara, G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J. Pharmacol. Exp. Ther. 1986, 239, 219–228. [Google Scholar]
- Rose, J.H.; Karkhanis, A.N.; Chen, R.; Gioia, D.; Lopez, M.F.; Becker, H.C.; McCool, B.A.; Jones, S.R. Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int. J. Neuropsychopharmacol. 2016, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Karkhanis, A.N.; Huggins, K.N.; Rose, J.H.; Jones, S.R. Switch from excitatory to inhibitory actions of ethanol on dopamine levels after chronic exposure: Role of kappa opioid receptors. Neuropharmacology 2016, 110, 190–197. [Google Scholar] [CrossRef]
- Diana, M.; Pistis, M.; Muntoni, A.; Gessa, G. Mesolimbic dopaminergic reduction outlasts ethanol withdrawal syndrome: Evidence of protracted abstinence. Neuroscience 1996, 71, 411–415. [Google Scholar] [CrossRef]
- Carrara-Nascimento, P.F.; Hoffmann, L.B.; Flório, J.C.; Planeta, C.S.; Camarini, R. Effects of Ethanol Exposure during Adolescence or Adulthood on Locomotor Sensitization and Dopamine Levels in the Reward System. Front. Behav. Neurosci. 2020, 14, 31. [Google Scholar] [CrossRef]
- Badanich, K.A.; Maldonado, A.M.; Kirstein, C.L. Chronic Ethanol Exposure during Adolescence Increases Basal Dopamine in the Nucleus Accumbens Septi during Adulthood. Alcohol. Clin. Exp. Res. 2007, 31, 895–900. [Google Scholar] [CrossRef]
- Shnitko, T.A.; Spear, L.P.; Robinson, D.L. Adolescent binge-like alcohol alters sensitivity to acute alcohol effects on dopamine release in the nucleus accumbens of adult rats. Psychopharmacology 2016, 233, 361–371. [Google Scholar] [CrossRef]
- Vetter-O’Hagen, C.S.; Spear, L.P. The effects of gonadectomy on age- and sex-typical patterns of ethanol consumption in Sprague-Dawley rats. Alcohol. Clin. Exp. Res. 2011, 35, 2039–2049. [Google Scholar] [CrossRef]
- Spear, L.P. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol. Behav. 2015, 148, 122–130. [Google Scholar] [CrossRef]
- Sulzer, D.; Cragg, S.J.; Rice, M.E. Striatal dopamine neurotransmission: Regulation of release and uptake. Basal. Ganglia 2016, 6, 123–148. [Google Scholar] [CrossRef]
- Svingos, A.L.; Chavkin, C.; Colago, E.E.; Pickel, V.M. Major coexpression of kappa-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synapse 2001, 42, 185–192. [Google Scholar] [CrossRef]
- Di Chiara, G.; Imperato, A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J. Pharmacol. Exp. Ther. 1988, 244, 1067–1080. [Google Scholar] [PubMed]
- Karkhanis, A.N.; Rose, J.H.; Weiner, J.L.; Jones, S.R. Early-Life Social Isolation Stress Increases Kappa Opioid Receptor Responsiveness and Downregulates the Dopamine System. Neuropsychopharmacology 2016, 41, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Spanagel, R.; Herz, A.; Shippenberg, T.S. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl. Acad. Sci. USA 1992, 89, 2046–2050. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Zapata, A.; Justice, J.B.; Vaughan, R.A.; Sharpe, L.G.; Shippenberg, T.S. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J. Neurosci. 2000, 20, 9333–9340. [Google Scholar] [CrossRef]
- Walker, B.M.; Koob, G.F. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology 2008, 33, 643–652. [Google Scholar] [CrossRef]
- Morales, M.; Anderson, R.I.; Spear, L.P.; Varlinskaya, E.I. Effects of the kappa opioid receptor antagonist, nor-binaltorphimine, on ethanol intake: Impact of age and sex. Dev. Psychobiol. 2014, 56, 700–712. [Google Scholar] [CrossRef]
- Zorrilla, E.P. Multiparous species present problems (and possibilities) to developmentalists. Dev. Psychobiol. 1997, 30, 141–150. [Google Scholar] [CrossRef]
- Spear, L.P. Timing Eclipses Amount: The Critical Importance of Intermittency in Alcohol Exposure Effects. Alcohol. Clin. Exp. Res. 2020, 44, 806–813. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, Compact 6th ed.; Academic Press: New York, NY, USA, 2005. [Google Scholar]
- Yorgason, J.T.; España, R.A.; Jones, S.R. Demon voltammetry and analysis software: Analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J. Neurosci. Methods 2011, 202, 158–164. [Google Scholar] [CrossRef]
- Ferris, M.J.; Calipari, E.S.; Yorgason, J.T.; Jones, S.R. Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices. ACS Chem. Neurosci. 2013, 4, 693–703. [Google Scholar] [CrossRef]
- Tepper, J.M.; Trent, F.; Nakamura, S. Postnatal development of the electrical activity of rat nigrostriatal dopaminergic neurons. Dev. Brain Res. 1990, 54, 21–33. [Google Scholar] [CrossRef]
- Pitts, D.K.; Freeman, A.S.; Chiodo, L.A. Dopamine neuron ontogeny: Electrophysiol. Stud. Synap. 1990, 6, 309–320. [Google Scholar] [CrossRef]
- McCutcheon, J.E.; Marinelli, M. Age matters. Eur. J. Neurosci. 2009, 29, 997–1014. [Google Scholar] [CrossRef]
- Philpot, R.M.; Wecker, L.; Kirstein, C.L. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int. J. Dev. Neurosci. 2009, 27, 805–815. [Google Scholar] [CrossRef]
- Maldonado-Devincci, A.M.; Badanich, K.A.; Kirstein, C.L. Alcohol during adolescence selectively alters immediate and long-term behavior and neurochemistry. Alcohol 2010, 44, 57–66. [Google Scholar] [CrossRef]
- Yim, H.J.; Gonzales, R.A. Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol 2000, 22, 107–115. [Google Scholar] [CrossRef]
- Katunar, M.R.; Saez, T.; Brusco, A.; Antonelli, M.C. Immunocytochemical expression of dopamine-related transcription factors Pitx3 and Nurr1 in prenatally stressed adult rats. J. Neurosci. Res. 2009, 87, 1014–1022. [Google Scholar] [CrossRef]
- Prakash, N.; Wurst, W. Genetic networks controlling the development of midbrain dopaminergic neurons. J. Physiol. 2006, 575, 403–410. [Google Scholar] [CrossRef]
- Alavian, K.N.; Scholz, C.; Simon, H.H. Transcriptional regulation of mesencephalic dopaminergic neurons: The full circle of life and death. Mov. Disord. 2008, 23, 319–328. [Google Scholar] [CrossRef]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar] [CrossRef]
- Kim, E.U.; Varlinskaya, E.I.; Dannenhoffer, C.A.; Spear, L.P. Adolescent intermittent ethanol exposure: Effects on pubertal development, novelty seeking, and social interaction in adulthood. Alcohol 2019, 75, 19–29. [Google Scholar] [CrossRef]
- Engleman, E.A.; Keen, E.J.; Tilford, S.S.; Thielen, R.J.; Morzorati, S.L. Ethanol drinking reduces extracellular dopamine levels in the posterior ventral tegmental area of nondependent alcohol-preferring rats. Alcohol 2011, 45, 549–557. [Google Scholar] [CrossRef]
- Anderson, R.I.; Lopez, M.F.; Becker, H.C. Stress-Induced Enhancement of Ethanol Intake in C57BL/6J Mice with a History of Chronic Ethanol Exposure: Involvement of Kappa Opioid Receptors. Front. Cell. Neurosci. 2016, 10, 45. [Google Scholar] [CrossRef]
- Georges, F.; Normand, E.; Bloch, B.; Le Moine, C. Opioid receptor gene expression in the rat brain during ontogeny, with special reference to the mesostriatal system: An in situ hybridization study. Dev. Brain Res. 1998, 109, 187–199. [Google Scholar] [CrossRef]
- Winzer-Serhan, U.H.; Chen, Y.; Leslie, F.M. Expression of opioid peptides and receptors in striatum and substantia nigra during rat brain development. J. Chem. Neuroanat. 2003, 26, 17–36. [Google Scholar] [CrossRef]
- Kornblum, H.I.; Hurlbut, D.E.; Leslie, F.M. Postnatal development of multiple opioid receptors in rat brain. Dev. Brain Res. 1987, 37, 21–41. [Google Scholar] [CrossRef]
- Stiene-Martin, A.; Knapp, P.E.; Martin, K.; Gurwell, J.A.; Ryan, S.; Thornton, S.R.; Smith, F.L.; Hauser, K.F. Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: Impact on gliogenesis in vivo. Glia 2001, 36, 78–88. [Google Scholar] [CrossRef]
- De Vries, T.J.; Hogenboom, F.; Mulder, A.H.; Schoffelmeer, A.N. Ontogeny of mu-, delta- and kappa-opioid receptors mediating inhibition of neurotransmitter release and adenylate cyclase activity in rat brain. Dev. Brain Res. 1990, 54, 63–69. [Google Scholar] [CrossRef]
- Bordner, K.; Deak, T. Endogenous Opioids as Substrates for Ethanol Intake in the Neonatal Rat: The Impact of Prenatal Ethanol Exposure on the Opioid Family in the Early Postnatal Period. Physiol. Behav. 2015, 148, 100–110. [Google Scholar] [CrossRef]
- Nizhnikov, M.E.; Pautassi, R.M.; Carter, J.M.; Landin, J.D.; Varlinskaya, E.I.; Bordner, K.A.; Werner, D.F.; Spear, N.E. Brief Prenatal Ethanol Exposure Alters Behavioral Sensitivity to the Kappa Opioid Receptor Agonist (U62,066E) and Antagonist (Nor-BNI) and Reduces Kappa Opioid Receptor Expression. Alcohol. Clin. Exp. Res. 2014, 38, 1630–1638. [Google Scholar] [CrossRef]
- Nizhnikov, M.E.; Varlinskaya, E.I.; Petrov, E.S.; Spear, N.E. Reinforcing properties of ethanol in neonatal rats: Involvement of the opioid system. Behav. Neurosci. 2006, 120, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Pautassi, R.M.; Nizhnikov, M.E.; Acevedo, M.B.; Spear, N.E. Early role of the κ opioid receptor in ethanol-induced reinforcement. Physiol. Behav. 2012, 105, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Morales, R.S.; Spear, N.E.; Nizhnikov, M.E.; Molina, J.C.; Abate, P. Role of mu, delta and kappa opioid receptors in ethanol-reinforced operant responding in infant rats. Behav. Brain Res. 2012, 234, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Dees, W.L.; Hiney, J.K.; Srivastava, V.K. Regulation of prepubertal dynorphin secretion in the medial basal hypothalamus of the female rat. J. Neuroendocrinol. 2019, 31, e12810. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.K.; Hiney, J.K.; Dees, W.L. Alcohol Delays the Onset of Puberty in the Female Rat by Altering Key Hypothalamic Events. Alcohol. Clin. Exp. Res. 2018, 42, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Vetter-O’Hagen, C.S.; Spear, L.P. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev. Psychobiol. 2012, 54, 523–535. [Google Scholar] [CrossRef]
- McLaughlin, J.P.; Li, S.; Valdez, J.; Chavkin, T.A.; Chavkin, C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology 2006, 31, 1241–1248. [Google Scholar] [CrossRef]
- Karkhanis, A.N.; Locke, J.L.; McCool, B.A.; Weiner, J.L.; Jones, S.R. Social isolation rearing increases nucleus accumbens dopamine and norepinephrine responses to acute ethanol in adulthood. Alcohol. Clin. Exp. Res. 2014, 38, 2770–2779. [Google Scholar] [CrossRef]
- Voorn, P.; Docter, G.J.; Jongen-Rêlo, A.L.; Jonker, A.J. Rostrocaudal subregional differences in the response of enkephalin, dynorphin and substance P synthesis in rat nucleus accumbens to dopamine depletion. Eur. J. Neurosci. 1994, 6, 486–496. [Google Scholar] [CrossRef]
- Yorgason, J.T.; España, R.A.; Konstantopoulos, J.K.; Weiner, J.L.; Jones, S.R. Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur. J. Neurosci. 2013, 37, 1022–1031. [Google Scholar] [CrossRef]
- Cheng, H.G.; Anthony, J.C. Female-male differences in alcohol dependence levels: Evidence on newly incident adolescent and young-adult drinkers in the United States, 2002–2014. Int. J. Methods Psychiatr. Res. 2018, 27, e1717. [Google Scholar] [CrossRef]
- Waller, R.; Murray, L.; Shaw, D.S.; Forbes, E.E.; Hyde, L.W. Accelerated alcohol use across adolescence predicts early adult symptoms of alcohol use disorder via reward-related neural function. Psychol. Med. 2019, 49, 675–684. [Google Scholar] [CrossRef]
- Kvamme, T.L.; Schmidt, C.; Strelchuk, D.; Chang-Webb, Y.C.; Baek, K.; Voon, V. Sexually dimorphic brain volume interaction in college-aged binge drinkers. NeuroImage Clin. 2016, 10, 310–317. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spodnick, M.B.; Amirault, R.T.; Towner, T.T.; Varlinskaya, E.I.; Spear, L.P.; Karkhanis, A.N. Adolescent Intermittent Ethanol Exposure Effects on Kappa Opioid Receptor Mediated Dopamine Transmission: Sex and Age of Exposure Matter. Brain Sci. 2020, 10, 472. https://doi.org/10.3390/brainsci10080472
Spodnick MB, Amirault RT, Towner TT, Varlinskaya EI, Spear LP, Karkhanis AN. Adolescent Intermittent Ethanol Exposure Effects on Kappa Opioid Receptor Mediated Dopamine Transmission: Sex and Age of Exposure Matter. Brain Sciences. 2020; 10(8):472. https://doi.org/10.3390/brainsci10080472
Chicago/Turabian StyleSpodnick, Mary B., Raymond T. Amirault, Trevor T. Towner, Elena I. Varlinskaya, Linda P. Spear, and Anushree N. Karkhanis. 2020. "Adolescent Intermittent Ethanol Exposure Effects on Kappa Opioid Receptor Mediated Dopamine Transmission: Sex and Age of Exposure Matter" Brain Sciences 10, no. 8: 472. https://doi.org/10.3390/brainsci10080472
APA StyleSpodnick, M. B., Amirault, R. T., Towner, T. T., Varlinskaya, E. I., Spear, L. P., & Karkhanis, A. N. (2020). Adolescent Intermittent Ethanol Exposure Effects on Kappa Opioid Receptor Mediated Dopamine Transmission: Sex and Age of Exposure Matter. Brain Sciences, 10(8), 472. https://doi.org/10.3390/brainsci10080472





