Neurophysiological Correlates of a Single Session of Prefrontal tDCS in Patients with Prolonged Disorders of Consciousness: A Pilot Double-Blind Randomized Controlled Study
Abstract
1. Introduction
2. Materials and Methods
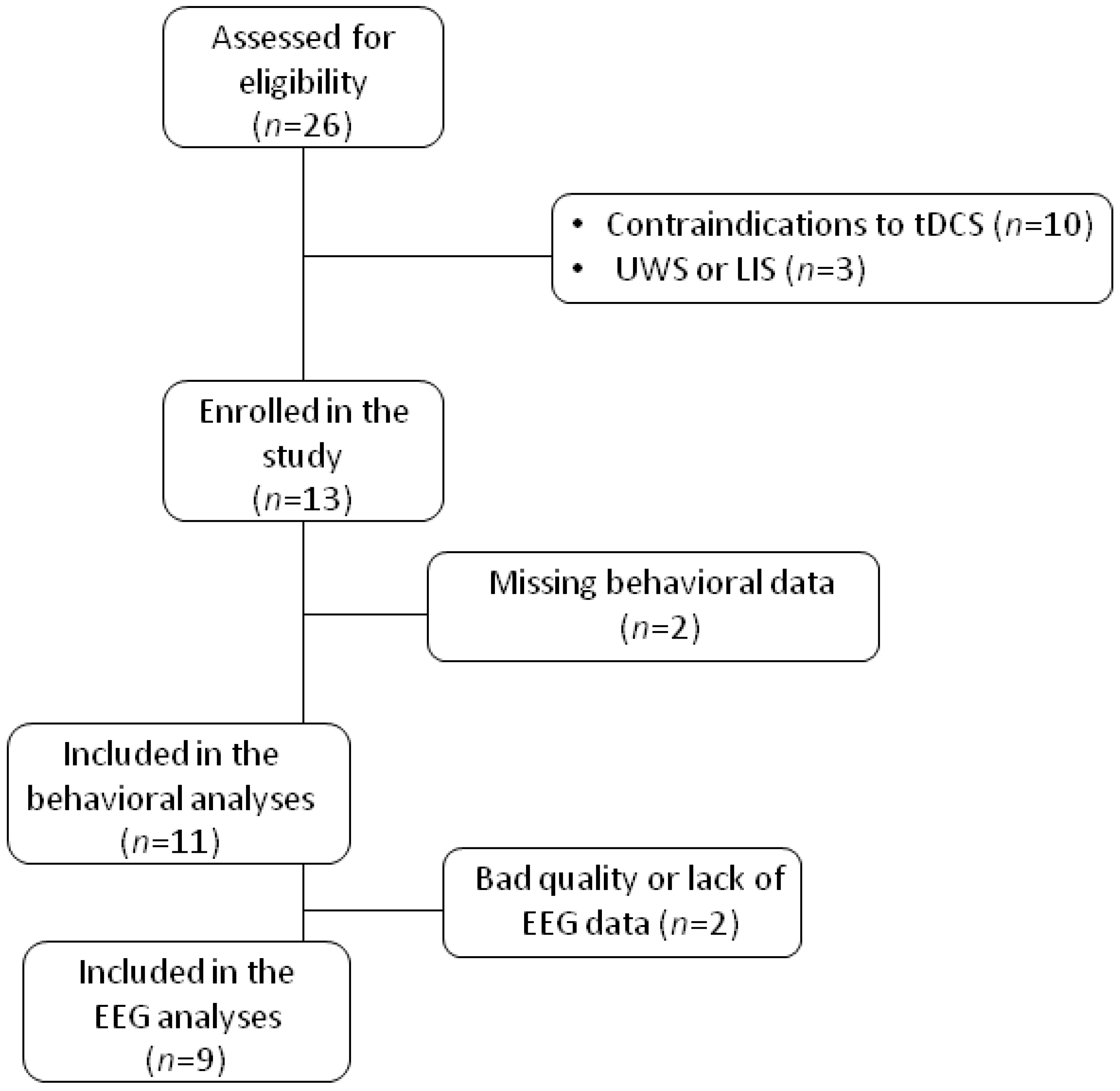
2.1. Patients
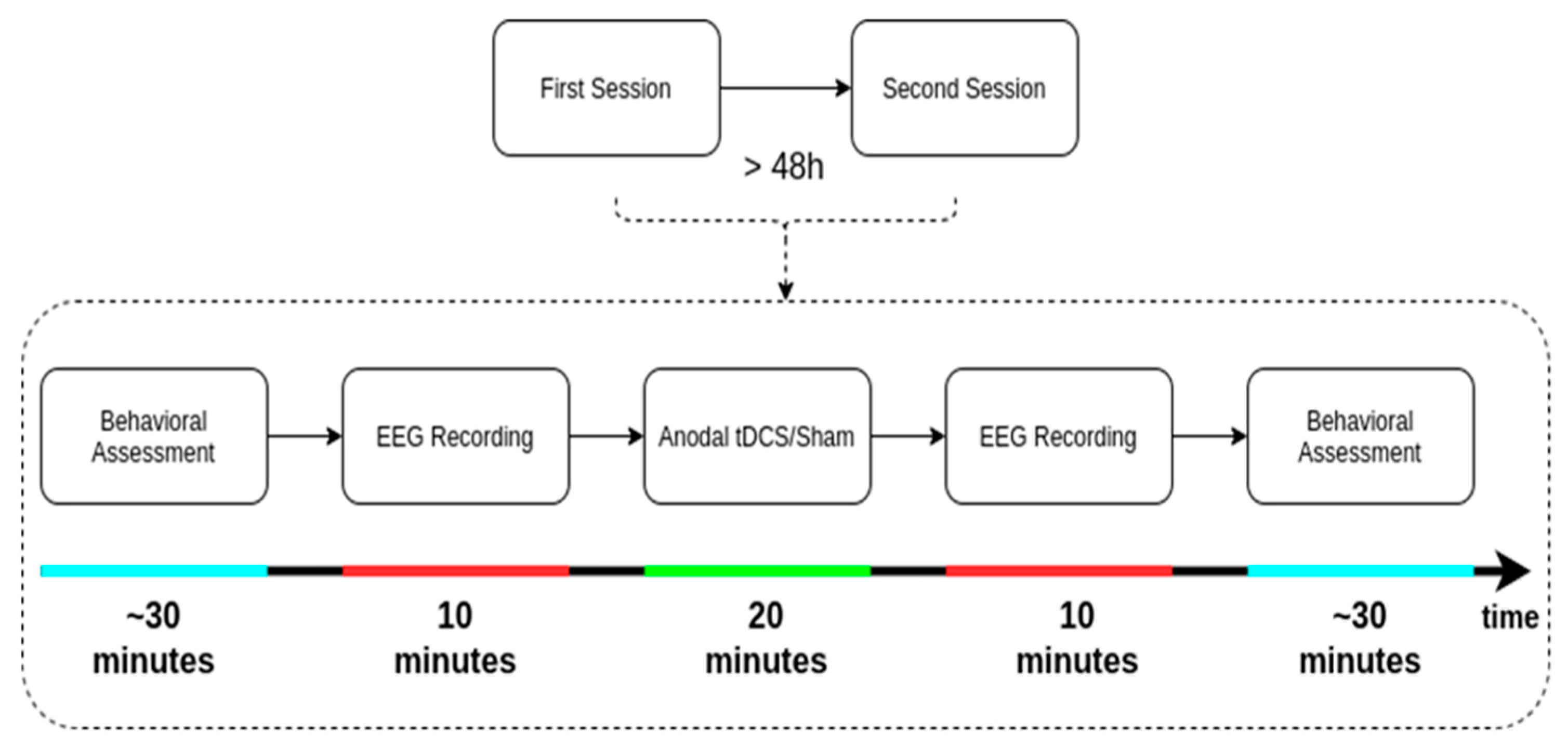
2.2. Data Acquisition
2.3. Data Analysis
2.3.1. EEG
Preprocessing
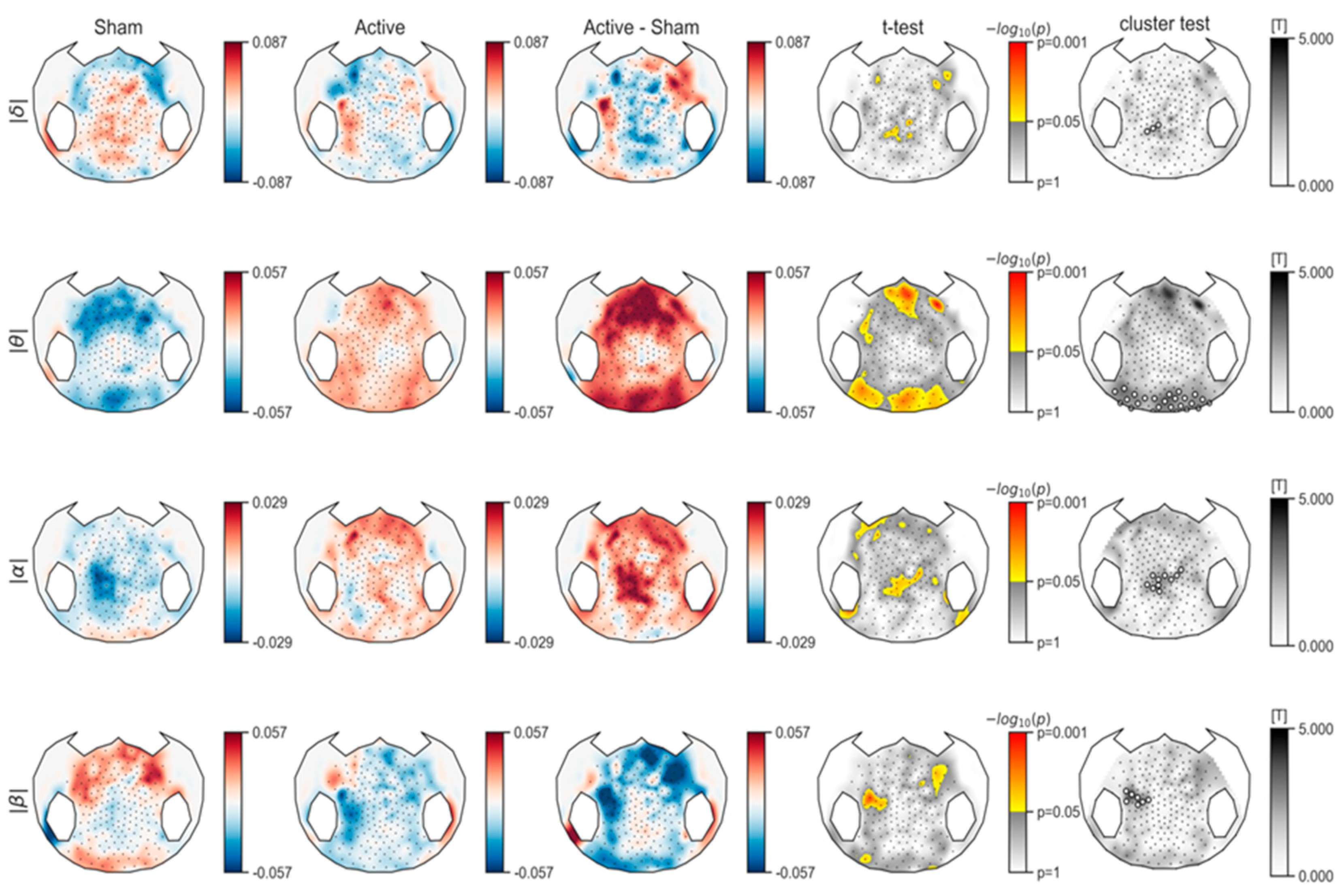
Spectral Power Analysis
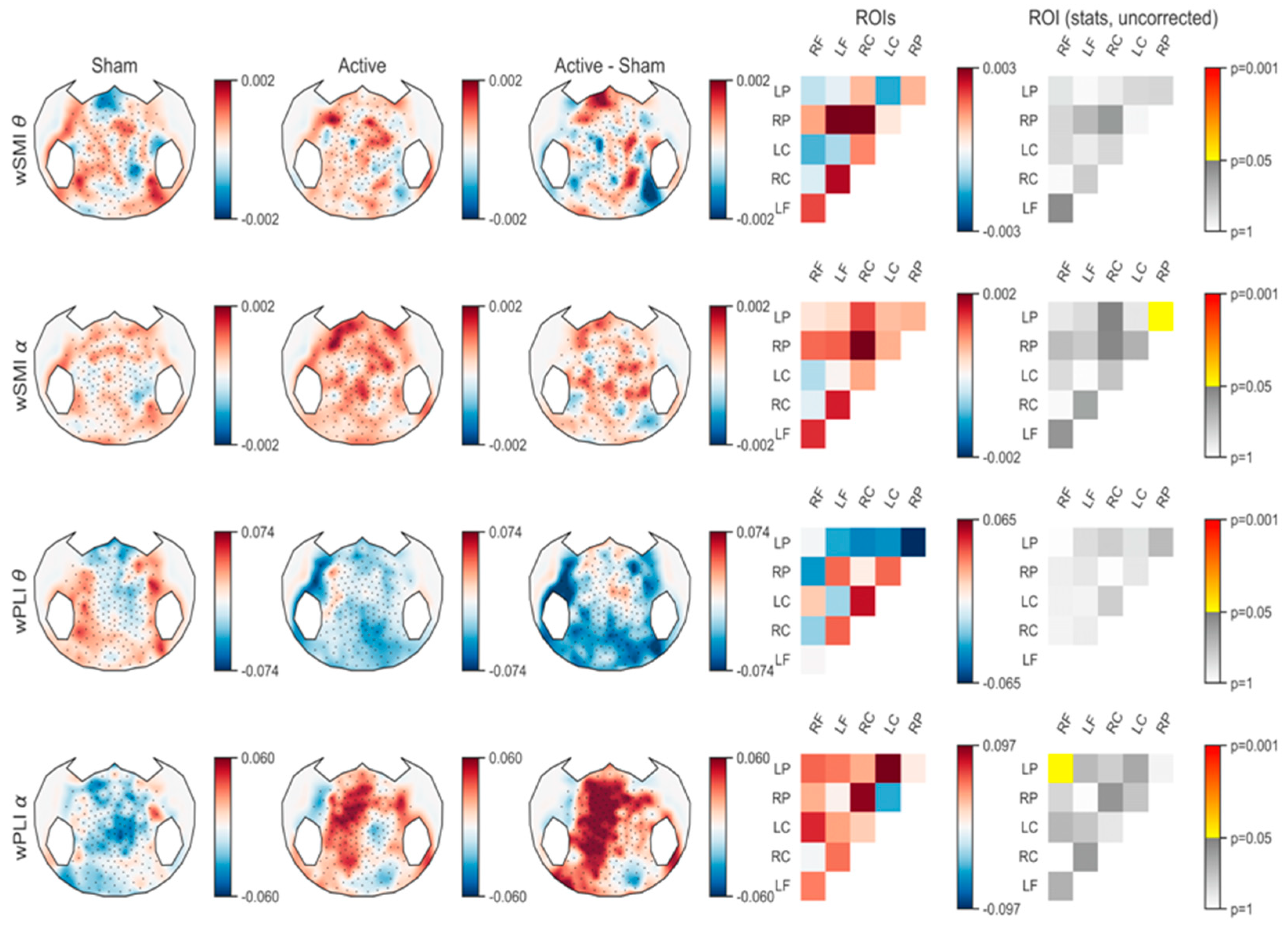
Connectivity Analysis
2.3.2. Behavioral
3. Results
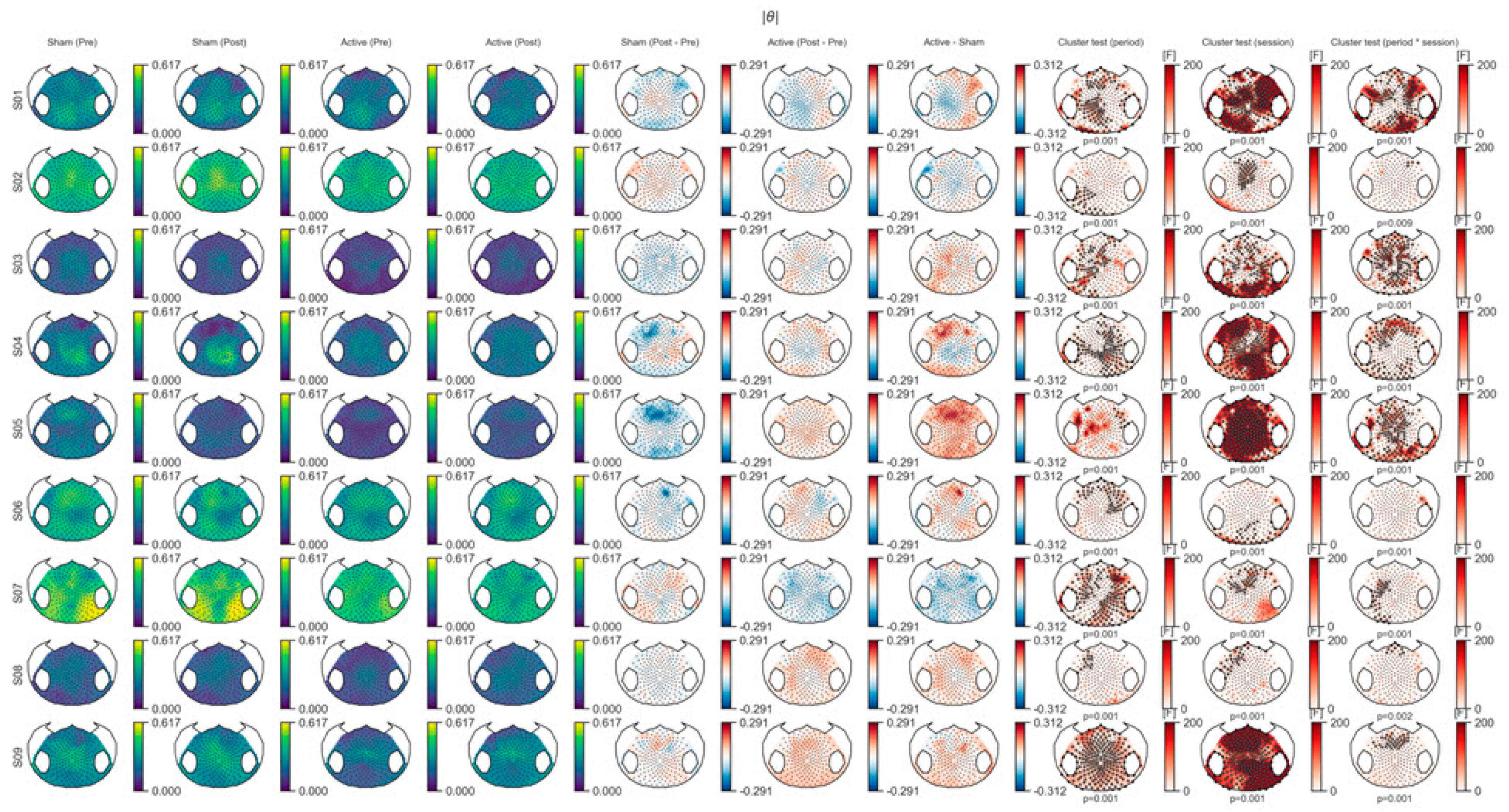
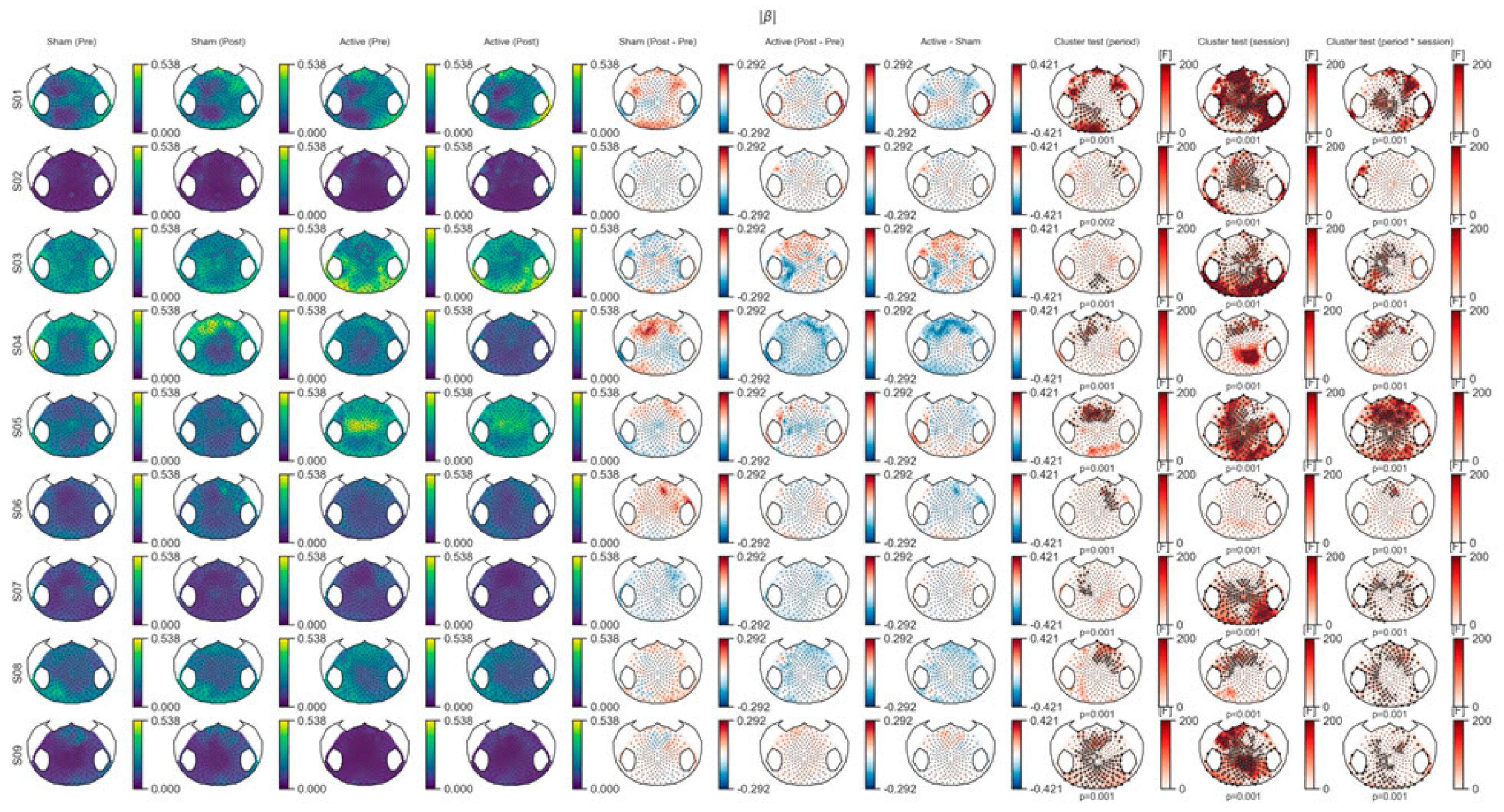
3.1. EEG Outcome
3.1.1. Spectral Power Analysis
3.1.2. Connectivity Analysis
3.2. Behavioral Outcome.
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A


| Weighted Symbolic Mutual Information_Theta | |
|---|---|
| LF<->RF | 0.06845148344321748 |
| RC<->RF | 0.8603674612115655 |
| RC<->LF | 0.30012883024173853 |
| LC<->RF | 0.39768910879384367 |
| LC<->LF | 0.6090831617266237 |
| LC<->RC | 0.38598873780886145 |
| RP<->RF | 0.38422905807924346 |
| RP<->LF | 0.19309897842414722 |
| RP<->RC | 0.09108255152855792 |
| RP<->LC | 0.816114568206544 |
| LP<->RF | 0.5348919229854945 |
| LP<->LF | 0.8593523263876319 |
| LP<->RC | 0.6223603957029509 |
| LP<->LC | 0.39911328745123653 |
| LP<->RP | 0.36409751901316506 |
| Weighted Symbolic Mutual Information_Alpha | |
|---|---|
| LF<->RF | 0.08164498533928806 |
| RC<->RF | 0.8506775723570095 |
| RC<->LF | 0.11615994116831124 |
| LC<->RF | 0.4157206733797898 |
| LC<->LF | 0.9454852579271487 |
| LC<->RC | 0.24167586742571967 |
| RP<->RF | 0.2047538024217198 |
| RP<->LF | 0.2890047170003517 |
| RP<->RC | 0.060096872178330714 |
| RP<->LC | 0.15465076061109098 |
| LP<->RF | 0.582422479565109 |
| LP<->LF | 0.41102120093594896 |
| LP<->RC | 0.05451530078455737 |
| LP<->LC | 0.5623741387523817 |
| LP<->RP * | 0.04864806793373383 |
| Weighted Phase Lag Index_Theta | |
|---|---|
| LF<->RF | 0.9949586043007148 |
| RC<->RF | 0.7746434675282614 |
| RC<->LF | 0.6373593093070036 |
| LC<->RF | 0.7050253843000385 |
| LC<->LF | 0.7491301165965596 |
| LC<->RC | 0.3081580496870976 |
| RP<->RF | 0.6722348661038704 |
| RP<->LF | 0.5557881408626988 |
| RP<->RC | 0.9490959896765098 |
| RP<->LC | 0.5655297057225542 |
| LP<->RF | 0.9629413407279742 |
| LP<->LF | 0.4343636029924479 |
| LP<->RC | 0.31223077250813464 |
| LP<->LC | 0.5176033393378308 |
| LP<->RP | 0.20326708891223635 |
| Weighted Phase Lag Index_Alpha | |
|---|---|
| LF<->RF | 0.15128490952536788 |
| RC<->RF | 0.9735390744662057 |
| RC<->LF | 0.094602176678944 |
| LC<->RF | 0.1791828499275001 |
| LC<->LF | 0.258877028158907 |
| LC<->RC | 0.5568189419533268 |
| RP<->RF | 0.3784138998772636 |
| RP<->LF | 0.9368265729563485 |
| RP<->RC | 0.08239789991160408 |
| RP<->LC | 0.2465064017316093 |
| LP<->RF * | 0.045140050121414615 |
| LP<->LF | 0.1945006416857746 |
| LP<->RC | 0.31111099148315785 |
| LP<->LC | 0.1273239178651476 |
| LP<->RP * | 0.7823403349079698 |
References
- Zhang, Y.; Song, W. Transcranial direct current stimulation in disorders of consciousness: A review. Int. J. Neurosci. 2018, 128, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Antal, A.; Nitsche, M.A. Physiology of transcranial direct current stimulation. J. ECT 2018, 34, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Bruno, M.-A.; Ledoux, D.; Demertzi, A.; Laureys, S. TDCS in patients with disorders of consciousness: Sham-controlled randomized double-blind study. Neurology 2014, 82, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Wannez, S.; Donneau, A.F.; Chatelle, C.; Gosseries, O.; Bruno, M.A.; Laureys, S. Controlled clinical trial of repeated prefrontal tDCS in patients with chronic minimally conscious state. Brain Inj. 2017, 31, 466–474. [Google Scholar] [CrossRef]
- Martens, G.; Lejeune, N.; O’Brien, A.T.; Fregni, F.; Martial, C.; Wannez, S.; Thibaut, A. Randomized controlled trial of home-based 4-week tDCS in chronic minimally conscious state. Brain Stimul. 2018, 11, 982–990. [Google Scholar] [CrossRef]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- The Multi-Society Task Force on PVS. Medical Aspects of the Persistent Vegetative State. N Engl. J. Med. 1994, 330, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Dolce, G. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Di Perri, C.; Chatelle, C.; Bruno, M.A.; Bahri, M.A.; Wannez, S.; Hustinx, R. Clinical response to tDCS depends on residual brain metabolism and grey matter integrity in patients with minimally conscious state. Brain Stimul. 2015, 8, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Chennu, S.; Chatelle, C.; Martens, G.; Annen, J.; Cassol, H.; Laureys, S. Theta network centrality correlates with tDCS response in disorders of consciousness. Brain Stimul. 2018, 11, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xia, X.; Kang, J.; Yang, Y.; He, J.; Li, X. TDCS modulates cortical excitability in patients with disorders of consciousness. Neuroimage Clin. 2017, 15, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xia, X.; Wang, Y.; Guo, Y.; Yang, Y.; He, J.; Li, X. Fronto-parietal coherence response to tDCS modulation in patients with disorders of consciousness. Int. J. Neurosci. 2018, 128, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bai, Y.; Xia, X.; Li, J.; Wang, X.; Dai, Y.; Zhang, H. Effects of long-lasting high-definition transcranial direct current stimulation in chronic disorders of consciousness: A pilot study. Front. Neurosci. 2019, 13, 412. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Du, J.; Huo, S.; Shan, G.; Li, R. Transcranial direct current stimulation in patients with prolonged disorders of consciousness: Combined behavioral and event-related potential evidence. Front. Neurol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Genna, C.; Formaggio, E.; Gregorio, C.; Storti, S.F.; Manganotti, P.; Piccione, F. Behavioural and electrophysiological effects of tDCS to prefrontal cortex in patients with disorders of consciousness. Clin. Neurophysiol. 2019, 130, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Hermann, B.; Raimondo, F.; Hirsch, L.; Huang, Y.; Denis-Valente, M.; Pérez, P.; Rohaut, B. Combined behavioral and electrophysiological evidence for a direct cortical effect of prefrontal tDCS on disorders of consciousness. Sci. Rep. 2020, 10, 4323. [Google Scholar] [CrossRef] [PubMed]
- Herwig, U.; Satrapi, P.; Schönfeldt-Lecuona, C. Using the International 10-20 EEG System for Positioning of Transcranial Magnetic Stimulation. Brain Topogr. 2003, 16, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Langlois, D.; Chartier, S.; Gosselin, D. An Introduction to Independent Component Analysis: InfoMax and FastICA algorithms. Tutor. Quant. Methods Psychol. 2010, 6, 31–38. [Google Scholar] [CrossRef]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- King, J.R.; Sitt, J.D.; Faugeras, F.; Rohaut, B.; El Karoui, I.; Cohen, L.; Dehaene, S. Information sharing in the brain indexes consciousness in noncommunicative patients. Curr. Biol. 2013, 23, 1914–1919. [Google Scholar] [CrossRef]
- Vinck, M.; Oostenveld, R.; Van Wingerden, M.; Battaglia, F.; Pennartz, C.M.A. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 2011, 55, 1548–1565. [Google Scholar] [CrossRef] [PubMed]
- Kayser, J.; Tenke, C.E. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin. Neurophysiol. 2006, 117, 348–368. [Google Scholar] [CrossRef] [PubMed]
- Sitt, J.D.; King, J.R.; El Karoui, I.; Rohaut, B.; Faugeras, F.; Gramfort, A.; Naccache, L. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014, 137, 2258–2270. [Google Scholar] [CrossRef] [PubMed]
- Chennu, S.; Annen, J.; Wannez, S.; Thibaut, A.; Chatelle, C.; Cassol, H.; Laureys, S. Brain networks predict metabolism, diagnosis and prognosis at the bedside in disorders of consciousness. Brain 2017, 140, 2120–2132. [Google Scholar] [CrossRef] [PubMed]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.; Strohmeier, D.; Brodbeck, C. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013, 7, 267. [Google Scholar] [CrossRef]
- Engemann, D.; Raimondo, F.; King, J.-R.; Jas, M.; Gramfort, A.; Dehaene, S.; Sitt, J. Automated Measurement and Prediction of Consciousness in Vegetative and Minimally Conscious Patients. In Proceedings of the ICML Work Stat Mach Learn Neurosci (Stamlins 2015), Lille, France, 10 July 2015. [Google Scholar]
- SPSS Statistic for Windows; Version 17.0; Statistical Software Platform; SPSS Inc.: Chicago, IL, USA.
- Imperatori, L.S.; Betta, M.; Cecchetti, L.; Canales-Johnson, A.; Ricciardi, E.; Siclari, F.; Bernardi, G. EEG functional connectivity metrics wPLI and wSMI account for distinct types of brain functional interactions. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Comsa, I.M.; Bekinschtein, T.A.; Chennu, S. Transient Topographical Dynamics of the Electroencephalogram Predict Brain Connectivity and Behavioural Responsiveness During Drowsiness. Brain Topogr. 2019, 32, 315–331. [Google Scholar] [CrossRef]
- Chennu, S.; O’Connor, S.; Adapa, R.; Menon, D.K.; Bekinschtein, T.A. Brain Connectivity Dissociates Responsiveness from Drug Exposure during Propofol-Induced Transitions of Consciousness. PLoS Comput. Biol. 2016, 12, 1–17. [Google Scholar] [CrossRef]
- Blain-Moraes, S.; Lee, U.C.; Ku, S.W.; Noh, G.J.; Mashour, G.A. Electroencephalographic effects of ketamine on power, cross-frequency coupling, and connectivity in the alpha bandwidth. Front. Syst. Neurosci. 2014, 8, 1–9. [Google Scholar] [CrossRef]
- Boly, M.; Massimini, M.; Tsuchiya, N.; Postle, B.R.; Koch, C.; Tononi, G. Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? Clinical and neuroimaging evidence. J. Neurosci. 2017, 37, 9603–9613. [Google Scholar] [CrossRef]
- Keeser, D.; Meindl, T.; Bor, J.; Palm, U.; Pogarell, O.; Mulert, C.; Padberg, F. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J. Neurosci. 2011, 31, 15284–15293. [Google Scholar] [CrossRef]
- Estraneo, A.; Pascarella, A.; Moretta, P.; Masotta, O.; Fiorenza, S.; Chirico, G.; Trojano, L. Repeated transcranial direct current stimulation in prolonged disorders of consciousness: A double-blind cross-over study. J. Neurol. Sci. 2017, 375, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Rorden, C.; Fridriksson, J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke 2010, 41, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Nitsche, M.; Marcolin, M.A.; Rigonatti, S.P.; Pascual-Leone, A. Treatment of major depression with transcranial direct current stimulation Ephedrine-induced emergence of bipolar symptoms. Bipolar. Disord 2006, 8, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Liouta, E.; Andreadis, N.; Korfias, S.; Ktonas, P.; Stranjalis, G.; Sakas, D.E. Transcranial direct current stimulation effects in disorders of consciousness. Arch. Phys. Med. Rehabil. 2014, 95, 283–289. [Google Scholar] [CrossRef]
| Patient | Gender | Age | Etiology | Structural Brain Lesions (MRI) | Time Since Injury (months) | Behavioral Analysis | EEG Analysis |
|---|---|---|---|---|---|---|---|
| S01 | M | 34 | TBI | Mature contusion damage extensively involving the left hemisphere, severe in the left frontal, (lateral and medial) temporal, and mesial parietal-occipital lobes | 5 | X | X |
| S02 | M | 45 | CA | Cortico-subcortical atrophy. Signs of rolandic and parieto-occipital ischemia (bilaterally) | 10 | X | |
| S03 | M | 52 | CA | Diffuse signal abnormality involving the periventricular white matter and and fronto-parieto-temporal and occipital cortex and subcortical white matter, mainly in the right hemisphere | 10 | X | X |
| S04 | M | 59 | CA | Diffuse and extensive white matter abnormality involving both hemispheres and severe and extensive cortico-subcortical atrophy | 11 | X | X |
| S05 | M | 31 | Meningitis | Diffuse signal abnormality affecting the temporal, frontoinsular, and frontobasal lobes bilaterally | 3 | X | X |
| S06 | M | 40 | TBI | Sequelae of hemorrhagic contusion in the left midbrain and diffuse signal abnormality affecting the left frontal lobe | 25 | X | X |
| S07 | M | 63 | Aneurysm | Mature midbrain damage, diffuse periventricular and frontal white matter abnormality, and diffuse cortico-subcortical atrophy | 5 | X | X |
| S08 | M | 23 | TBI | Established contusion lesions in the right frontal and right mesial temporal lobes and left basal ganglia | 3 | X | X |
| S09 | F | 47 | Aneurysm | Left temporo-occipital encephalomalacia with partial confluence with the left lateral ventricle associated with diffuse surrounding signal abnormality | 4 | X | X |
| S10 | F | 71 | Aneurysm | Sequelae of hemorrhagic infarcts in the right mesial occipital and left mesial frontal lobes | 13 | X | |
| S11 | F | 50 | Aneurysm | Diffuse and extensive white matter signal abnormalities in the fronto-parietal-insular and temporal lobes, mainly affecting the left hemisphere | 3 | X |
| Patient | tDCS Allocation | Pre-Sham CRS-R | Post-Sham CRS-R | Pre-Active CRS-R | Post-Active CRS-R |
|---|---|---|---|---|---|
| S01 | Active/sham | 8 (1-3-2-0-0-2) | 8 (1-3-2-0-0-2) | 9 (1-3-1-2-0-2) | 8 (1-3-1-1-0-2) |
| S03 | Sham/active | 9 (1-3-1-2-0-2) | 5 (1-1-1-1-0-1) | 8 (1-3-1-1-0-2) | 7 (1-3-1-1-0-1) |
| S04 | Sham/active | 7 (1-2-2-1-0-1) | 7 (1-3-0-1-0-2) | 6 (1-3-0-1-0-1) | 7 (2-1-0-2-0-2) |
| S05 | Sham/active | 11 (0-3-5-2-0-1) | 10 (0-3-5-1-0-1) | 12 (0-3-5-2-0-2) | 8 (0-1-5-1-0-1) |
| S06 | Active/sham | 9 (0-3-2-2-0-2) | 12 (3-3-2-2-0-2) | 9 (1-3-2-1-0-2) | 10 (1-4-2-1-0-2) |
| S07 | Active/sham | 16 (4-5-5-2-0-0) | 14 (3-4-5-2-0-0) | 8 (3-3-0-2-0-0) | 9 (3-3-0-2-1-0) |
| S08 | Sham/active | 18 (4-4-5-2-1-2) | 17 (4-5-5-2-0-2) | 19 (4-5-4-2-2-2) | 17 (4-5-4-0-2-2) |
| S09 | Sham/active | 7 (1-1-2-2-0-1) | 7 (1-1-2-2-0-1) | 6 (0-1-2-1-0-2) | 5 (1-1-1-1-0-1) |
| S10 | Sham/active | 8 (3-1-1-1-0-2) | 3 (0-0-2-1-0-0) | 3 (0-0-2-1-0-0) | 2 (0-0-1-1-0-0) |
| S11 | Sham/active | 2 (0-1-0-0-0-1) | 4 (0-3-0-0-0-1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrière, M.; Mortaheb, S.; Raimondo, F.; Annen, J.; Barra, A.; Binda Fossati, M.C.; Chatelle, C.; Hermann, B.; Martens, G.; Di Perri, C.; et al. Neurophysiological Correlates of a Single Session of Prefrontal tDCS in Patients with Prolonged Disorders of Consciousness: A Pilot Double-Blind Randomized Controlled Study. Brain Sci. 2020, 10, 469. https://doi.org/10.3390/brainsci10070469
Carrière M, Mortaheb S, Raimondo F, Annen J, Barra A, Binda Fossati MC, Chatelle C, Hermann B, Martens G, Di Perri C, et al. Neurophysiological Correlates of a Single Session of Prefrontal tDCS in Patients with Prolonged Disorders of Consciousness: A Pilot Double-Blind Randomized Controlled Study. Brain Sciences. 2020; 10(7):469. https://doi.org/10.3390/brainsci10070469
Chicago/Turabian StyleCarrière, Manon, Sepehr Mortaheb, Federico Raimondo, Jitka Annen, Alice Barra, Maria C. Binda Fossati, Camille Chatelle, Bertrand Hermann, Géraldine Martens, Carol Di Perri, and et al. 2020. "Neurophysiological Correlates of a Single Session of Prefrontal tDCS in Patients with Prolonged Disorders of Consciousness: A Pilot Double-Blind Randomized Controlled Study" Brain Sciences 10, no. 7: 469. https://doi.org/10.3390/brainsci10070469
APA StyleCarrière, M., Mortaheb, S., Raimondo, F., Annen, J., Barra, A., Binda Fossati, M. C., Chatelle, C., Hermann, B., Martens, G., Di Perri, C., Laureys, S., & Thibaut, A. (2020). Neurophysiological Correlates of a Single Session of Prefrontal tDCS in Patients with Prolonged Disorders of Consciousness: A Pilot Double-Blind Randomized Controlled Study. Brain Sciences, 10(7), 469. https://doi.org/10.3390/brainsci10070469






