Joint Neuropsychological Assessment through Coma/Near Coma and Level of Cognitive Functioning Assessment Scales Reduces Negative Findings in Pediatric Disorders of Consciousness
Abstract
1. Introduction
- To explore the agreement between the CNCS and LOCFAS;
- To quantify the practical advantage of administering both CNCS and LOCFAS instead of only one tool.
2. Materials and Methods
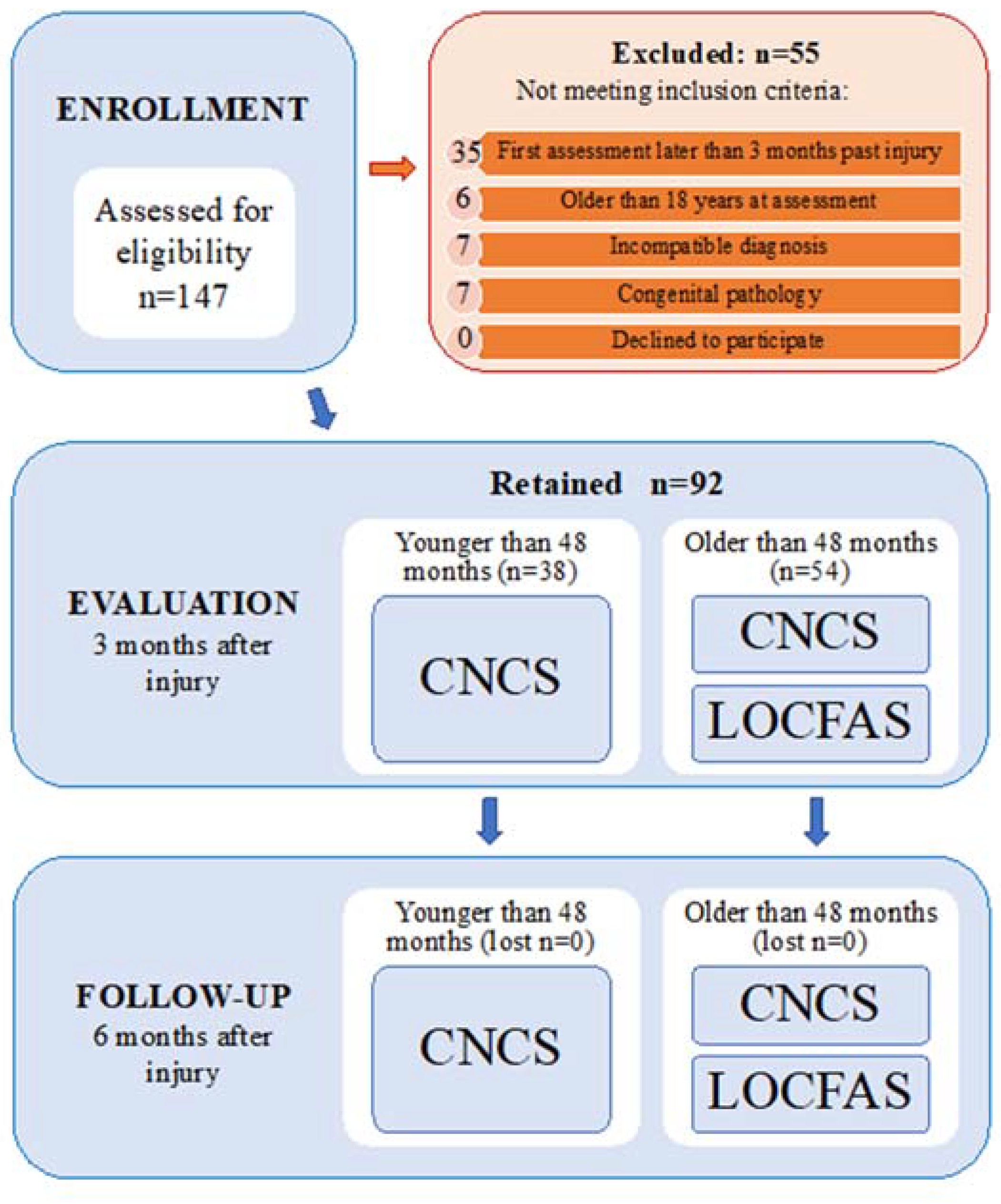
2.1. Participants
2.2. Measures
2.3. Data Analysis
3. Results
3.1. Patients
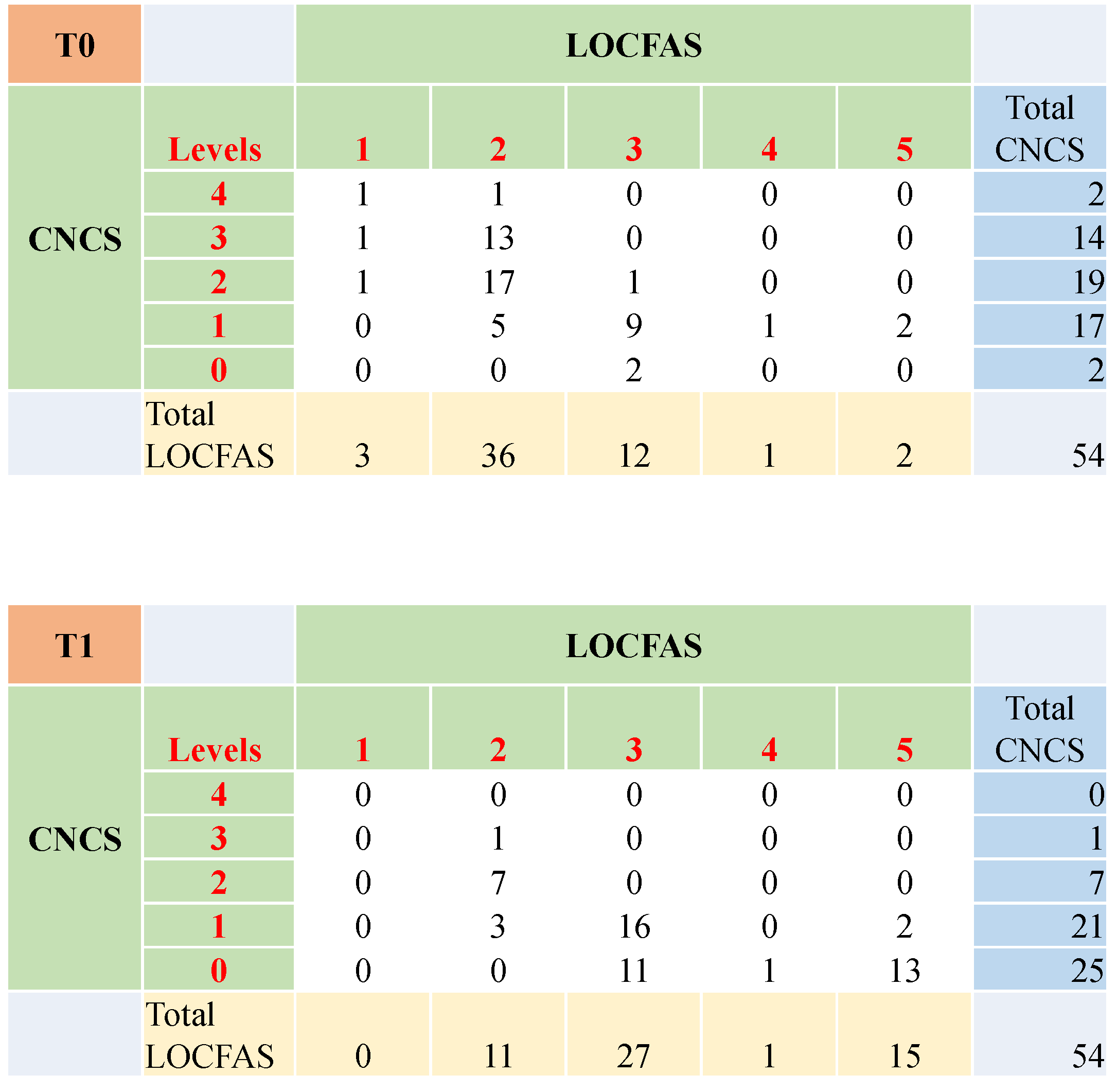
3.2. The Level of Awareness and Responsivity at Three and Six Months after Injury
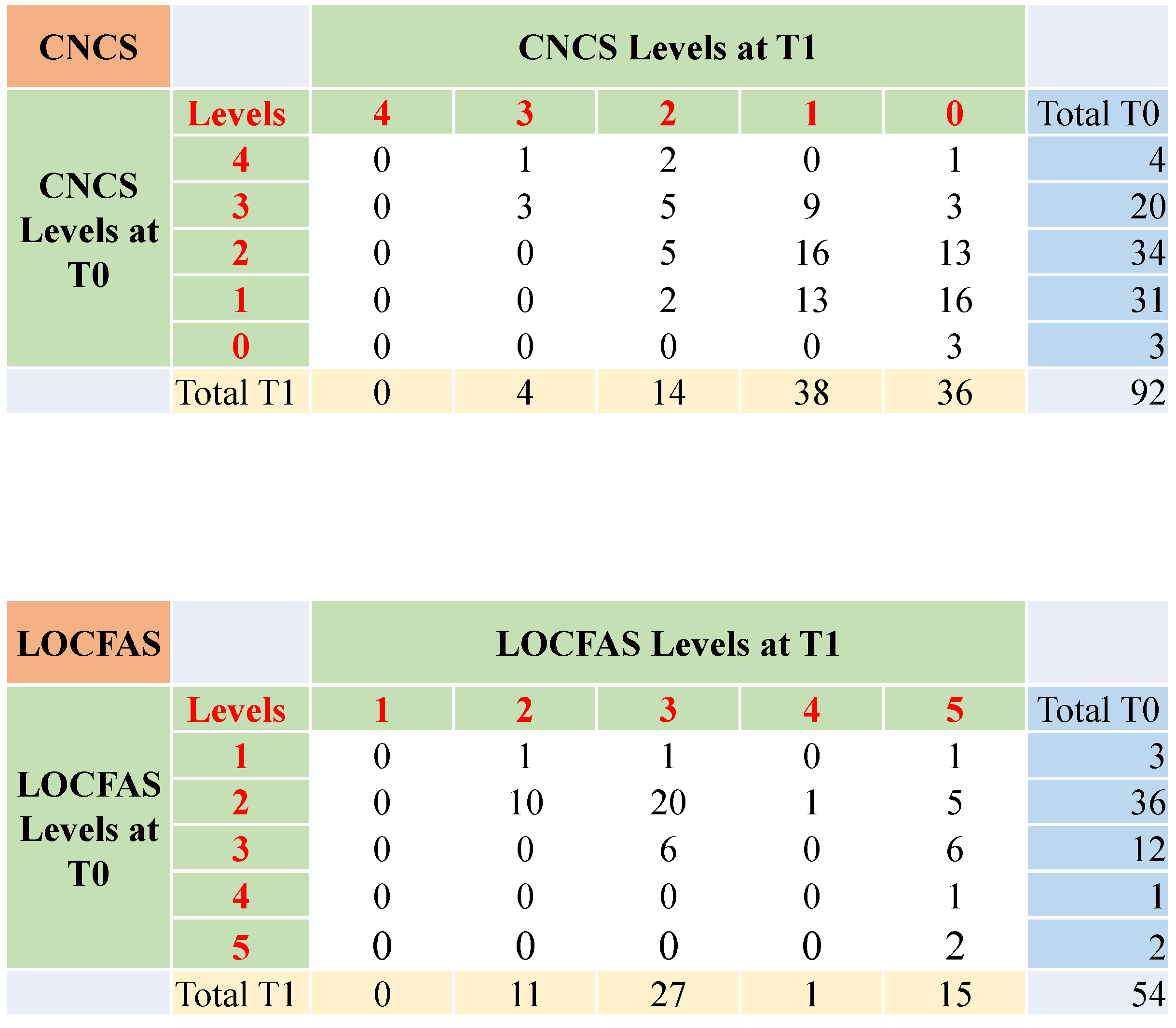
3.3. The Evolution of the State of Consciousness from Three to Six Months after Injury
3.4. The Evolution of the State of Consciousness in Patients with Stable Score at Three and Six Months after Injury
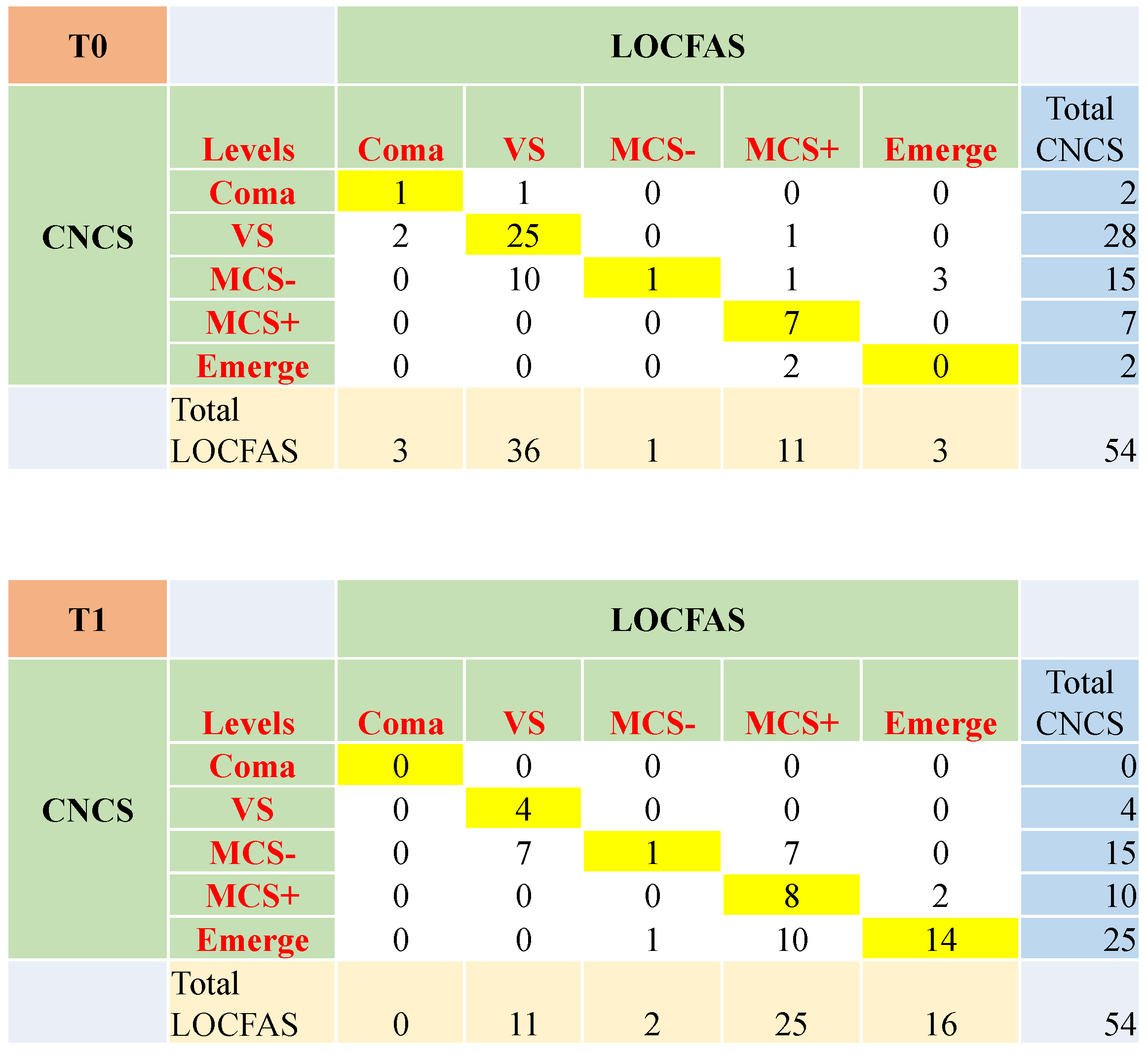
3.5. Clinical Agreement between CNCS and LOCFAS
3.6. The Evolution of the State of Consciousness with Respect to the Initial Ability to Follow Commands
3.7. The Evolution of the State of Consciousness in the Sample Divided by Etiology
3.8. Effect of Age
3.9. Correlations between the Clinical Characteristics and the CNCS and LOCFAS Scales
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Practice guideline update recommendations summary: Disorders of consciousness. Neurology 2018, 99, 1699–1709. [Google Scholar] [CrossRef]
- Peterson, A.; Cruse, D.; Naci, L.; Weijer, C.; Owen, A.M. Risk, diagnostic error, and the clinical science of consciousness. NeuroImage Clin. 2015, 7, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Seel, R.T.; Sherer, M.; Whyte, J.; Katz, D.I.; Giacino, J.T.; Rosenbaum, A.M.; Hammond, F.M.; Kalmar, K.; Pape, T.L.B.; Zafonte, R.; et al. Assessment scales for disorders of consciousness: Evidence-based recommendations for clinical practice and research. Arch. Phys. Med. Rehabil. 2010, 91, 1795–1813. [Google Scholar] [CrossRef] [PubMed]
- Eken, C.; Kartal, M.; Bacanli, A.; Eray, O. Comparison of the Full Outline of Unresponsiveness Score Coma Scale and the Glasgow Coma Scale in an emergency setting population. Eur. J. Emerg. Med. 2009, 16, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Schnakers, C.; Majerus, S.; Giacino, J.; Vanhaudenhuyse, A.; Bruno, M.A.; Boly, M.; Moonen, G.; Damas, P.; Lambermont, B.; Lamy, M.; et al. A French validation study of the Coma Recovery Scale-Revised (CRS-R). Brain Inj. 2008, 22, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.M.; Bamlet, W.R.; Maramattom, B.V.; Manno, E.M.; McClelland, R.L. Validation of a new coma scale: The FOUR score. Ann. Neurol. 2005, 58, 585–593. [Google Scholar] [CrossRef]
- Hall, K.; Cope, D.; Rappaport, M. Glasgow Outcome Scale and Disability Rating Scale: Comparative usefulness in following recovery in traumatic head injury. Arch. Phys. Med. Rehabil. 1985, 66, 35–37. [Google Scholar]
- Stanczak, D.E.; White, J.G.; Gouview, W.D.; Moehle, K.A.; Daniel, M.; Novack, T.; Long, C.J. Assessment of level of consciousness following severe neurological insult. A comparison of the psychometric qualities of the Glasgow Coma Scale and the Comprehensive Level of Consciousness Scale. J. Neurosurg. 1984, 60, 955–960. [Google Scholar] [CrossRef]
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; von Wild, K.R.; Zeman, A.; et al. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Bates, D. The vegetative state: Guidance on diagnosis and management. Clin. Med. (Northfield. Il) 2003, 3, 249. [Google Scholar]
- Bruno, M.A.; Vanhaudenhuyse, A.; Thibaut, A.; Moonen, G.; Laureys, S. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: Recent advances in our understanding of disorders of consciousness. J. Neurol. 2011, 258, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Eilander, H.J.; Wijnen, V.J.M.; Scheirs, J.G.M.; de Kort, P.L.M.; Prevo, A.J.H. Children and young adults in a prolonged unconscious state due to severe brain injury: Outcome after an early intensive neurorehabilitation programme. Brain Inj. 2005, 19, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.; Kramer, M.E.; Slomine, B.S.; Suskauer, S.J. Emergence to the Conscious State During Inpatient Rehabilitation After Traumatic Brain Injury in Children and Young Adults: A Case Series. J. Head Trauma Rehabil. 2014, 29, 44–48. [Google Scholar] [CrossRef]
- Molteni, E.; Slomine, B.S.; Castelli, E.; Zasler, N.; Schnakers, C.; Estraneo, A. International survey on diagnostic and prognostic procedures in pediatric disorders of consciousness. Brain Inj. 2019, 33, 1–12. [Google Scholar] [CrossRef]
- Philbin, M.K.; Klaas, P. Hearing and Behavioral Responses to Sound in Full-Term Newborns. J. Perinatol. 2000, 20, S68–S76. [Google Scholar] [CrossRef]
- Alvarez, G.; Suskauer, S.J.; Slomine, B. Clinical Features of Disorders of Consciousness in Young Children. Arch. Phys. Med. Rehabil. 2019, 100, 687–694. [Google Scholar] [CrossRef]
- Kriel, R.L.; Krach, L.E.; Panser, L.A. Closed head injury: Comparison of children younger and older than 6 years of age. Pediatr. Neurol. 1989, 5, 296–300. [Google Scholar] [CrossRef]
- Donders, J.; Warschausky, S. Neurobehavioral outcomes after early versus late childhood traumatic brain injury. J. Head Trauma Rehabil. 2007, 22, 296–302. [Google Scholar] [CrossRef]
- Haley, S.M.; Fragala-Pinkham, M.A.; Ni, P.S.; Skrinar, A.M.; Kaye, E.M. Pediatric physical functioning reference curves. Pediatr. Neurol. 2004, 31, 333–341. [Google Scholar] [CrossRef]
- Shaklai, S.; Peretz, R.; Spasser, R.; Simantov, M.; Groswasser, Z. Long-term functional outcome after moderate-to-severe paediatric traumatic brain injury. Brain Inj. 2014, 28, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Anderson, V.; Godfrey, C.; Rosenfeld, J.V.; Catroppa, C. Predictors of cognitive function and recovery 10 years after traumatic brain injury in young children. Pediatrics 2012, 129, e254–e261. [Google Scholar] [CrossRef] [PubMed]
- Msall, M.E.; DiGaudio, K.; Duffy, L.C.; LaForest, S.; Braun, S.; Granger, C. V WeeFIM. Normative sample of an instrument for tracking functional independence in children. Clin. Pediatr. (Phila) 1994, 33, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Suskauer, S.J.; Slomine, B.S.; Inscore, A.B.; Lewelt, A.J.; Kirk, J.W.; Salorio, C.F. Injury severity variables as predictors of WeeFIM scores in pediatric TBI: Time to follow commands is best. J. Pediatr. Rehabil. Med. 2009, 2, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.; Slomine, B.; De Matt, E.; Salorio, C.; Suskauer, S. Time to follow commands remains the most useful injury severity variable for predicting WeeFIM® scores 1 year after paediatric TBI. Brain Inj. 2013, 27, 1056–1062. [Google Scholar] [CrossRef]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef]
- Slomine, B.S.; Suskauer, S.J.; Nicholson, R.; Giacino, J.T.; Slomine, B.S.; Suskauer, S.J.; Nicholson, R.; Giacino, J.T. Preliminary validation of the coma recovery scale for pediatrics in typically developing young children. Brain Inj. 2019, 33, 1640–1645. [Google Scholar] [CrossRef]
- Rappaport, M.; Dougherty, A.M.; Kelting, D.L. Evaluation of coma and vegetative states. Arch. Phys. Med. Rehabil. 1992, 73, 628–634. [Google Scholar]
- Rappaport, M. The Coma/Near Coma Scale. The center for outcome measurement in brain injury. Available online: https://www.tbims.org/combi/cnc (assessed on 10 January 2020).
- Patrick, P.D.; Blackman, J.A.; Mabry, J.L.; Buck, M.L.; Gurka, M.J.; Conaway, M.R. Dopamine agonist therapy in low-response children following traumatic brain injury. J. Child Neurol. 2006, 20, 879–885. [Google Scholar] [CrossRef]
- McMahon, M.A.; Vargus-Adams, J.N.; Michaud, L.J.; Bean, J. Effects of amantadine in children with impaired consciousness caused by acquired brain injury: A pilot study. Am. J. Phys. Med. Rehabil. 2009, 88, 525–532. [Google Scholar] [CrossRef]
- Hagen, C.; Malkmus, D.; Durham, P. The Rancho Los Amigos Levels of Cognitive Functioning. Commun. Disord. Serv. Available online: https://www.neuroskills.com/education-and-resources/rancho-los-amigos-revised/ (assessed on 10 January 2020).
- Villa, F.; Colombo, K.; Pastore, V.; Locatelli, F.; Molteni, E.; Galbiati, S.; Galbiati, S.; Strazzer, S. LOCFAS-assessed evolution of cognitive and behavioral functioning in a sample of pediatric patients with severe acquired brain injury in the postacute phase. J. Child Neurol. 2015, 9, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Liscio, M.; Adduci, A.; Galbiati, S.; Poggi, G.; Sacchi, D.; Strazzer, S.; Castelli, E.; Flannery, J. Cognitive-behavioural stimulation protocol for severely brain-damaged patients in the post-acute stage in developmental age. Disabil. Rehabil. 2008, 30, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Flannery, J. Using the levels of cognitive functioning assessment scale with patients with traumatic brain injury in an acute care setting. Rehabil. Nurs. 1998, 23, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Aubinet, C.; Larroque, S.K.; Heine, L.; Martial, C.; Majerus, S.; Laureys, S.; Di Perri, C. Clinical subcategorization of minimally conscious state according to resting functional connectivity. Hum. Brain Mapp. 2018, 39, 4519–4532. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of Coma and Impared Conciousness. Lancet 1974, 304, 81–84. [Google Scholar] [CrossRef]
- Brink, J.D.; Imbus, C.; Woo-Sam, J. Physical recovery after severe closed head trauma in children and adolescents. J. Pediatr. 1980, 97, 721–727. [Google Scholar] [CrossRef]
- Stover, S.L.; Zeiger, H.E., Jr. Head injury in children and teenagers; functional recovery correlated with the duration of coma. Arch. Phys. Med. Rehabil. 1976, 57, 201–205. [Google Scholar]
- The Multi-Society Task Force on PVS Medical aspects of the persistent vegetative state. N. Engl. J. Med. 1994, 330, 1499–1508. [CrossRef]
- Garland, E.L. Pain Processing in the Human Nervous System. A Selective Review of Nociceptive and Biobehavioral Pathways. Prim. Care Clin. Off. Pract. 2012, 39, 561–571. [Google Scholar] [CrossRef]
- Iannetti, G.D.; Mouraux, A. From the neuromatrix to the pain matrix (and back). Exp. Brain Res. 2010, 205, 1–12. [Google Scholar] [CrossRef]
- Schnakers, C.; Zasler, N. Assessment and Management of Pain in Patients with Disorders of Consciousness. PM R 2015, 7, S270–S277. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.A.; Schnakers, C.; Boly, M.; Hustinx, R.; Vanhaudenhuyse, A.; Kirsch, M.; Bernard, C.; Moonen, G.; Laureys, S. Subcategorizing the minimally conscious state based on cerebral metabolism PET studies. In Proceedings of the 19th Meeting of the European Neurological Society, Milan, Italy, 5–9 June 2009. [Google Scholar]
- Godbolt, A.K.; Stenberg, M.; Jakobsson, J.; Sorjonen, K.; Krakau, K.; Stålnacke, B.M.; Nygren DeBoussard, C. Subacute complications during recovery from severe traumatic brain injury: Frequency and associations with outcome. BMJ Open 2015, 5, e007208. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ortega, J.F.; Prieto-Palomino, M.A.; Garcia-Caballero, M.; Galeas-Lopez, J.L.; Quesada-Garcia, G.; Baguley, I.J. Paroxysmal Sympathetic Hyperactivity after Traumatic Brain Injury: Clinical and Prognostic Implications. J. Neurotrauma 2011, 29, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, L.; Rogano, S.; Quintieri, M.; Leto, E.; Dolce, G. Decreasing incidence of paroxysmal sympathetic hyperactivity syndrome in the vegetative state. J. Rehabil. Med. 2012, 44, 502–504. [Google Scholar] [CrossRef]
- Pozzi, M.; Conti, V.; Locatelli, F.; Galbiati, S.; Radice, S.; Clementi, E.; Strazzer, S. Paroxysmal Sympathetic Hyperactivity in Pediatric Rehabilitation. J. Head Trauma Rehabil. 2016, 30, 357–363. [Google Scholar] [CrossRef]
- Mélotte, E.; Maudoux, A.; Delhalle, S.; Martial, C.; Antonopoulos, G.; Larroque, S.K.; Wannez, S.; Faymonville, M.E.; Kaux, J.F.; Laureys, S.; et al. Is oral feeding compatible with an unresponsive wakefulness syndrome? J. Neurol. 2018, 265, 954–961. [Google Scholar] [CrossRef]
- Corrigan, J.D.; Selassie, A.W.; Orman, J.A. The epidemiologyo of traumatic brain injury. J. Head Trauma Rehabil. 2010, 21, 375–378. [Google Scholar]
| Clinical Status | CNCS Level | CNCS Additional Criteria | LOCFAS Level | LOCFAS Additional Criteria |
|---|---|---|---|---|
| Emerge | Level 0 | - | Level 5 | |
| Level 4 | ||||
| MCS+ | Level 1 | And ‘Command responsivity’ is 0 | Level 3 | If specific score at item ‘Execution of commands’ is 2, 3 or 4 |
| MCS- | Level 1 | And ‘Command responsivity’ is 2 or 4 | Level 3 | If specific score at item ‘Execution of commands’ is 1 |
| Level 2 | If ‘Auditory’ is 0, or if ‘Visual (item n.3 or 4)’ is 0, or both | |||
| Vegetative State | Level 2 | If ‘Auditory’ is not 0 and ‘Visual (item n.3 or 4)’ is not 0 | Level 2 | |
| Level 3 | ||||
| Coma | Level 4 | Level 1 | ||
| Total Sample (n = 92) | Traumatic (TBI) (n = 44) | Non-Traumatic (NTBI) (n = 48) | Statistics | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p (t-Test) | |
| Age at injury (months) | 87.0 | 66.1 | 127.7 | 65.0 | 50.1 | 41.2 | <1 × 10−4 ** |
| Median | Range | Median | Range | Median | Range | p (Wilcoxon test) | |
| GCS score | 4 | 3–8 | 4 | 3–7 | 4 | 3–8 | 0.892 |
| GOS-E score | 2 | 2–3 | 2 | 2–3 | 2 | 2–3 | 0.141 |
| n | % | n | % | n | % | p (2) | |
| Gender | |||||||
| Male | 60 | 65.2 | 29 | 65.9 | 31 | 64.6 | 0.535 |
| Female | 32 | 34.8 | 15 | 34.1 | 17 | 35.4 | |
| Need for neurosurgery | 48 | 52.2 | 32 | 72.7 | 17 | 35.4 | <1 × 10−4 ** |
| Tracheotomy | 46 | 50.0 | 27 | 61.4 | 20 | 41.7 | 0.060 |
| Feeding disorders | |||||||
| Absence of disorders | 1 | 1.1 | 0 | 0.0 | 1 | 2.1 | 0.251 |
| Dysphagia | 5 | 5.4 | 4 | 9.1 | 2 | 4.2 | |
| Nasogastric tube (NGT) | 38 | 41.3 | 14 | 31.8 | 24 | 50.0 | |
| Percutaneous endoscopic gastrostomy (PEG) | 48 | 52.2 | 26 | 59.1 | 21 | 43.7 | |
| Paroxysmal sympathetic hyperactivity episodes | 38 | 41.3 | 20 | 45.5 | 18 | 37.5 | 0.529 |
| Motor impairment | |||||||
| Absence of impairment | 1 | 1.1 | 1 | 2.3 | 0 | 0.0 | 0.370 |
| Motor retardation | 1 | 1.1 | 1 | 2.3 | 0 | 0.0 | |
| Quadriparesis | 89 | 96.7 | 42 | 95.4 | 47 | 97.9 | |
| Ataxia | 1 | 1.1 | 0 | 0.0 | 1 | 2.1 | |
| Previous rehabilitation | 4 | 4.3 | 2 | 4.5 | 2 | 4.2 | 0.572 |
| Traumatic (TBI) | Non-Traumatic (NTBI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Individual Parameters | T0 | T1 | Z (p) | T0 | T1 | Z (p) | ||||
| Median | Mode | Median | Mode | Median | Mode | Median | Mode | ||||
| 1 | Auditory | 2 | 2 | 0 | 0 | 3.5 (0.001) * | 2 | 2 | 0 | 0 | 4.2 (<0.001) ** |
| 2 | Command responsivity | 4 | 4 | 0 | 0 | 4.3 (<0.001) ** | 4 | 4 | 2 | 0 | 4.5 (<0.001) ** |
| 3 | Visual | 2 | 4 | 0 | 0 | 4.4 (<0.001) ** | 4 | 4 | 2 | 0 | 4.4 (<0.001) ** |
| 4 | Visual | 2 | 4 | 0 | 0 | 4.2 (<0.001) ** | 4 | 4 | 2 | 0 | 4.0 (<0.001) ** |
| 5 | Threat | 2 | 4 | 0 | 0 | 3.5 (<0.001) ** | 4 | 4 | 0 | 0 | 4.3 (<0.001) ** |
| 6 | Olfactory | 4 | 4 | 2 | 0 | 4.0 (<0.001) ** | 2 | 4 | 2 | 0 | 2.1 (0.033) * |
| 7 | Tactile | 4 | 4 | 2 | 2 | 4.8 (<0.001) ** | 4 | 4 | 2 | 2 | 4.7 (<0.001) ** |
| 8 | Tactile | 2 | 0 | 0 | 0 | 4.2 (<0.001) ** | 0 | 0 | 0 | 0 | 2.1 (0.038) * |
| 9 | Pain | 0 | 0 | 0 | 0 | 1.5 (0.132) | 0 | 0 | 0 | 0 | 3.1 (0.002) * |
| 10 | Pain | 0 | 0 | 0 | 0 | 2.3 (0.021) * | 0 | 0 | 0 | 0 | 3.3 (0.001) * |
| 11 | Vocalization | 4 | 4 | 2 | 2 | 4.1 (<0.001) ** | 3 | 4 | 2 | 2 | 4.3 (<0.001) ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molteni, E.; Colombo, K.; Pastore, V.; Galbiati, S.; Recla, M.; Locatelli, F.; Galbiati, S.; Fedeli, C.; Strazzer, S. Joint Neuropsychological Assessment through Coma/Near Coma and Level of Cognitive Functioning Assessment Scales Reduces Negative Findings in Pediatric Disorders of Consciousness. Brain Sci. 2020, 10, 162. https://doi.org/10.3390/brainsci10030162
Molteni E, Colombo K, Pastore V, Galbiati S, Recla M, Locatelli F, Galbiati S, Fedeli C, Strazzer S. Joint Neuropsychological Assessment through Coma/Near Coma and Level of Cognitive Functioning Assessment Scales Reduces Negative Findings in Pediatric Disorders of Consciousness. Brain Sciences. 2020; 10(3):162. https://doi.org/10.3390/brainsci10030162
Chicago/Turabian StyleMolteni, Erika, Katia Colombo, Valentina Pastore, Susanna Galbiati, Monica Recla, Federica Locatelli, Sara Galbiati, Claudia Fedeli, and Sandra Strazzer. 2020. "Joint Neuropsychological Assessment through Coma/Near Coma and Level of Cognitive Functioning Assessment Scales Reduces Negative Findings in Pediatric Disorders of Consciousness" Brain Sciences 10, no. 3: 162. https://doi.org/10.3390/brainsci10030162
APA StyleMolteni, E., Colombo, K., Pastore, V., Galbiati, S., Recla, M., Locatelli, F., Galbiati, S., Fedeli, C., & Strazzer, S. (2020). Joint Neuropsychological Assessment through Coma/Near Coma and Level of Cognitive Functioning Assessment Scales Reduces Negative Findings in Pediatric Disorders of Consciousness. Brain Sciences, 10(3), 162. https://doi.org/10.3390/brainsci10030162





