Abstract
Parkinson’s disease (PD) is a common neurodegenerative disorder; however, well-established biochemical markers have not yet been identified. This review article covers several candidate cerebrospinal fluid (CSF) biomarkers for PD based on the recent literature and meta-analysis data. The decrease of α-synuclein in PD is supported by meta-analyses with modest reproducibility, and a decrease of amyloid β42 is seen as a prognostic marker for cognitive decline. Tau, phosphorylated tau (p-tau), and neurofilament light chains have been used to discriminate PD from other neurodegenerative disorders. This article also describes more hopeful biochemical markers, such as neurotransmitters, oxidative stress markers, and other candidate biomarkers.
1. Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders, and is characterized by resting tremors, bradykinesia, rigidity, and postural instability, as well as cognitive symptoms [1]. Pathologically, this disorder is defined by degeneration of the substantia nigra and the presence of Lewy bodies, which contain abundant amounts of α-synuclein. Researchers have also found that gene mutations of α-synuclein cause familial forms of PD. Therefore, α-synuclein is considered to play a major role in the pathogenesis of PD.
The current clinical diagnostic criteria of PD are based on existence of parkinsonism and the exclusion of other disorders, and all the tests for PD, such as magnetic resonance imaging or nuclear medicine imaging are positioned as supportive tools [2]. Therefore, good biomarkers for PD with high sensitivity and specificity are desired.
The cerebrospinal fluid (CSF) biomarkers for PD have been investigated widely to elucidate the pathophysiology and to support differentiating PD from normal subjects or other neurological disorders, such as multiple system atrophy (MSA) [3] or progressive supranuclear palsy (PSP) [4]. CSF biomarkers are also sought for the evaluation of disease activity/progression, severity, staging, comorbidity (i.e., cognitive dysfunction), and prognosis.
Confirmed CSF biomarkers have not yet been established; however, some candidate molecules (for example, α-synuclein) have been inspected as potential ones. In this article, we aimed to provide a comprehensive overview for the biomarkers for PD and referred to recent meta-analyses for several biomarkers.
2. Overview and Classification of the CSF Biomarkers of PD
A previous review divided the biomarker candidates into six categories (A, neurotransmitters and neuromodulators; B, oxidative stress markers; C, inflammatory and immunological markers; D, growth factors; E, proteins involved in PD pathology; and F, others) [5]. Under each category, the authors focused on their usability in these four clinical domains (1, distinguishing PD from controls; 2, distinguishing PD from other neurodegenerative diseases; 3, representing the disease severity or cognitive abilities; and 4, being of prognostic value regarding the disease severity and cognitive abilities).
Our review also adopted this categorization; however, the clinical usability was divided into three clinical domains (1, 2, and 3 + 4). This categorization is tentative and conceptual, and exceptions are possible (e.g., one marker can belong to two categories, or be not categorical). We also annotated the putative functions, roles, or pathophysiological interpretations for each marker in Table 1 of this article to provide perspectives on this field. Any single marker cannot cover all these domains as yet. This is unsurprising as the central nervous system in PD is exposed to various pathological changes (aging, synuclein-, amyloid-, tau-pathology, etc.). Therefore, many recent studies have adopted combinations of several CSF biomarkers.

Table 1.
Candidate cerebrospinal fluid biomarkers for distinguishing Parkinson’s disease from controls.
3. Materials and Methods
This study is based on a literature search in the database PubMed with the keywords (Parkinson AND cerebrospinal fluid AND biomarker AND (review OR meta-analysis)) on 24 May 2020. Non-English publications were excluded from this review because of the difficulty of assessing the contents as available open resources. Only the most relevant or earliest studies were included in this review and duplicated contents were omitted as well as studies with a small sample size (n < 20).
Of the 255 articles identified in the database, 63 were included in this study (Figure 1). The search also found 15 meta-analysis articles referring to α-synuclein, neurofilament light chains, cytokines (interleukin (IL)-1β, IL-6, and transforming growth factor (TGF)-β1), amyloid Aβ42, tau, and phosphorylated tau.
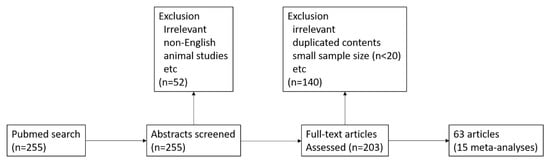
Figure 1.
Flowchart of the article selection process.
We also focused on the following markers in particular: (1) α-synuclein and its related molecules; (2) dopamine, neurotransmitters, and the metabolites; (3) oxidative stress markers; (4) amyloid beta as a predictor for cognition; and (5) tau, phosphorylated tau, and neurofilament light chains as discriminators for atypical Parkinsonism.
4. Results
The results are summarized in Table 1 (the biomarkers for distinguishing PD from controls), Table 2 (the biomarkers for distinguishing PD from other neurodegenerative disorders), and Table 3 (the biomarkers for representing disease severity or cognitive abilities). Then, we made detailed comments for each marker category.

Table 2.
Candidate cerebrospinal fluid biomarkers for distinguishing Parkinson’s disease.

Table 3.
Candidate cerebrospinal fluid biomarkers for representing disease severity or cognitive abilities.
4.1. Neurotransmitters and Neuromodulators: Focusing on Dopamine and the Metabolites
The depletion of dopaminergic neurons in the substantia nigra is an essential pathology in PD, and noradrenergic neurons in locus ceruleus were also observed. Dopamine and noradrenaline decreases are also potential candidates of PD, as well as the metabolites, including dihydroxyphenyl acetic acid (DOPAC) and homovanillic acid (HVA). However, the levels of these compounds actually have different sources and meanings [7]. CSF homovanillic acid is rather distantly related to neuronal dopamine stores and reflects several intervening processes. As dopaminergic neurons do not contain catechol-O-methyltransferase, CSF homovanillic acid depends on the uptake and intracellular O-methylation in extra-dopaminergic cells. Thus, in PD, the striatal content of homovanillic acid is not as severely decreased as that of dopamine. CSF dopamine also may not provide an accurate reflection of central dopamine deficiency. Dopamine in extracellular fluid is derived mainly from exocytotic release in response to pathway traffic and the escape of neuronal reuptake by the cell membrane dopamine transporter.
As dopaminergic neurons are lost, pathway traffic to the remaining terminals likely increases compensatorily, thereby augmenting dopamine delivery from those terminals to the extracellular fluid; thus, CSF dopamine may underestimate the extent of the loss of neuronal dopamine stores. CSF dopamine concentrations are infinitesimal—sometimes below the detection limit of the assay method [7]. Meta-analysis articles were not found in the PubMed database in this category. CSF catecholamine would be influenced by levodopa intake, and, therefore, the measurements should be performed for a levodopa-naïve or washed-out state.
Other marker changes (anandamide, neuromodulin (GAP43), 3-hydroxykynurenine, orexin, and 5-hydroxyindole acetic acid (5-HIAA)) were also reported [6,8,9,45,54].
4.2. Oxidative Stress Markers
Numerous markers have been investigated to evaluate oxidative stress in PD [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,55] (Table 1 and Table 2); however, meta-analysis showed that only five CSF oxidative stress markers, 8-hydroxy-2’-deoxyguanosine (8-OHdG), Mn, Cu, Zn, and Fe, could be evaluated, and no significant difference was found between PD patients and controls [13]. On the other hand, the meta-analysis showed that patients with PD had significantly higher levels of blood oxidative stress markers compared with healthy control subjects for ferritin, 8-OHdG, nitrite, and malondialdehyde; meanwhile, the concentrations of uric acid, catalase, glutathione, and total-cholesterol were significantly lower in PD patients compared with healthy control subjects [13]. Oxidative stress markers could be influenced by numerous confounding factors, such as age, sex, diets, drugs, supplements, smoking, exercise, and comorbidities (hypertension, diabetes mellitus, inflammation, ischemia, etc.).
Other markers (advanced oxidized protein products (self-oxidized), ceruloplasmin ferroxidase activity, oxidized Q10, Cu/Zn-superoxide dismutase, DJ-1, glutathione S-transferase Pi, glutathione (oxidized), hydroxy radical (·OH), lipid peroxidation, nitrites, nitrates, silicic acid, xanthine, 3-nitrotyrosine products, and 8-hydroxyguanosine (8-OHG)) have also been investigated [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,55].
4.3. Inflammatory and Immunological Markers
Researchers have suggested that cytokine-mediated inflammation plays a key role in the onset and/or development of PD. One meta-analysis reported that IL-1β, IL-6, and TGF-β were elevated in PD [27] and the work also revealed the unique inflammatory response profile in the central nervous system of patients with Alzheimer’s disease, PD, and amyotrophic lateral sclerosis. Other markers (β2-microglobulin, Immunoglobulin G (IgG) ratio (CSF/serum), interferon (IFN)-γ, prostaglandin E2, soluble CD (cluster of differentiation) 14, tumor necrosis factor (TNF)-α, and differentially sialylated isoforms of Serpin A1 and Flt3 (Fms-related tyrosine kinase 3) ligand) have also been investigated [6,19,25,26,28,29,56].
4.4. Growth Factors
Only sparse data was available for this area. Brain-derived neurotrophic factor (BDNF) was significantly different (decreased) between the control and both neurodegenerative groups (PD and Alzheimer’s disease) but not between neurodegenerative groups [25]. CSF progranulin was investigated in PD, amyotrophic lateral sclerosis and controls, but no difference was found among the groups [30].
4.5. Proteins Involved in PD Pathology
4.5.1. α-Synuclein and Its Related Molecules
α-synuclein is considered one of the most important targets in this field, and many studies have investigated CSF α-synuclein. The reported control/normal CSF α-synuclein values varied widely; however, meta-analyses showed that the CSF levels of α-synuclein decreased in patients with PD [31,32,33,34]. One possible reason for the conflicting results is the contamination of CSF by α-synuclein from the blood, as α-synuclein is abundant in whole blood, plasma, and serum, in which its levels are up to 102–103 times higher than those found in the CSF [64]. Synuclein oligomer and phosphorylated α-synuclein are also candidate molecules supported by a meta-analysis [34]. The meta-analyses showed that the sensitivity and specificity of α-synuclein were 0.72–0.88% and 0.40–0.65, respectively [32,34]. The sensitivity and specificity of α-synuclein oligomer were 0.71 and 0.64, respectively [34] (Table 4).

Table 4.
Summary of the values of sensitivity, specificity, or the effect size of each marker as reported by the meta-analysis studies.
A recent study demonstrated that CSF α-synuclein decreased early in the disease, preceding motor PD. CSF α-synuclein does not correlate with the progression and therefore does not reflect ongoing dopaminergic neurodegeneration. Decreased CSF α-synuclein may be an indirect index of changes in the balance between α-synuclein secretion, solubility, or aggregation in the brain, reflecting its overall turnover [65]. This corresponds with another finding that the Hoehn–Yahr stage was not correlated with the CSF α-synuclein level, and that the striatal binding ratio on dopamine transporter imaging with 123I-ioflupane decreased in the PD group, but this was not correlated with the CSF α-synuclein level [66]. Another area of interest regarding CSF α-synuclein is its relationship with cognitive function; however, the research remains inconclusive or less influential [66].
Technical caution and pitfalls for the measurement of α-synuclein should be considered for appropriate use. Perianalytical considerations for biomarker studies in PD have been summarized [67]. α-synuclein is abundant in erythrocytes and, therefore contained in the serum, plasma, and whole blood, and, thus, blood contamination should be avoided cautiously. CSF hemoglobin is often referred to as a marker for blood contamination. The use of traumatic needles, discarding the first five drops of CSF, and collection with polypropylene or siliconized tubes are recommended for sampling for CSF. There does not appear to be any diurnal fluctuation.
Why α-synuclein decreases in the CSF of PD remains unknown. Low production, aggregation in the brain, and/or increased clearance of α-synuclein would be possible.
4.5.2. Amyloid Beta, Tau, and Phosphorylated Tau as Predictors for Cognition
Cognitive decline in PD is well known, and several studies reported a relationship between amyloid-beta and cognition. Another recent topic of research interest is the relationship between PD, amyloid β42 (Aβ42), tau, and p-tau, particularly from the viewpoint of cognitive function. One meta-analysis showed that the CSF Aβ42 level in PD with the cognitive impairment (CI) cohort was lower than that in PD with the normal cognition (NC) cohort. Reduced Aβ42 as well as elevated t-tau and p-tau were observed in the PD with dementia (PDD) cohort compared with the PDNC cohort. Therefore, the meta-analysis concluded that amyloid pathology and tauopathy may participate in the development of PDD, which is similar to Alzheimer’s disease [35].
It is unknown whether α-synuclein and amyloid beta can interact; however, a previous study reported no significant correlation between α-synuclein and amyloid beta [66]. There is no robust evidence regarding the contribution of α-synuclein to cognitive function. These matters should be investigated in further research.
4.5.3. Neurofilament Light Chains for Discriminating PD from Other Neurological Disorders
Neurofilament light chains (NFL) also have the potential to differentiate PD from MSA or PSP, and this is supported by meta-analyses [57,58,59,60]. NFL elevation was observed in MSA, PSP, and corticobasal degeneration. In terms of the comparison between PD and atypical parkinsonian disorders, the sensitivity and specificity of NFL were 0.82 and 0.85, respectively [59] (Table 4).
4.5.4. Other Proteins
Other proteins (neurosin, glial fibrillary acidic protein (GFAP), clusterin, neurofilament heavy chains, YKL-40 (CHI3L1), soluble neuron-glial antigen 2 (NG2) proteoglycan, ubiquitin, ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCHL-1), apolipoprotein A1, apolipoprotein A2, apolipoprotein ε, transthyretin, glycan isoforms of transferrin (serum-type/brain-type ratio), neuron-specific enolase, myelin basic protein, heart fatty acid binding protein, glucocerebrosidase activity, and neprilysin activity) have also been investigated [18,25,28,36,37,38,39,40,41,42,43,44,46,62,63].
Others
Many other candidate markers have been reported (Table 1) [6,9,18,25,47,48,49,50,51,52,53,61]; however, these candidates also include preliminary ones with small sample data. These markers require verification and interpretation of the background. Pathophysiological meanings have been proposed for each marker, but these should also be inspected.
Recent studies focused on the hypothesis that epigenetic mechanisms contribute to PD development and progression. Epigenetics refers to the regulatory mechanisms of gene expression that are not mediated by the DNA sequence itself, but by chemical or allosteric DNA modifications or by the action of regulatory noncoding RNAs. MicroRNAs (miRNAs) are small noncoding RNAs that serve as posttranscriptional regulators of gene expression. They bind to messenger RNA (mRNA) and promote mRNA degradation and/or decrease the translation. One meta-analysis identified four studies for miRNA of CSF; however, no significant signals were found in the CSF, although the study identified 13 significantly differentially expressed miRNAs in the brain (n = 3) and blood (n = 10) [51]. The most compelling finding was miRNA hsa-miR-132-3p binding to the mRNA of the α-synuclein gene (SNCA).
5. Discussion
Cumulative data, including meta-analyses, support that a decrease of CSF α-synuclein is observed in PD and that a decrease of amyloid β42 is a predictor of cognitive decline in PD. Tau, phosphorylated tau, and neurofilament light chains were able to discriminate PD from other neurodegenerative disorders. The elevation of cytokines (IL-1β, IL-6, and TGF-β) in PD suggested pro-inflammatory mechanisms in the pathogenesis of PD. CSF DOPAC appeared to provide a sensitive means to identify PD. This review could not identify reliable CSF oxidative stress markers in the literature thus far.
Further studies are necessary to reveal α-synuclein abnormalities, which may include the transcription, translation, epigenetic regulation, and kinetics (production, distribution, post-translational modification, oligomerization, aggregation, clearance, etc.). The standardization of measurement techniques is also required to use the practical application of these markers.
This review has some limitations. First, the abovementioned meta-analyses showed statistical significance; however, heterogeneity was present in each meta-analysis. Overlapping of the data between PD and the counterpart is present in any marker; therefore, they should be applied carefully. Second, these CSF biomarker samplings were performed mainly while the patients were alive, and, therefore, the diagnosis at the time of CSF sampling was established with clinical criteria but not as a histopathological diagnosis.
It is inevitable to have the limitation of pre-mortem differential diagnosis of AD and dementia with Lewy bodies (DLB). Certain studies adopted post-mortem CSF sampling with neuropathologically diagnosed PD subjects [18]; however, post-mortem changes may happen in the CSF or even in the brain, and the post-mortem data should be compared with longitudinal information. Certain patients showed a mixed pathology of AD and Lewy pathology [68]. The employment of other surrogate markers, such as amyloid positron emission tomography (PET), tau PET, or dopamine transporter imaging, would be necessary for the verification of the clinical diagnosis, as well as other modalities, such as myocardium metaiodobenzylguanidine (MIBG) and dopamine transporter (DAT) scintigraphy. Longitudinal data sampling is also needed but limited [65].
6. Conclusions
In conclusion, the meta-analyses for cerebrospinal fluid biomarkers of PD showed: (1) a decrease of α-synuclein was a marker of PD, (2) a decrease of amyloid β42 was a marker of cognitive decline in PD, (3) the elevation of tau, phosphorylated tau, and neurofilament light chains differentiated between PD and the related disorders, and (4) the elevation of cytokines (IL-1β, IL-6, and TGF-β) was observed in PD. Many other candidate biomarkers were also scrutinized. These findings will aid in the accurate diagnosis of PD and other neurodegenerative disorders and facilitate understanding of the pathogeneses of these conditions. Further studies will be needed to obtain more precise measurements of the biochemical biomarker levels. Any single biomarker cannot lead to a ‘snap-shot’ diagnosis. Combinations of several biomarkers would help with more accurate diagnoses and evaluation of the pathophysiology.
CSF biomarkers would aid in understanding the pathophysiological mechanisms, improve the diagnostic accuracy, and facilitate the future development of novel therapies, including disease-modifying drugs.
Finally, we refer to the limitation of this article. This work is a narrative review but not a systematic review. Therefore, we admit that this article could contain some selection bias although each report was fairly scrutinized as much as possible. The aim of this work is to provide the latest comprehensive review regarding cerebrospinal fluid biomarkers of PD, to grade the reliance of each marker according to meta-analyses, and to focus on each marker or marker category with respect to future research directions. We agree that the systematic review method is a more reliable scientific approach. However, referring to all reports offsets the conciseness of this article, and even dopamine and the metabolites, which are generally regarded as central issues in the pathophysiology of PD, have no meta-analysis yet. Hence, we did not adopt the systematic review approach in this article.
Author Contributions
Conceptualization, T.K.; Methodology, T.K.; Software, T.K.; Validation, T.K.; Formal Analysis, T.K.; Investigation, T.K.; Resources, T.K.; Data Curation, T.K.; Writing—Original Draft Preparation, T.K.; Writing—Review & Editing, J.S. and K.T.; Supervision, O.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kalinderi, K.; Bostantjopoulou, S.; Fidani, L. The genetic background of Parkinson’s disease: Current progress and future prospects. Acta Neurol. Scand. 2016, 134, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.; Wenning, G.K.; Low, P.A.; Brooks, D.J.; Mathias, C.J.; Trojanowski, J.Q.; Wood, N.W.; Colosimo, C.; Dürr, A.; Fowler, C.J.; et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008, 71, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Movement Disorder Society-endorsed PSP Study Group. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.D.; Binzer, M.; Stenager, E.; Gramsbergen, J.B. Cerebrospinal fluid biomarkers for Parkinson’s disease-a systematic review. Acta Neurol. Scand. 2017, 135, 34–56. [Google Scholar] [CrossRef]
- Pisani, V.; Moschella, V.; Bari, M.; Fezza, F.; Galati, S.; Bernardi, G.; Stanzione, P.; Pisani, A.; Maccarrone, M. Dynamic changes of anandamide in the cerebrospinal fluid of Parkinson’s disease patients. Mov. Disord. 2010, 25, 920–924. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Holmes, C.; Sharabi, Y. Cerebrospinal fluid biomarkers of central catecholamine deficiency in Parkinson’s disease and other synucleinopathies. Brain 2012, 135, 1900–1913. [Google Scholar] [CrossRef]
- Sjögren, M.; Minthon, L.; Davidsson, P.; Granérus, A.-K.; Clarberg, A.; Vanderstichele, H.; Vanmechelen, E.; Wallin, A.; Blennow, K. CSF levels of tau, beta-amyloid(1-42) and GAP-43 in frontotemporal dementia, other types of dementia and normal aging. J. Neural. Transm. (Vienna) 2000, 107, 563–579. [Google Scholar]
- Lewitt, P.A.; Li, J.; Lu, M.; Beach, T.G.; Adler, C.H.; Guo, L.; Arizona Parkinson’s Disease Consortium. 3-hydroxykynurenine and other Parkinson’s disease biomarkers discovered by metabolomic analysis. Mov. Disord. 2013, 28, 1653–1660. [Google Scholar] [CrossRef]
- García-Moreno, J.M.; Martín de Pablos, A.; García-Sánchez, M.I.; Méndez-Lucena, C.; Damas-Hermoso, F.; Rus, M.; Chacón, J.; Fernández, E. May serum levels of advanced oxidized protein products serve as a prognostic marker of disease duration in patients with idiopathic Parkinson’s disease? Antioxid. Redox. Signal. 2013, 18, 1296–1302. [Google Scholar] [CrossRef]
- Boll, M.C.; Alcaraz-Zubeldia, M.; Montes, S.; Rios, C. Free copper, ferroxidase and SOD1 activities, lipid peroxidation and NO(x) content in the CSF. A different marker profile in four neurodegenerative diseases. Neurochem. Res. 2008, 33, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Isobe, C.; Murata, T.; Sato, C.; Terayama, Y. Increase of oxidized/total coenzyme Q-10 ratio in cerebrospinal fluid in patients with Parkinson’s disease. J. Clin. Neurosci. 2007, 14, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Liu, M.Y.; Zhong, X.; Wei, M.J. Decreased circulating Zinc levels in Parkinson’s disease: A meta-analysis study. Sci. Rep. 2017, 7, 3902. [Google Scholar] [CrossRef]
- Waragai, M.; Wei, J.; Fujita, M.; Nakai, M.; Ho, G.J.; Masliah, E.; Akatsu, H.; Yamada, T.; Hashimoto, M. Increased level of DJ-1 in the cerebrospinal fluids of sporadic Parkinson’s disease. Biochem. Biophys. Res. Commun. 2006, 345, 967–972. [Google Scholar] [CrossRef]
- Hong, Z.; Shi, M.; Chung, K.A.; Quinn, J.F.; Peskind, E.R.; Galasko, D.; Jankovic, J.; Zabetian, C.P.; Leverenz, J.B.; Baird, G.; et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain 2010, 133, 713–726. [Google Scholar] [CrossRef]
- Herbert, M.K.; Eeftens, J.M.; Aerts, M.B.; Esselink, R.A.; Bloem, B.R.; Kuiperij, H.B.; Verbeek, M.M. CSF levels of DJ-1 and tau distinguish MSA patients from PD patients and controls. Park. Relat. Disord. 2014, 20, 112–115. [Google Scholar] [CrossRef]
- Maarouf, C.L.; Beach, T.G.; Adler, C.H.; Shill, H.A.; Sabbagh, M.N.; Wu, T.; Walker, D.G.; Kokjohn, T.A.; Roher, A.E.; Arizona PD Consortium. Cerebrospinal fluid biomarkers of neuropathologically diagnosed Parkinson’s disease subjects. Neurol. Res. 2012, 34, 669–676. [Google Scholar] [CrossRef]
- Yu, S.Y.; Zuo, L.J.; Wang, F.; Chen, Z.J.; Hu, Y.; Wang, Y.J.; Wang, X.M.; Zhang, W. Potential biomarkers relating pathological proteins, neuroinflammatory factors and free radicals in PD patients with cognitive impairment: A cross-sectional study. BMC Neurol. 2014, 14, 113. [Google Scholar] [CrossRef]
- Forte, G.; Bocca, B.; Senofonte, O.; Petrucci, F.; Brusa, L.; Stanzione, P.; Zannino, S.; Violante, N.; Alimonti, A.; Sancesario, G. Trace and major elements in whole blood, serum, cerebrospinal fluid and urine of patients with Parkinson’s disease. J. Neural. Transm. (Vienna) 2004, 111, 1031–1040. [Google Scholar]
- LeWitt, P.; Schultz, L.; Auinger, P.; Lu, M.; Parkinson Study Group DATATOP Investigators. CSF xanthine, homovanillic acid, and their ratio as biomarkers of Parkinson’s disease. Brain Res. 2011, 1408, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; García-Moreno, J.M.; Martín de Pablos, A.; Chacón, J. May the evaluation of nitrosative stress through selective increase of 3-nitrotyrosine proteins other than nitroalbumin and dominant tyrosine-125/136 nitrosylation of serum α-synuclein serve for diagnosis of sporadic Parkinson’s disease? Antioxid. Redox. Signal. 2013, 19, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Isobe, C.; Murata, T.; Sato, C.; Tohgi, H. Alteration of 8-hydroxyguanosine concentrations in the cerebrospinal fluid and serum from patients with Parkinson’s disease. Neurosci. Lett. 2003, 336, 105–108. [Google Scholar] [CrossRef]
- Crotty, G.F.; Ascherio, A.; Schwarzschild, M.A. Targeting urate to reduce oxidative stress in Parkinson disease. Exp. Neurol. 2017, 298, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sokal, I.; Peskind, E.R.; Quinn, J.F.; Jankovic, J.; Kenney, C.; Chung, K.A.; Millard, S.P.; Nutt, J.G.; Montine, T.J. CSF multianalyte profile distinguishes Alzheimer and Parkinson diseases. Am. J. Clin. Pathol. 2008, 129, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Pisani, V.; Stefani, A.; Pierantozzi, M.; Natoli, S.; Stanzione, P.; Franciotta, D.; Pisani, A. Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson’s disease. J. Neuroinflamm. 2012, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, Y.; Cao, Z.; Liu, Q.; Cheng, Y. Cerebrospinal Fluid Inflammatory Cytokine Aberrations in Alzheimer’s Disease, Parkinson’s Disease and Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Front. Immunol. 2018, 9, 2122. [Google Scholar] [CrossRef]
- Olsson, B.; Constantinescu, R.; Holmberg, B.; Andreasen, N.; Blennow, K.; Zetterberg, H. The glial marker YKL-40 is decreased in synucleinopathies. Mov. Disord. 2013, 28, 1882–1885. [Google Scholar] [CrossRef]
- Jesse, S.; Lehnert, S.; Jahn, O.; Parnetti, L.; Soininen, H.; Herukka, S.K.; Steinacker, P.; Tawfik, S.; Tumani, H.; von Arnim, C.A.; et al. Differential sialylation of serpin A1 in the early diagnosis of Parkinson’s disease dementia. PLoS ONE 2012, 7, e48783. [Google Scholar] [CrossRef]
- Steinacker, P.; Fang, L.; Kuhle, J.; Petzold, A.; Tumani, H.; Ludolph, A.C.; Otto, M.; Brettschneider, J. Soluble beta-amyloid precursor protein is related to disease progression in amyotrophic lateral sclerosis. PLoS ONE 2011, 6, e23600. [Google Scholar] [CrossRef]
- Sako, W.; Murakami, N.; Izumi, Y.; Kaji, R. Reduced alpha-synuclein in cerebrospinal fluid in synucleinopathies: Evidence from a meta-analysis. Mov. Disord. 2014, 29, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Tang, H.; Nie, K.; Wang, L.; Zhao, J.; Gan, R.; Huang, J.; Zhu, R.; Feng, S.; Duan, Z.; et al. Cerebrospinal fluid alpha-synuclein as a biomarker for Parkinson’s disease diagnosis: A systematic review and meta-analysis. Int. J. Neurosci. 2015, 125, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Han, Z.M.; Liu, Q.F.; Tang, W.; Ye, K.; Yao, Y.Y. Use of CSF α-synuclein in the differential diagnosis between Alzheimer’s disease and other neurodegenerative disorders. Int. Psychogeriatr. 2015, 27, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, P.; Giannandrea, D.; Biscetti, L.; Abraha, I.; Chiasserini, D.; Orso, M.; Calabresi, P.; Parnetti, L. Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2017, 32, 1389–1400. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Y.; Gong, D. Changes of cerebrospinal fluid Aβ42, t-tau, and p-tau in Parkinson’s disease patients with cognitive impairment relative to those with normal cognition: A meta-analysis. Neurol. Sci. 2017, 38, 1953–1961. [Google Scholar] [CrossRef]
- Wennström, M.; Surova, Y.; Hall, S.; Nilsson, C.; Minthon, L.; Boström, F.; Hansson, O.; Nielsen, H.M. Low CSF levels of both α-synuclein and the α-synuclein cleaving enzyme neurosin in patients with synucleinopathy. PLoS ONE 2013, 8, e53250. [Google Scholar] [CrossRef]
- Nielsen, H.M.; Hall, S.; Surova, Y.; Nägga, K.; Nilsson, C.; Londos, E.; Minthon, L.; Hansson, O.; Wennström, M. Low levels of soluble NG2 in cerebrospinal fluid from patients with dementia with Lewy bodies. J. Alzheimers. Dis. 2014, 40, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Süssmuth, S.D.; Uttner, I.; Landwehrmeyer, B.; Pinkhardt, E.H.; Brettschneider, J.; Petzold, A.; Kramer, B.; Schulz, J.B.; Palm, C.; Otto, M.; et al. Differential pattern of brain-specific CSF proteins tau and amyloid-β in Parkinsonian syndromes. Mov. Disord. 2010, 25, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Öhrfelt, A.; Constantinescu, R.; Andreasson, U.; Surova, Y.; Bostrom, F.; Nilsson, C.; Håkan, W.; Decraemer, H.; Någga, K.; et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch. Neurol. 2012, 69, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J.; Petzold, A.; Süssmuth, S.D.; Landwehrmeyer, G.B.; Ludolph, A.C.; Kassubek, J.; Tumani, H. Neurofilament heavy-chain NfH(SMI35) in cerebrospinal fluid supports the differential diagnosis of Parkinsonian syndromes. Mov. Disord. 2006, 21, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- Oeckl, P.; Steinacker, P.; von Arnim, C.A.; Straub, S.; Nagl, M.; Feneberg, E.; Weishaupt, J.H.; Ludolph, A.C.; Otto, M. Intact protein analysis of ubiquitin in cerebrospinal fluid by multiple reaction monitoring reveals differences in Alzheimer’s disease and frontotemporal lobar degeneration. J. Proteome. Res. 2014, 13, 4518–4525. [Google Scholar] [CrossRef]
- Mondello, S.; Constantinescu, R.; Zetterberg, H.; Andreasson, U.; Holmberg, B.; Jeromin, A. CSF α-synuclein and UCH-L1 levels in Parkinson’s disease and atypical parkinsonian disorders. Park. Relat. Disord. 2014, 20, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.; Brønnick, K.; Aarsland, D.; Blennow, K.; Zetterberg, H.; Ballard, C.; Kurz, M.W.; Andreasson, U.; Tysnes, O.B.; Larsen, J.P.; et al. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson’s disease: The Norwegian ParkWest study. J. Neurol. Neurosurg. Psychiatry. 2010, 81, 1080–1086. [Google Scholar] [CrossRef]
- Hoshi, K.; Matsumoto, Y.; Ito, H.; Saito, K.; Honda, T.; Yamaguchi, Y.; Hashimoto, Y. A unique glycan-isoform of transferrin in cerebrospinal fluid: A potential diagnostic marker for neurological diseases. Biochim. Biophys. Acta. Gen. Subj. 2017, 1861, 2473–2478. [Google Scholar] [CrossRef] [PubMed]
- Abdo, W.F.; De Jong, D.; Hendriks, J.C.; Horstink, M.W.; Kremer, B.P.; Bloem, B.R.; Verbeek, M.M. Cerebrospinal fluid analysis differentiates multiple system atrophy from Parkinson’s disease. Mov. Disord. 2004, 19, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Parnetti, L.; Chiasserini, D.; Persichetti, E.; Eusebi, P.; Varghese, S.; Qureshi, M.M.; Dardis, A.; Deganuto, M.; De Carlo, C.; Castrioto, A.; et al. Cerebrospinal fluid lysosomal enzymes and alpha-synuclein in Parkinson’s disease. Mov. Disord. 2014, 29, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Trupp, M.; Jonsson, P.; Ohrfelt, A.; Zetterberg, H.; Obudulu, O.; Malm, L.; Wuolikainen, A.; Linder, J.; Moritz, T.; Blennow, K.; et al. Metabolite and peptide levels in plasma and CSF differentiating healthy controls from patients with newly diagnosed Parkinson’s disease. J. Park. Dis. 2014, 4, 549–560. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Molina, J.A.; Vargas, C.; Gómez, P.; De Bustos, F.; Zurdo, M.; Gómez-Escalonilla, C.; Barcenilla, B.; Berbel, A.; Camacho, A.; et al. Normal cerebrospinal fluid levels of insulin in patients with Parkinson’s disease. J. Neural. Transm. (Vienna) 2000, 107, 445–449. [Google Scholar] [CrossRef]
- Van den Berg, M.M.J.; Krauskopf, J.; Ramaekers, J.G.; Kleinjans, J.C.S.; Prickaerts, J.; Briedé, J.J. Circulating microRNAs as potential biomarkers for psychiatric and neurodegenerative disorders. Prog. Neurobiol. 2020, 185, 101732. [Google Scholar] [CrossRef]
- Schulz, J.; Takousis, P.; Wohlers, I.; Itua, I.O.G.; Dobricic, V.; Rücker, G.; Binder, H.; Middleton, L.; Ioannidis, J.P.A.; Perneczky, R.; et al. Meta-analyses identify differentially expressed micrornas in Parkinson’s disease. Ann. Neurol. 2019, 85, 835–851. [Google Scholar] [CrossRef]
- Gui, Y.; Liu, H.; Zhang, L.; Lv, W.; Hu, X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 2015, 6, 37043–37053. [Google Scholar] [CrossRef] [PubMed]
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosome Determinants of Physiological Aging and Age-Related Neurodegenerative Diseases. Front. Aging. Neurosci. 2019, 11, 232. [Google Scholar] [CrossRef]
- Nagatsu, T. Prolyl oligopeptidase and dipeptidyl peptidase II/dipeptidyl peptidase IV ratio in the cerebrospinal fluid in Parkinson’s disease: Historical overview and future prospects. J. Neural. Transm. (Vienna) 2017, 124, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Inoue, Y.; Kanbayashi, T.; Nomura, T.; Kusumi, M.; Nakashima, K. CSF orexin levels of Parkinson’s disease, dementia with Lewy bodies, progressive supranuclear palsy and corticobasal degeneration. J. Neurol. Sci. 2006, 250, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Takeda, A.; Onodera, H.; Kimpara, T.; Hisanaga, K.; Sato, N.; Nunomura, A.; Castellani, R.J.; Perry, G.; Smith, M.A.; et al. Systemic increase of oxidative nucleic acid damage in Parkinson’s disease and multiple system atrophy. Neurobiol. Dis. 2002, 9, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Bradner, J.; Hancock, A.M.; Chung, K.A.; Quinn, J.F.; Peskind, E.R.; Galasko, D.; Jankovic, J.; Zabetian, C.P.; Kim, H.M.; et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann. Neurol. 2011, 69, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Sako, W.; Murakami, N.; Izumi, Y.; Kaji, R. Neurofilament light chain level in cerebrospinal fluid can differentiate Parkinson’s disease from atypical parkinsonism: Evidence from a meta-analysis. J. Neurol. Sci. 2015, 352, 84–87. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Y.; Gong, D. Cerebrospinal fluid levels of neurofilament light chain in multiple system atrophy relative to Parkinson’s disease: A meta-analysis. Neurol. Sci. 2017, 38, 407–414. [Google Scholar] [CrossRef]
- Ge, F.; Ding, J.; Liu, Y.; Lin, H.; Chang, T. Cerebrospinal fluid NFL in the differential diagnosis of parkinsonian disorders: A meta-analysis. Neurosci. Lett. 2018, 685, 35–41. [Google Scholar] [CrossRef]
- Bridel, C.; van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; the NFL Group; Alvarez-Cermeño, J.C.; Andreasson, U.; Axelsson, M.; Bäckström, D.C.; et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 1035–1048. [Google Scholar] [CrossRef]
- Leverenz, J.B.; Watson, G.S.; Shofer, J.; Zabetian, C.P.; Zhang, J.; Montine, T.J. Cerebrospinal fluid biomarkers and cognitive performance in non-demented patients with Parkinson’s disease. Park. Relat. Disord. 2011, 17, 61–64. [Google Scholar] [CrossRef]
- Bäckström, D.C.; Eriksson Domellöf, M.; Linder, J.; Olsson, B.; Öhrfelt, A.; Trupp, M.; Zetterberg, H.; Blennow, K.; Forsgren, L. Cerebrospinal Fluid Patterns and the Risk of Future Dementia in Early, Incident Parkinson Disease. JAMA Neurol. 2015, 72, 1175–1182. [Google Scholar] [CrossRef]
- Maetzler, W.; Stoycheva, V.; Schmid, B.; Schulte, C.; Hauser, A.K.; Brockmann, K.; Melms, A.; Gasser, T.; Berg, D. Neprilysin activity in cerebrospinal fluid is associated with dementia and amyloid-β42 levels in Lewy body disease. J. Alzheimers Dis. 2010, 22, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; El-Agnaf, O.M.; Marcus, K.; Trenkwalder, C.; Schlossmacher, M.G. Quantification of α-synuclein in cerebrospinal fluid as a biomarker candidate: Review of the literature and considerations for future studies. Biomark. Med. 2010, 4, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; Caspell-Garcia, C.J.; Coffey, C.S.; Taylor, P.; Singleton, A.; Shaw, L.M.; Trojanowski, J.Q.; Frasier, M.; Simuni, T.; Iranzo, A.; et al. Longitudinal analyses of cerebrospinal fluid α-Synuclein in prodromal and early Parkinson’s disease. Mov. Disord. 2019, 34, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Sawada, J.; Kikuchi-Takeguchi, S.; Kano, K.; Takahashi, K.; Saito, T.; Okizaki, A.; Hasebe, N. Cerebrospinal fluid levels of alpha-synuclein, amyloid β, tau, phosphorylated tau, and neuron-specific enolase in patients with Parkinson’s disease, dementia with Lewy bodies or other neurological disorders: Their relationships with cognition and nuclear medicine imaging findings. Neurosci. Lett. 2020, 715, 134564. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; Batrla, R.; El-Agnaf, O.; Galasko, D.R.; Lashuel, H.A.; Merchant, K.M.; Shaw, L.M.; Selkoe, D.J.; Umek, R.; Vanderstichele, H.; et al. A user’s guide for α-synuclein biomarker studies in biological fluids: Perianalytical considerations. Mov. Disord. 2017, 32, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).