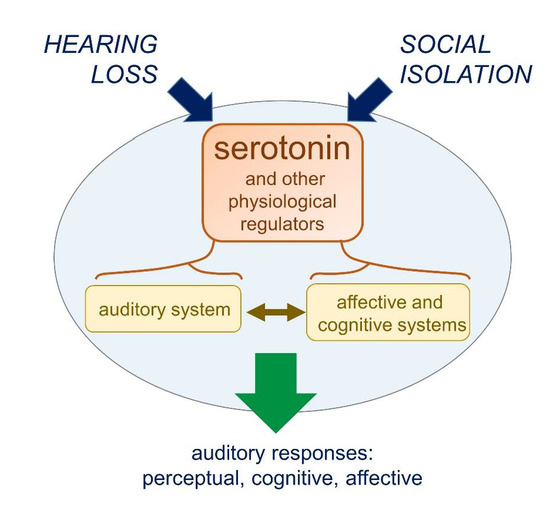
Silence, Solitude, and Serotonin: Neural Mechanisms Linking Hearing Loss and Social Isolation
Abstract
1. Social Isolation Can Occur with Hearing Loss
2. Defining Serotonin–Auditory Interactions
3. Plasticity in the Serotonergic System
4. Hearing Loss and the Serotonergic System
5. Social Isolation and the Auditory System
6. Social Isolation Influences Serotonin in the Auditory System
7. Hearing Loss and Social Isolation have Extra-Auditory Effects
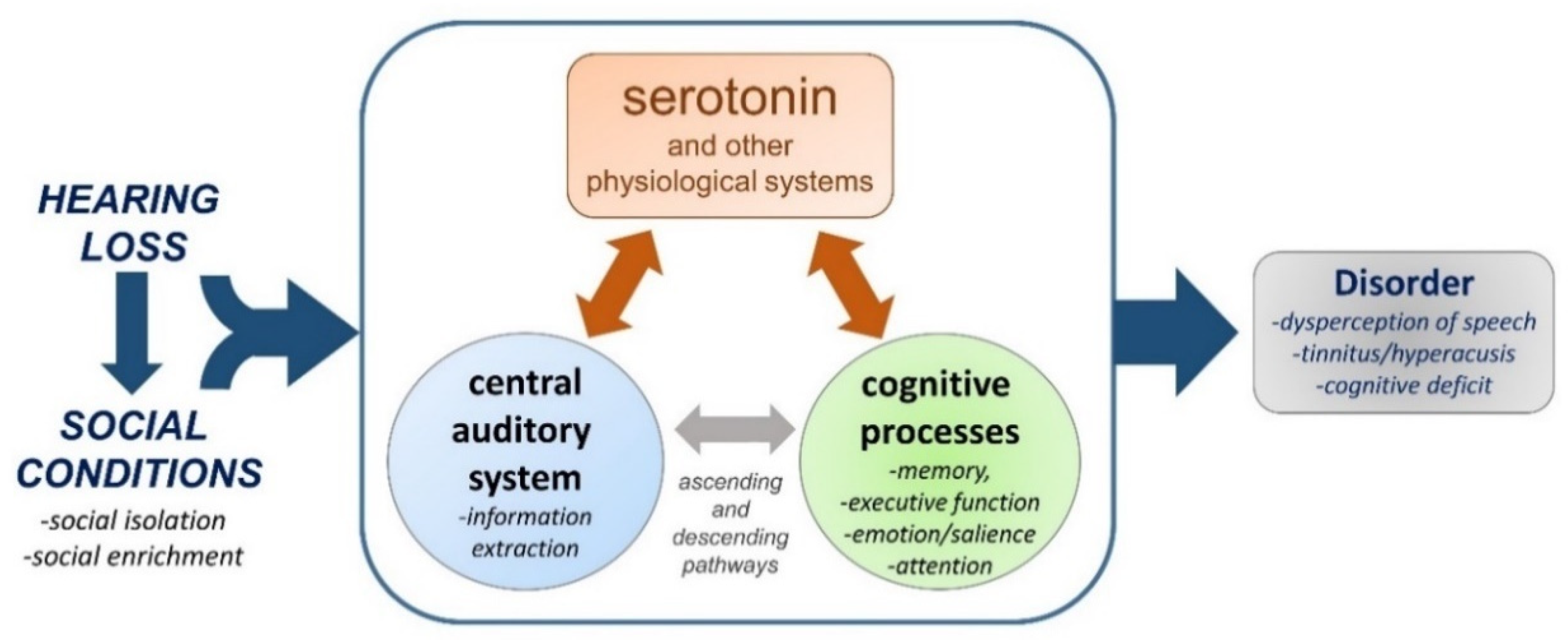
8. Do Hearing Loss and Social Isolation Physiologically Converge?
9. Concluding Thoughts
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gilmartin, H.M.; Grota, P.G.; Sousa, K. Isolation: A concept analysis. Nurs. Forum. 2013, 48, 54–60. [Google Scholar] [CrossRef]
- Strawbridge, W.J.; Wallhagen, M.I.; Shema, S.J.; Kaplan, G.A. Negative consequences of hearing impairment in old age: A longitudinal analysis. Gerontologist 2000, 40, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Vas, V.; Akeroyd, M.A.; Hall, D.A. A data-driven synthesis of research evidence for domains of hearing loss, as reported by adults with hearing loss and their communication partners. Trends Hear. 2017, 21, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Harper, M.; Pedersen, E.; Goman, A.; Suen, J.J.; Price, C.; Applebaum, J.; Hoyer, M.; Lin, F.R.; Reed, N.S. Hearing Loss, Loneliness, and Social Isolation: A Systematic Review. Otolaryngol. Head Neck Surg. 2020, 162, 622–633. [Google Scholar] [CrossRef]
- Arlinger, S. Negative consequences of uncorrected hearing loss—A review. Int. J. Audiol. 2003, 42, 17–20. [Google Scholar] [CrossRef]
- Mick, P.; Parfyonov, M.; Wittich, W.; Phillips, N.; Guthrie, D.; Kathleen Pichora-Fuller, M. Associations between sensory loss and social networks, participation, support, and loneliness: Analysis of the Canadian Longitudinal Study on Aging. Can. Fam. Physician 2018, 64, e33–e41. [Google Scholar]
- Ramage-Morin, P.L. Hearing difficulties and feelings of social isolation among Canadians aged 45 or older. Health Rep. 2016, 27, 3–12. [Google Scholar]
- Pronk, M.; Deeg, D.J.H.; Smits, C.; Twisk, J.W.; van Tilburg, T.G.; Festen, J.M.; Kramer, S.E. Hearing loss in older persons: Does the rate of decline affect psychosocial health? J. Aging Health 2014, 26, 703–723. [Google Scholar] [CrossRef]
- Stam, M.; Smit, J.H.; Twisk, J.W.R.; Lemke, U.; Smits, C.; Festen, J.M.; Kramer, S.E. Change in psychosocial health status over 5 years in relation to adults’ hearing ability in noise. Ear Hear. 2016, 37, 680–689. [Google Scholar] [CrossRef]
- Choi, J.S.; Betz, J.; Li, L.; Blake, C.R.; Sung, Y.K.; Contrera, K.J.; Lin, F.R. Association of using hearing aids or cochlear implants with changes in depressive symptoms in older adults. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 652. [Google Scholar] [CrossRef]
- Mick, P.; Pichora-Fuller, M.K. Is hearing loss associated with poorer health in older adults who might benefit from hearing screening? Ear Hear. 2016, 37, e194–e201. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Li, L.; Blake, C.; Betz, J.; Lin, F.R. Association of hearing loss and loneliness in older adults. J. Aging Health 2016, 28, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Contrera, K.J.; Sung, Y.K.; Betz, J.; Li, L.; Lin, F.R. Change in loneliness after intervention with cochlear implants or hearing aids: Loneliness after hearing loss treatment. Laryngoscope 2017, 127, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Hay-McCutcheon, M.J.; Reed, P.E.; Cheimariou, S. Positive social interaction and hearing loss in older adults living in rural and urban communities. J. Speech Lang. Hear. Res. 2018, 61, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, N.R. A review of social isolation: An important but underassessed condition in older adults. J. Prim. Prev. 2012, 33, 137–152. [Google Scholar] [CrossRef]
- Tambs, K. Moderate effects of hearing loss on mental health and subjective well-being: Results from the Nord-Trøndelag hearing loss study. Psychosom. Med. 2004, 66, 776–782. [Google Scholar] [CrossRef]
- Dawes, P.; Emsley, R.; Cruickshanks, K.J.; Moore, D.R.; Fortnum, H.; Edmondson-Jones, M.; McCormack, A.; Munro, K.J. Hearing loss and cognition: The role of hearing aids, social isolation and depression. PLoS ONE 2015, 10, e0119616. [Google Scholar] [CrossRef]
- Simning, A.; Fox, M.L.; Barnett, S.L.; Sorensen, S.; Conwell, Y. Depressive and anxiety symptoms in older adults with auditory, vision, and dual sensory impairment. J. Aging Health 2019, 31, 1353–1375. [Google Scholar] [CrossRef]
- Syka, J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol. Rev. 2002, 82, 601–636. [Google Scholar] [CrossRef]
- Mulders, W.H.A.M.; Robertson, D. Progressive centralization of midbrain hyperactivity after acoustic trauma. Neuroscience 2011, 192, 753–760. [Google Scholar] [CrossRef]
- Mulders, W.H.A.M.; Robertson, D. Development of hyperactivity after acoustic trauma in the guinea pig inferior colliculus. Hear. Res. 2013, 298, 104–108. [Google Scholar] [CrossRef]
- Lesicko, A.M.H.; Hristova, T.S.; Maigler, K.C.; Llano, D.A. Connectional modularity of top-down and bottom-up multimodal inputs to the lateral cortex of the mouse inferior colliculus. J. Neurosci. 2016, 36, 11037–11050. [Google Scholar] [CrossRef]
- Eggermont, J.J. Acquired hearing loss and brain plasticity. Hear. Res. 2017, 343, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Teichert, M.; Liebmann, L.; Hübner, C.A.; Bolz, J. Homeostatic plasticity and synaptic scaling in the adult mouse auditory cortex. Sci. Rep. 2017, 7, 17423. [Google Scholar] [CrossRef] [PubMed]
- Balaram, P.; Hackett, T.A.; Polley, D.B. Synergistic transcriptional changes in ampa and gabaa receptor genes support compensatory plasticity following unilateral hearing loss. Neuroscience 2019, 407, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Rodger, J.; Mulders, W.H.A.M.; Robertson, D. Tonotopic changes in GABA receptor expression in guinea pig inferior colliculus after partial unilateral hearing loss. Brain Res. 2010, 1342, 24–32. [Google Scholar] [CrossRef]
- Manohar, S.; Ramchander, P.V.; Salvi, R.; Seigel, G.M. Synaptic reorganization response in the cochlear nucleus following intense noise exposure. Neuroscience 2019, 399, 184–198. [Google Scholar] [CrossRef]
- Qiu, C.; Salvi, R.; Ding, D.; Burkard, R. Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: Evidence for increased system gain. Hear. Res. 2000, 139, 153–171. [Google Scholar] [CrossRef]
- Chambers, A.R.; Salazar, J.J.; Polley, D.B. Persistent thalamic sound processing despite profound cochlear denervation. Front. Neural Circuits 2016, 10. [Google Scholar] [CrossRef]
- Schrode, K.M.; Muniak, M.A.; Kim, Y.-H.; Lauer, A.M. Central compensation in auditory brainstem after damaging noise exposure. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Xu, H.; Kotak, V.C.; Sanes, D.H. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J. Neurosci. 2007, 27, 9417–9426. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, M.; Beitel, R.E. Behavioral training restores temporal processing in auditory cortex of long-deaf cats. J. Neurophysiol. 2011, 106, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, S.-H.; Peng, K.; Liao, X.-M. Long-term impairment of sound processing in the auditory midbrain by daily short-term exposure to moderate noise. Neural Plast. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Green, D.B.; Mattingly, M.M.; Ye, Y.; Gay, J.D.; Rosen, M.J. Brief stimulus exposure fully remediates temporal processing deficits induced by early hearing loss. J. Neurosci. 2017, 37, 7759–7771. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.R. The role of neural plasticity in tinnitus. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2007; Volume 166, pp. 37–544. ISBN 978-0-444-53167-4. [Google Scholar]
- Noreña, A.J. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci. Biobehav. Rev. 2011, 35, 1089–1109. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Weiner, B.D.; Zhang, L.S.; Cho, S.-J.; Bao, S. Homeostatic plasticity drives tinnitus perception in an animal model. Proc. Natl. Acad. Sci. USA 2011, 108, 14974–14979. [Google Scholar] [CrossRef]
- Wang, H.; Brozoski, T.J.; Caspary, D.M. Inhibitory neurotransmission in animal models of tinnitus: Maladaptive plasticity. Hear. Res. 2011, 279, 111–117. [Google Scholar] [CrossRef]
- Richardson, B.D.; Brozoski, T.J.; Ling, L.L.; Caspary, D.M. Targeting inhibitory neurotransmission in tinnitus. Brain Res. 2012, 1485, 77–87. [Google Scholar] [CrossRef]
- Gold, J.R.; Bajo, V.M. Insult-induced adaptive plasticity of the auditory system. Front. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Auerbach, B.D.; Rodrigues, P.V.; Salvi, R.J. Central gain control in tinnitus and hyperacusis. Front. Neurol. 2014, 5. [Google Scholar] [CrossRef]
- Sardone, R.; Battista, P.; Panza, F.; Lozupone, M.; Griseta, C.; Castellana, F.; Capozzo, R.; Ruccia, M.; Resta, E.; Seripa, D.; et al. The age-related central auditory processing disorder: Silent impairment of the cognitive ear. Front. Neurosci. 2019, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- Cousillas, H.; George, I.; Mathelier, M.; Richard, J.-P.; Henry, L.; Hausberger, M. Social experience influences the development of a central auditory area. Naturwissenschaften 2006, 93, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Cousillas, H.; George, I.; Henry, L.; Richard, J.-P.; Hausberger, M. Linking social and vocal brains: Could social segregation prevent a proper development of a central auditory area in a female songbird? PLoS ONE 2008, 3, e2194. [Google Scholar] [CrossRef] [PubMed]
- George, I.; Alcaix, S.; Henry, L.; Richard, J.-P.; Cousillas, H.; Hausberger, M. Neural correlates of experience-induced deficits in learned vocal communication. PLoS ONE 2010, 5, e14347. [Google Scholar] [CrossRef]
- Yanagihara, S.; Yazaki-Sugiyama, Y. Auditory experience-dependent cortical circuit shaping for memory formation in bird song learning. Nat. Commun. 2016, 7, 11946. [Google Scholar] [CrossRef]
- Maul, K.K.; Voss, H.U.; Parra, L.C.; Salgado-Commissariat, D.; Ballon, D.; Tchernichovski, O.; Helekar, S.A. The development of stimulus-specific auditory responses requires song exposure in male but not female zebra finches. Dev. Neurobiol. 2010, 70, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Njegovan, M.; Weisman, R. Pitch discrimination in field- and isolation-reared black-capped chickadees (Parus atricapillus). J. Comp. Psychol. 1997, 111, 294–301. [Google Scholar] [CrossRef]
- Sturdy, C.B.; Phillmore, L.S.; Sartor, J.J.; Weisman, R.G. Reduced social contact causes auditory perceptual deficits in zebra finches. Taeniopygia Guttata Anim. Behav. 2001, 62, 1207–1218. [Google Scholar] [CrossRef]
- Weisman, R.G.; Njegovan, M.G.; Williams, M.T.; Cohen, J.S.; Sturdy, C.B. A behavior analysis of absolute pitch: Sex, experience, and species. Behav. Process. 2004, 66, 289–307. [Google Scholar] [CrossRef]
- Kuhl, P.K.; Tsao, F.-M.; Liu, H.-M. Foreign-language experience in infancy: Effects of short-term exposure and social interaction on phonetic learning. Proc. Natl. Acad. Sci. USA 2003, 100, 9096–9101. [Google Scholar] [CrossRef]
- Schofield, B.R.; Hurley, L. Circuits for Modulation of Auditory Function. In The Mammalian Auditory Pathways; Oliver, D.L., Cant, N.B., Fay, R.R., Popper, A.N., Eds.; Springer Handbook of Auditory Research; Springer International Publishing: Cham, Switzerland, 2018; Volume 65, pp. 235–267. ISBN 978-3-319-71796-8. [Google Scholar]
- Mizutani, H.; Hori, T.; Takahashi, T. 5-HT1B receptor-mediated presynaptic inhibition at the calyx of Held of immature rats. Eur. J. Neurosci. 2006, 24, 1946–1954. [Google Scholar] [CrossRef]
- Leão, R.M.; Von Gersdorff, H. Noradrenaline increases high-frequency firing at the calyx of held synapse during development by inhibiting glutamate release. J. Neurophysiol. 2002, 87, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.W.; Rasband, M.N.; Meseguer, V.; Kramer, R.H.; Golding, N.L. Serotonin modulates spike probability in the axon initial segment through HCN channels. Nat. Neurosci. 2016, 19, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-Q.; Trussell, L.O. Serotonergic modulation of sensory representation in a central multisensory circuit is pathway specific. Cell Rep. 2017, 20, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.M.; Sullivan, M.R. From behavioral context to receptors: Serotonergic modulatory pathways in the IC. Front. Neural Circuits 2012, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Pawlisch, B.A.; Macedo-Lima, M.; Remage-Healey, L. Norepinephrine enhances song responsiveness and encoding in the auditory forebrain of male zebra finches. J. Neurophysiol. 2018, 119, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.M. Activation of the serotonin 1A receptor alters the temporal characteristics of auditory responses in the inferior colliculus. Brain Res. 2007, 1181, 21–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoyt, J.M.; Perkel, D.J.; Portfors, C.V. Dopamine acts via D2-like receptors to modulate auditory responses in the inferior colliculus. eNeuro 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-Q.; Trussell, L.O. Serotonergic regulation of excitability of principal cells of the dorsal cochlear nucleus. J. Neurosci. 2015, 35, 4540–4551. [Google Scholar] [CrossRef]
- Hall, I.C.; Sell, G.L.; Hurley, L.M. Social regulation of serotonin in the auditory midbrain. Behav. Neurosci. 2011, 125, 501–511. [Google Scholar] [CrossRef]
- Keesom, S.M.; Hurley, L.M. Socially induced serotonergic fluctuations in the male auditory midbrain correlate with female behavior during courtship. J. Neurophysiol. 2016, 115, 1786–1796. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L. Neuromodulatory Feedback to the Inferior Colliculus. In The Oxford Handbook of the Auditory Brainstem; Kandler, K., Ed.; Oxford University Press: Oxford, UK, 2019; pp. 576–610. ISBN 978-0-19-084906-1. [Google Scholar]
- Fone, K.C.F.; Porkess, M.V. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2008, 32, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Lukkes, J.L.; Watt, M.J.; Lowry, C.A.; Forster, G.L. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front. Behav. Neurosci. 2009, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.R.; McCormick, C.M.; Pellis, S.M.; Lukkes, J.L. Impact of adolescent social experiences on behavior and neural circuits implicated in mental illnesses. Neurosci. Biobehav. Rev. 2017, 76, 280–300. [Google Scholar] [CrossRef]
- Klepper, A.; Herbert, H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res. 1991, 557, 190–201. [Google Scholar] [CrossRef]
- Thompson, A.M.; Schofield, B.R. Afferent projections of the superior olivary complex. Microsc. Res. Tech. 2000, 51, 330–354. [Google Scholar] [CrossRef]
- Hurley, L.M.; Thompson, A.M. Serotonergic innervation of the auditory brainstem of the Mexican free-tailed bat, Tadarida Brasiliensis. J. Comp. Neurol. 2001, 435, 78–88. [Google Scholar] [CrossRef]
- Kiser, D.; SteemerS, B.; Branchi, I.; Homberg, J.R. The reciprocal interaction between serotonin and social behaviour. Neurosci. Biobehav. Rev. 2012, 36, 786–798. [Google Scholar] [CrossRef]
- Schaefer, A.; Burmann, I.; Regenthal, R.; Arélin, K.; Barth, C.; Pampel, A.; Villringer, A.; Margulies, D.S.; Sacher, J. Serotonergic modulation of intrinsic functional connectivity. Curr. Biol. 2014, 24, 2314–2318. [Google Scholar] [CrossRef]
- Fakhoury, M. Revisiting the serotonin hypothesis: Implications for major depressive disorders. Mol. Neurobiol. 2016, 53, 2778–2786. [Google Scholar] [CrossRef]
- Jacob, S.N.; Nienborg, H. Monoaminergic neuromodulation of sensory processing. Front. Neural Circuits 2018, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T. The role of the serotonergic system in motor control. Neurosci. Res. 2018, 129, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Conio, B.; Martino, M.; Magioncalda, P.; Escelsior, A.; Inglese, M.; Amore, M.; Northoff, G. Opposite effects of dopamine and serotonin on resting-state networks: Review and implications for psychiatric disorders. Mol. Psychiatry 2020, 25, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Harris-Warrick, R.M. Neuromodulation and flexibility in central pattern generator networks. Curr. Opin. Neurobiol. 2011, 21, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Marder, E. Neuromodulation of neuronal circuits: Back to the future. Neuron 2012, 76, 1–11. [Google Scholar] [CrossRef]
- Nusbaum, M.P.; Blitz, D.M.; Marder, E. Functional consequences of neuropeptide and small-molecule co-transmission. Nat. Rev. Neurosci. 2017, 18, 389–403. [Google Scholar] [CrossRef]
- DeFelipe, J.; Hendry, S.H.C.; Hashikawa, T.; Jones, E.G. Synaptic relationships of serotonin-inmmunoreactive terminal baskets on GABA neurons in the cat auditory cortex. Cereb. Cortex 1991, 1, 117–133. [Google Scholar] [CrossRef]
- Niederkofler, V.; Asher, T.E.; Okaty, B.W.; Rood, B.D.; Narayan, A.; Hwa, L.S.; Beck, S.G.; Miczek, K.A.; Dymecki, S.M. Identification of serotonergic neuronal modules that affect aggressive behavior. Cell Rep. 2016, 17, 1934–1949. [Google Scholar] [CrossRef]
- Petersen, C.L.; Koo, A.; Patel, B.; Hurley, L.M. Serotonergic innervation of the auditory midbrain: Dorsal raphe subregions differentially project to the auditory midbrain in male and female mice. Brain Struct. Funct. 2020, in press. [Google Scholar]
- Thompson, G.C.; Thompson, A.M.; Garrett, K.M.; Britton, B.H. Serotonin and serotonin receptors in the central auditory system. Otolaryngol. Head Neck Surg. 1994, 110, 93–102. [Google Scholar] [CrossRef]
- Peruzzi, D.; Dut, A. GABA, serotonin and serotonin receptors in the rat inferior colliculus. Brain Res. 2004, 998, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.R.; Kwon, J.H.; Navarro, M.; Hurley, L.M. Acoustic trauma triggers upregulation of serotonin receptor genes. Hear. Res. 2014, 315, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Tadros, S.F.; D’Souza, M.; Zettel, M.L.; Zhu, X.; Lynch-Erhardt, M.; Frisina, R.D. Serotonin 2B receptor: Upregulated with age and hearing loss in mouse auditory system. Neurobiol. Aging 2007, 28, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.G.; Asako, M.; Lomax, C.A.; MacDonald, J.W.; Tong, L.; Lomax, M.I.; Altschuler, R.A. Deafness-related plasticity in the inferior colliculus: Gene expression profiling following removal of peripheral activity: Gene expression in the IC following deafness. J. Neurochem. 2005, 93, 1069–1086. [Google Scholar] [CrossRef]
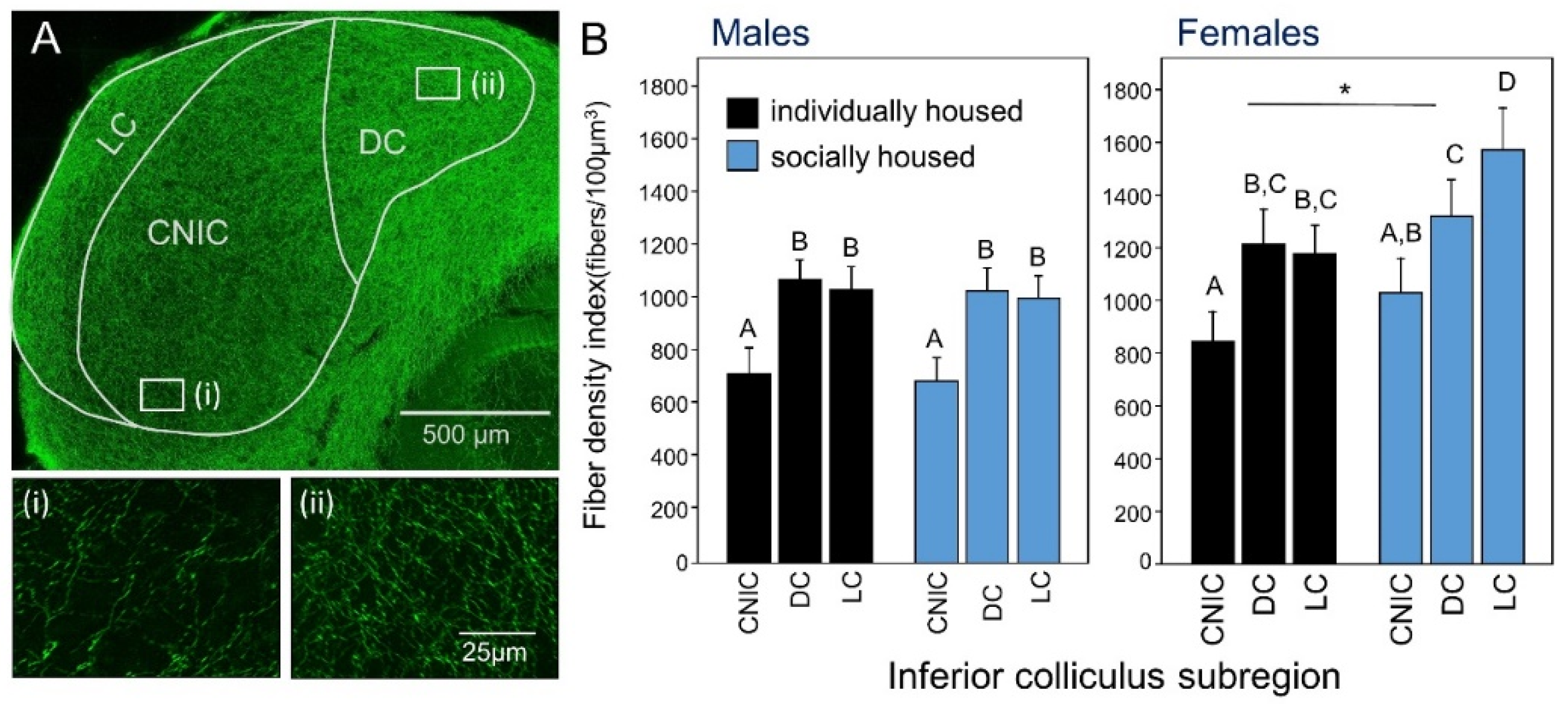
- Keesom, S.M.; Morningstar, M.D.; Sandlain, R.; Wise, B.M.; Hurley, L.M. Social isolation reduces serotonergic fiber density in the inferior colliculus of female, but not male, mice. Brain Res. 2018, 1694, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.C.; Rebec, G.V.; Hurley, L.M. Serotonin in the inferior colliculus fluctuates with behavioral state and environmental stimuli. J. Exp. Biol. 2010, 213, 1009–1017. [Google Scholar] [CrossRef][Green Version]
- Cransac, H.; Cottet-Emard, J.-M.; Hellström, S.; Peyrin, L. Specific sound-induced noradrenergic and serotonergic activation in central auditory structures. Hear. Res. 1998, 118, 151–156. [Google Scholar] [CrossRef]
- Hall, I.C.; Sell, G.L.; Chester, E.M.; Hurley, L.M. Stress-evoked increases in serotonin in the auditory midbrain do not directly result from elevations in serum corticosterone. Behav. Brain Res. 2012, 226, 41–49. [Google Scholar] [CrossRef] [PubMed]
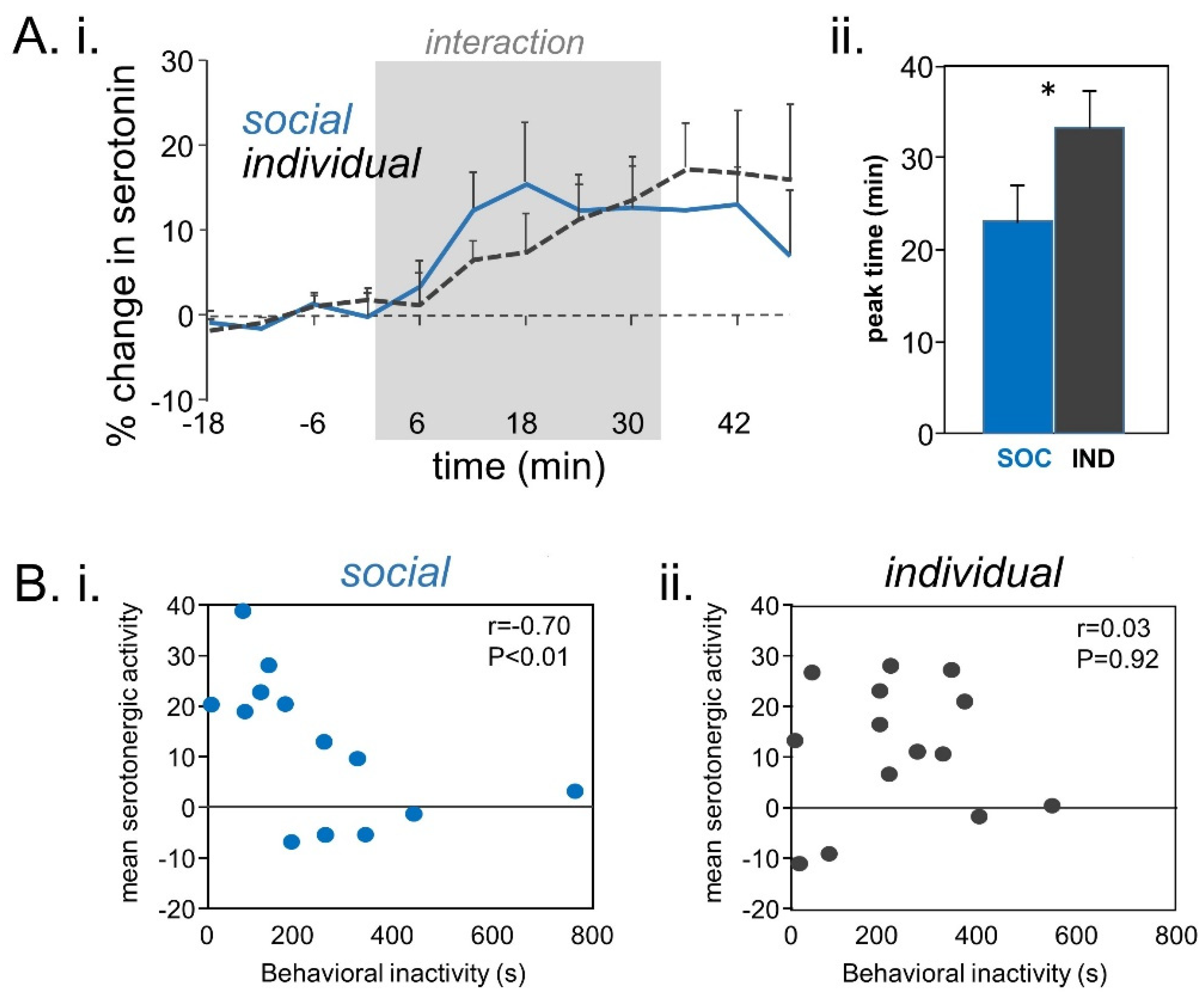
- Hanson, J.L.; Hurley, L.M. Context-dependent fluctuation of serotonin in the auditory midbrain: The influence of sex, reproductive state and experience. J. Exp. Biol. 2014, 217, 526–535. [Google Scholar] [CrossRef]
- Wang, H.-T.; Luo, B.; Huang, Y.-N.; Zhou, K.-Q.; Chen, L. Sodium salicylate suppresses serotonin-induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hear. Res. 2008, 236, 42–51. [Google Scholar] [CrossRef]
- Hanson, J.L.; Hurley, L.M. Serotonin, estrus, and social context influence c-Fos immunoreactivity in the inferior colliculus. Behav. Neurosci. 2016, 130, 600–613. [Google Scholar] [CrossRef]
- Papesh, M.A.; Hurley, L.M. Modulation of auditory brainstem responses by serotonin and specific serotonin receptors. Hear. Res. 2016, 332, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Gopal, K.V.; Bishop, C.E.; Carney, L. Auditory measures in clinically depressed individuals. II. Auditory evoked potentials and behavioral speech tests. Int. J. Audiol. 2004, 43, 499–505. [Google Scholar] [CrossRef]
- Gopal, K.V.; Briley, K.A.; Goodale, E.S.; Hendea, O.M. Selective serotonin reuptake inhibitors treatment effects on auditory measures in depressed female subjects. Eur. J. Pharmacol. 2005, 520, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Juckel, G. Serotonin: From sensory processing to schizophrenia using an electrophysiological method. Behav. Brain Res. 2015, 277, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Selinger, L.; Zarnowiec, K.; Via, M.; Clemente, I.C.; Escera, C. Involvement of the serotonin transporter gene in accurate subcortical speech encoding. J. Neurosci. 2016, 36, 10782–10790. [Google Scholar] [CrossRef]
- Cruz, O.L.M.; Kasse, C.A.; Sanchez, M.; Barbosa, F.; Barros, F.A. Serotonin reuptake inhibitors in auditory processing disorders in elderly patients: Preliminary results. Laryngoscope 2004, 114, 1656–1659. [Google Scholar] [CrossRef]
- Polanski, J.F.; Soares, A.D.; Pereira, L.D.; Laercio de Mendonça Cruz, O. The effect of citalopram versus a placebo on central auditory processing in the elderly. Otol. Neurotol. 2017, 38, 1233–1239. [Google Scholar] [CrossRef]
- Simpson, J.J.; Davies, W.E. A review of evidence in support of a role for 5-HT in the perception of tinnitus. Hear. Res. 2000, 145, 1–7. [Google Scholar] [CrossRef]
- Robinson, S.K.; Viirre, E.S.; Stein, M.B. Antidepressant therapy in tinnitus. Hear. Res. 2007, 226, 221–231. [Google Scholar] [CrossRef]
- Baldo, P.; Doree, C.; Lazzarini, R.; Molin, P.; McFerran, D. Antidepressants for patients with tinnitus. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2006; p. CD003853. [Google Scholar]
- Vicente-Torres, M.A.; Dávila, D.; Bartolomé, M.V.; Carricondo, F.; Gil-Loyzaga, P. Biochemical evidence for the presence of serotonin transporters in the rat cochlea. Hear. Res. 2003, 182, 43–47. [Google Scholar] [CrossRef]
- Felix, R.A.; Elde, C.J.; Nevue, A.A.; Portfors, C.V. Serotonin modulates response properties of neurons in the dorsal cochlear nucleus of the mouse. Hear. Res. 2017, 344, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ebert, U.; Ostwald, J. Serotonin modulates auditory information processing in the cochlear nucleus of the rat. Neurosci. Lett. 1992, 145, 51–54. [Google Scholar] [CrossRef]
- Fitzgerald, K.K.; Sanes, D.H. Serotonergic modulation of synapses in the developing gerbil lateral superior olive. J. Neurophysiol. 1999, 81, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Thompson, G.C. Serotonin-immunoreactive neurons in the postnatal MAO-A KO mouse lateral superior olive project to the inferior colliculus. Neurosci. Lett. 2009, 460, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Baldan Ramsey, L.C.; Sinha, S.R.; Hurley, L.M. 5-HT1A and 5-HT1B receptors differentially modulate rate and timing of auditory responses in the mouse inferior colliculus. Eur. J. Neurosci. 2010, 32, 368–379. [Google Scholar] [CrossRef]
- Bohorquez, A.; Hurley, L.M. Activation of serotonin 3 receptors changes in vivo auditory responses in the mouse inferior colliculus. Hear. Res. 2009, 251, 29–38. [Google Scholar] [CrossRef][Green Version]
- Miko, I.J.; Sanes, D.H. Transient gain adjustment in the inferior colliculus is serotonin- and calcium-dependent. Hear. Res. 2009, 251, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.C.; McCormick, D.A. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature 1989, 340, 715–718. [Google Scholar] [CrossRef]
- Cervantes-Ramírez, V.; Canto-Bustos, M.; Aguilar-Magaña, D.; Pérez-Padilla, E.A.; Góngora-Alfaro, J.L.; Pineda, J.C.; Atzori, M.; Salgado, H. Citalopram reduces glutamatergic synaptic transmission in the auditory cortex via activation of 5-HT1A receptors. Neuroreport 2019, 30, 1316–1322. [Google Scholar] [CrossRef]
- García-Oscos, F.; Torres-Ramírez, O.; Dinh, L.; Galindo-Charles, L.; Pérez Padilla, E.A.; Pineda, J.C.; Atzori, M.; Salgado, H. Activation of 5-HT receptors inhibits GABAergic transmission by pre-and post-synaptic mechanisms in layer II/III of the juvenile rat auditory cortex: SYNAPTIC MECHANISMS IN RAT AUDITORY CORTEX. Synapse 2015, 69, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Takesian, A.E.; Bogart, L.J.; Lichtman, J.W.; Hensch, T.K. Inhibitory circuit gating of auditory critical-period plasticity. Nat. Neurosci. 2018, 21, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.Y.; Soutar, C.N.; Dringenberg, H.C. Gating of long-term potentiation (LTP) in the thalamocortical auditory system of rats by serotonergic (5-HT) receptors. Brain Res. 2018, 1683, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Suga, N. Serotonergic Modulation of Plasticity of the Auditory Cortex Elicited by Fear Conditioning. J. Neurosci. 2007, 27, 4910–4918. [Google Scholar] [CrossRef]
- Papesh, M.A.; Hurley, L.M. Plasticity of serotonergic innervation of the inferior colliculus in mice following acoustic trauma. Hear. Res. 2012, 283, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Li, I.-H.; Shih, J.-H.; Jhao, Y.-T.; Chen, H.-C.; Chiu, C.-H.; Chen, C.-F.; Huang, Y.-S.; Shiue, C.-Y.; Ma, K.-H. Regulation of noise-induced loss of serotonin transporters with resveratrol in a rat model using 4-[18f]-ADAM/small-animal positron emission tomography. Molecules 2019, 24, 1344. [Google Scholar] [CrossRef]
- Cotel, F.; Exley, R.; Cragg, S.J.; Perrier, J.-F. Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Proc. Natl. Acad. Sci. USA 2013, 110, 4774–4779. [Google Scholar] [CrossRef]
- Sari, Y. Serotonin receptors: From protein to physiological function and behavior. Neurosci. Biobehav. Rev. 2004, 28, 565–582. [Google Scholar] [CrossRef]
- Babb, J.A.; Masini, C.V.; Day, H.E.W.; Campeau, S. Stressor-specific effects of sex on HPA axis hormones and activation of stress-related neurocircuitry. Stress 2013, 16, 664–677. [Google Scholar] [CrossRef]
- Eraslan, E.; Akyazi, İ.; Ergül-Ekiz, E.; Matur, E. Noise stress-induced changes in mRNA levels of corticotropin-releasing hormone family molecules and glucocorticoid receptors in the rat brain. Folia Biol. 2015, 61, 66–73. [Google Scholar]
- Rao, D.; Basura, G.J.; Roche, J.; Daniels, S.; Mancilla, J.G.; Manis, P.B. Hearing loss alters serotonergic modulation of intrinsic excitability in auditory cortex. J. Neurophysiol. 2010, 104, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.H.; Lowry, C.A. Corticotropin-releasing factor-related peptides, serotonergic systems, and emotional behavior. Front. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.L.; Ata, A.E.; Jackson, N.L.; Rahn, E.J.; Ramaker, R.C.; Cooper, S.; Kerman, I.A.; Clinton, S.M. Differential stress induced c-Fos expression and identification of region-specific miRNA-mRNA networks in the dorsal raphe and amygdala of high-responder/low-responder rats. Behav. Brain Res. 2017, 319, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Woolley, S.M.N. Early experience shapes vocal neural coding and perception in songbirds. Dev. Psychobiol. 2012, 54, 612–631. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.H.; Guillette, L.M.; Lee, D.; McMillan, N.; Hoang, J.; Sturdy, C.B. Experience affects immediate early gene expression in response to conspecific call notes in black-capped chickadees (Poecile atricapillus). Behav. Brain Res. 2015, 287, 49–58. [Google Scholar] [CrossRef]
- Bolhuis, J.J.; Zijlstra, G.G.; den Boer-Visser, A.M.; Van Der Zee, E.A. Localized neuronal activation in the zebra finch brain is related to the strength of song learning. Proc. Natl. Acad. Sci. USA 2000, 97, 2282–2285. [Google Scholar] [CrossRef] [PubMed]
- Eda-Fujiwara, H.; Imagawa, T.; Matsushita, M.; Matsuda, Y.; Takeuchi, H.-A.; Satoh, R.; Watanabe, A.; Zandbergen, M.A.; Manabe, K.; Kawashima, T.; et al. Localized brain activation related to the strength of auditory learning in a parrot. PLoS ONE 2012, 7, e38803. [Google Scholar] [CrossRef] [PubMed]
- Gobes, S.M.H.; Zandbergen, M.A.; Bolhuis, J.J. Memory in the making: Localized brain activation related to song learning in young songbirds. Proc. Roy. Soc. B Biol. Sci. 2010, 277, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- London, S.E.; Clayton, D.F. Functional identification of sensory mechanisms required for developmental song learning. Nat. Neurosci. 2008, 11, 579–586. [Google Scholar] [CrossRef]
- George, I.; Cousillas, H.; Richard, J.-P.; Hausberger, M. Experience with adults shapes multisensory representation of social familiarity in the brain of a songbird. PLoS ONE 2012, 7, e38764. [Google Scholar] [CrossRef]
- Arakawa, H. Ethological approach to social isolation effects in behavioral studies of laboratory rodents. Behav. Brain Res. 2018, 341, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.L.; Pickel, V.M. Adolescent isolation rearing produces a prepulse inhibition deficit correlated with expression of the NMDA GluN1 subunit in the nucleus accumbens. Brain Struct. Funct. 2018, 223, 3169–3181. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, W.; Sun, L.; Du, W.; Zhou, H.; Shao, F. Chronic clozapine treatment improves the alterations of prepulse inhibition and BDNF mRNA expression in the medial prefrontal cortex that are induced by adolescent social isolation. Behav. Pharmacol. 2019, 30, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Min, L.; Li, M.; Shao, F.; Wang, W. Transcriptomic analysis reveals oxidative phosphorylation activation in an adolescent social isolation rat model. Brain Res. Bull. 2018, 142, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-M.; Ding, Y.; Jia, H.-X.; Li, L. Different effects of isolation-rearing and neonatal MK-801 treatment on attentional modulations of prepulse inhibition of startle in rats. Psychopharmacology 2016, 233, 3089–3102. [Google Scholar] [CrossRef]
- Dai, H.; Okuda, T.; Sakurai, E.; Kuramasu, A.; Kato, M.; Jia, F.; Xu, J.; Iinuma, K.; Sato, I.; Yanai, K. Blockage of histamine H1 receptor attenuates social isolation-induced disruption of prepulse inhibition: A study in H1 receptor gene knockout mice. Psychopharmacology 2005, 183, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Koda, K.; Ago, Y.; Yano, K.; Nishimura, M.; Kobayashi, H.; Fukada, A.; Takuma, K.; Matsuda, T. Involvement of decreased muscarinic receptor function in prepulse inhibition deficits in mice reared in social isolation. Br. J. Pharmacol. 2011, 162, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Higashino, K.; Ago, Y.; Umeki, T.; Hasebe, S.; Onaka, Y.; Hashimoto, H.; Takuma, K.; Matsuda, T. Rivastigmine improves isolation rearing-induced prepulse inhibition deficits via muscarinic acetylcholine receptors in mice. Psychopharmacology 2016, 233, 521–528. [Google Scholar] [CrossRef]
- Powell, S.B.; Geyer, M.A.; Preece, M.A.; Pitcher, L.K.; Reynolds, G.P.; Swerdlow, N.R. Dopamine depletion of the nucleus accumbens reverses isolation-induced deficits in prepulse inhibition in rats. Neuroscience 2003, 119, 233–240. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Singer, P.; Feldon, J.; Yee, B.K. The postweaning social isolation in C57BL/6 mice: Preferential vulnerability in the male sex. Psychopharmacology 2008, 197, 613–628. [Google Scholar] [CrossRef]
- Marriott, A.L.; Tasker, R.A.; Ryan, C.L.; Doucette, T.A. Alterations to prepulse inhibition magnitude and latency in adult rats following neonatal treatment with domoic acid and social isolation rearing. Behav. Brain Res. 2016, 298, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Screven, L.A.; Dent, M.L. Perception of Ultrasonic Vocalizations by Socially Housed and Isolated Mice. eNeuro 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Keesom, S.M.; Sloss, B.G.; Erbowor-Becksen, Z.; Hurley, L.M. Social experience alters socially induced serotonergic fluctuations in the inferior colliculus. J. Neurophysiol. 2017, 118, 3230–3241. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.P.; Norvelle, A.; Choi, D.C.; Walton, J.C.; Albers, H.E.; Huhman, K.L. Social housing and social isolation: Impact on stress indices and energy balance in male and female Syrian hamsters (Mesocricetus auratus). Physiol. Behav. 2017, 177, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, Y.; Takeda, H.; Muto, S.; Nakagawa, K.; Ohnishi, S.; Sadakane, C.; Nahata, M.; Hattori, T.; Asaka, M. Decreased plasma ghrelin contributes to anorexia following novelty stress. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E685–E696. [Google Scholar] [CrossRef]
- Whitaker-Azmitia, P.; Zhou, F.; Hobin, J.; Borella, A. Isolation-rearing of rats produces deficits as adults in the serotonergic innervation of hippocampus. Peptides 2000, 21, 1755–1759. [Google Scholar] [CrossRef]
- Lehmann, K.; Lesting, J.; Polascheck, D.; Teuchert-Noodt, G. Serotonin fibre densities in subcortical areas: Differential effects of isolated rearing and methamphetamine. Brain Res. Dev. Brain Res. 2003, 147, 143–152. [Google Scholar] [CrossRef]
- Braun, K.; Lange, E.; Metzger, M.; Poeggel, G. Maternal separation followed by early social deprivation affects the development of monoaminergic fiber systems in the medial prefrontal cortex of Octodon degus. Neuroscience 2000, 95, 309–318. [Google Scholar] [CrossRef]
- Kuramochi, M.; Nakamura, S. Effects of postnatal isolation rearing and antidepressant treatment on the density of serotonergic and noradrenergic axons and depressive behavior in rats. Neuroscience 2009, 163, 448–455. [Google Scholar] [CrossRef]
- Ago, Y.; Araki, R.; Tanaka, T.; Sasaga, A.; Nishiyama, S.; Takuma, K.; Matsuda, T. Role of social encounter-induced activation of prefrontal serotonergic systems in the abnormal behaviors of isolation-reared mice. Neuropsychopharmacology 2013, 38, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- McNeal, N.; Anderson, E.M.; Moenk, D.; Trahanas, D.; Matuszewich, L.; Grippo, A.J. Social isolation alters central nervous system monoamine content in prairie voles following acute restraint. Soc. Neurosci. 2018, 13, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Fulford, A.J.; Marsden, C.A. Conditioned release of 5-hydroxytryptamine in vivo in the nucleus accumbens following isolation-rearing in the rat. Neuroscience 1998, 83, 481–487. [Google Scholar] [CrossRef]
- Fulford, A.J.; Marsden, C.A. An intact dopaminergic system is required for context-conditioned release of 5-HT in the nucleus accumbens of postweaning isolation-reared rats. Neuroscience 2007, 149, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Lapiz, M.D.S.; Fulford, A.; Muchimapura, S.; Mason, R.; Parker, T.; Marsden, C.A. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci. Behav. Physiol. 2003, 33, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Dalley, J.W.; Theobald, D.E.; Pereira, E.A.C.; Li, P.M.M.C.; Robbins, T.W. Specific abnormalities in serotonin release in the prefrontal cortex of isolation-reared rats measured during behavioural performance of a task assessing visuospatial attention and impulsivity. Psychopharmacology 2002, 164, 329–340. [Google Scholar] [CrossRef]
- Bickerdike, M.J.; Wright, I.K.; Marsden, C.A. Social isolation attenuates rat forebrain 5-HT release induced by KCI stimulation and exposure to a novel environment. Behav. Pharmacol. 1993, 4, 231–236. [Google Scholar] [CrossRef]
- Muchimapura, S.; Fulford, A.J.; Mason, R.; Marsden, C.A. Isolation rearing in the rat disrupts the hippocampal response to stress. Neuroscience 2002, 112, 697–705. [Google Scholar] [CrossRef]
- dos Santos, L.; de Andrade, T.G.; Graeff, F.G. Social separation and diazepam withdrawal increase anxiety in the elevated plus-maze and serotonin turnover in the median raphe and hippocampus. J. Psychopharmacol. 2009. [Google Scholar] [CrossRef]
- Brenes, J.C.; Fornaguera, J. The effect of chronic fluoxetine on social isolation-induced changes on sucrose consumption, immobility behavior, and on serotonin and dopamine function in hippocampus and ventral striatum. Behav. Brain Res. 2009, 198, 199–205. [Google Scholar] [CrossRef]
- Brenes, J.C.; Padilla, M.; Fornaguera, J. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav. Brain Res. 2009, 197, 125–137. [Google Scholar] [CrossRef]
- Heidbreder, C.A.; Weiss, I.C.; Domeney, A.M.; Pryce, C.; Homberg, J.; Hedou, G.; Feldon, J.; Moran, M.C.; Nelson, P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 2000, 100, 749–768. [Google Scholar] [CrossRef]
- Kang, H.-H.; Wang, C.-H.; Chen, H.-C.; Li, I.-H.; Cheng, C.-Y.; Liu, R.-S.; Huang, W.-S.; Shiue, C.-Y.; Ma, K.-H. Investigating the effects of noise-induced hearing loss on serotonin transporters in rat brain using 4-[18F]-ADAM/small animal PET. Neuroimage 2013, 75, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Leaver, A.M.; Renier, L.; Chevillet, M.A.; Morgan, S.; Kim, H.J.; Rauschecker, J.P. Dysregulation of limbic and auditory networks in tinnitus. Neuron 2011, 69, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Leaver, A.M.; Seydell-Greenwald, A.; Turesky, T.K.; Morgan, S.; Kim, H.J.; Rauschecker, J.P. Cortico-limbic morphology separates tinnitus from tinnitus distress. Front. Syst. Neurosci. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Leaver, A.M.; Seydell-Greenwald, A.; Rauschecker, J.P. Auditory–limbic interactions in chronic tinnitus: Challenges for neuroimaging research. Hear. Res. 2016, 334, 49–57. [Google Scholar] [CrossRef]
- Leaver, A.M.; Turesky, T.K.; Seydell-Greenwald, A.; Morgan, S.; Kim, H.J.; Rauschecker, J.P. Intrinsic network activity in tinnitus investigated using functional MRI: Intrinsic Networks in Tinnitus. Hum. Brain Mapp. 2016, 37, 2717–2735. [Google Scholar] [CrossRef]
- Xu, X.-M.; Jiao, Y.; Tang, T.-Y.; Zhang, J.; Lu, C.-Q.; Salvi, R.; Teng, G.-J. Sensorineural hearing loss and cognitive impairments: Contributions of thalamus using multiparametric MRI. J. Magn. Reson. Imaging 2019, 50, 787–797. [Google Scholar] [CrossRef]
- Xu, X.-M.; Jiao, Y.; Tang, T.-Y.; Zhang, J.; Salvi, R.; Teng, G.-J. Inefficient Involvement of Insula in Sensorineural Hearing Loss. Front. Neurosci. 2019, 13, 133. [Google Scholar] [CrossRef]
- Xu, X.-M.; Jiao, Y.; Tang, T.-Y.; Lu, C.-Q.; Zhang, J.; Salvi, R.; Teng, G.-J. Altered spatial and temporal brain connectivity in the salience network of sensorineural hearing loss and tinnitus. Front. Neurosci. 2019, 13, 246. [Google Scholar] [CrossRef]
- Xu, X.-M.; Jiao, Y.; Tang, T.-Y.; Zhang, J.; Lu, C.-Q.; Luan, Y.; Salvi, R.; Teng, G.-J. Dissociation between cerebellar and cerebral neural activities in humans with long-term bilateral sensorineural hearing loss. Neural Plast. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Carpenter-Thompson, J.R.; Akrofi, K.; Schmidt, S.A.; Dolcos, F.; Husain, F.T. Alterations of the emotional processing system may underlie preserved rapid reaction time in tinnitus. Brain Res. 2014, 1567, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.T.; Carpenter-Thompson, J.R.; Schmidt, S.A. The effect of mild-to-moderate hearing loss on auditory and emotion processing networks. Front. Syst. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Picou, E.M. How hearing loss and age affect emotional responses to nonspeech sounds. J. Speech Lang. Hear. Res. 2016, 59, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Picou, E.M.; Buono, G.H. Emotional responses to pleasant sounds are related to social disconnectedness and loneliness independent of hearing loss. Trends Hear. 2018, 22. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K.; Kaufer, D. Stress, social behavior, and resilience: Insights from rodents. Neurobiol. Stress 2015, 1, 116–127. [Google Scholar] [CrossRef]
- Canlon, B.; Meltser, I.; Johansson, P.; Tahera, Y. Glucocorticoid receptors modulate auditory sensitivity to acoustic trauma. Hear. Res. 2007, 226, 61–69. [Google Scholar] [CrossRef]
- Tabuchi, K.; Nakamagoe, M.; Nishimura, B.; Hayashi, K.; Nakayama, M.; Hara, A. Protective effects of corticosteroids and neurosteroids on cochlear injury. Med. Chem. 2011, 7, 140–144. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Carola, V.; Pascucci, T.; Puglisi-Allegra, S.; Cabib, S.; Lesch, K.-P.; Parmigiani, S.; Palanza, P.; Gross, C. Increased vulnerability to psychosocial stress in heterozygous serotonin transporter knockout mice. Dis. Models Mech. 2010, 3, 459–470. [Google Scholar] [CrossRef]
- Iurescia, S.; Seripa, D.; Rinaldi, M. Looking beyond the 5-HTTLPR polymorphism: Genetic and epigenetic layers of regulation affecting the serotonin transporter gene expression. Mol. Neurobiol. 2017, 54, 8386–8403. [Google Scholar] [CrossRef]
- Lin, F.R.; Metter, E.J.; O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L. Hearing loss and incident dementia. Arch. Neurol. 2011, 68, 214–220. [Google Scholar] [CrossRef]
- Lin, F.R.; Thorpe, R.; Gordon-Salant, S.; Ferrucci, L. Hearing loss prevalence and risk factors among older adults in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Deal, J.A.; Sharrett, A.R.; Albert, M.S.; Coresh, J.; Mosley, T.H.; Knopman, D.; Wruck, L.M.; Lin, F.R. Hearing impairment and cognitive decline: A pilot study conducted within the atherosclerosis risk in communities neurocognitive study. Am. J. Epidemiol. 2015, 181, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Deal, J.A.; Betz, J.; Yaffe, K.; Harris, T.; Purchase-Helzner, E.; Satterfield, S.; Pratt, S.; Govil, N.; Simonsick, E.M.; Lin, F.R.; et al. Hearing impairment and incident dementia and cognitive decline in older adults: The health abc study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Harrison Bush, A.L.; Edwards, J.D.; Lister, J.J.; Lin, F.R.; Betz, J. Peripheral hearing and cognition: Evidence from the staying keen in later life (SKILL) study. Ear Hear. 2015, 36, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Humes, L.E.; Young, L.A. Sensory-Cognitive Interactions in Older Adults. Ear Hear. 2016, 37, 52S–61S. [Google Scholar] [CrossRef]
- Armstrong, N.M.; An, Y.; Resnick, S.M.; Doshi, J.; Erus, G.; Davatzikos, C.; Ferrucci, L.; Deal, J.A.; Lin, F.R. Association of midlife hearing impairment with late-life temporal lobe volume loss. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 794–802. [Google Scholar] [CrossRef]
- Arlinger, S.; Lunner, T.; Lyxell, B.; Kathleen Pichora-Fuller, M. The emergence of Cognitive Hearing Science. Scand. J. Psychol. 2009, 50, 371–384. [Google Scholar] [CrossRef]
- Fulton, S.E.; Lister, J.J.; Bush, A.L.H.; Edwards, J.D.; Andel, R. Mechanisms of the hearing-cognition relationship. Semin. Hear. 2015, 36, 140–149. [Google Scholar] [CrossRef]
- Humes, L.E.; Dubno, J.R.; Gordon-Salant, S.; Lister, J.J.; Cacace, A.T.; Cruickshanks, K.J.; Gates, G.A.; Wilson, R.H.; Wingfield, A. Central presbycusis: A review and evaluation of the evidence. J. Am. Acad. Audiol. 2012, 23, 635–666. [Google Scholar] [CrossRef]
- Maharani, A.; Dawes, P.; Nazroo, J.; Tampubolon, G.; Pendleton, N. Sense-Cog WP1 Group Associations between self-reported sensory impairment and risk of cognitive decline and impairment in the health and retirement study cohort. J. Gerontol. B Psychol. Sci. Soc. Sci. 2020, 75, 1230–1242. [Google Scholar] [CrossRef]
- Marsh, J.E.; Ljung, R.; Nöstl, A.; Threadgold, E.; Campbell, T.A. Failing to get the gist of what’s being said: Background noise impairs higher-order cognitive processing. Front Psychol. 2015, 6, 548. [Google Scholar] [CrossRef]
- Pichora-Fuller, M.K. Use of supportive context by younger and older adult listeners: Balancing bottom-up and top-down information processing. Int. J. Audiol. 2008, 47, S72–S82. [Google Scholar] [CrossRef] [PubMed]
- Pichora-Fuller, M.K.; Kramer, S.E.; Eckert, M.A.; Edwards, B.; Hornsby, B.W.Y.; Humes, L.E.; Lemke, U.; Lunner, T.; Matthen, M.; Mackersie, C.L.; et al. Hearing impairment and cognitive energy: The Framework for Understanding Effortful Listening (FUEL). Ear Hear. 2016, 37, 5S–27S. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Albert, M. Hearing loss and dementia - who is listening? Aging Ment. Health 2014, 18, 671–673. [Google Scholar] [CrossRef]
- Lin, F.R.; Ferrucci, L.; An, Y.; Goh, J.O.; Doshi, J.; Metter, E.J.; Davatzikos, C.; Kraut, M.A.; Resnick, S.M. Association of hearing impairment with brain volume changes in older adults. Neuroimage 2014, 90, 84–92. [Google Scholar] [CrossRef]
- Maharani, A.; Pendleton, N.; Leroi, I. Hearing impairment, loneliness, social isolation, and cognitive function: Longitudinal analysis using English longitudinal study on ageing. Am. J. Geriat. Psychiat. 2019, 27, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Gumaste, A.; Adams, G.K.; Chong, K.K.; Nguyen, M.; Shepard, K.N.; Liu, R.C. Familiarity with social sounds alters c-Fos expression in auditory cortex and interacts with estradiol in locus coeruleus. Hear. Res. 2018, 366, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Reyes, B.A.S.; Zhang, X.-Y.; Dufourt, E.C.; Bhatnagar, S.; Valentino, R.J.; Van Bockstaele, E.J. Neurochemically distinct circuitry regulates locus coeruleus activity during female social stress depending on coping style. Brain Struct. Funct. 2019, 224, 1429–1446. [Google Scholar] [CrossRef] [PubMed]
- Zerbi, V.; Floriou-Servou, A.; Markicevic, M.; Vermeiren, Y.; Sturman, O.; Privitera, M.; von Ziegler, L.; Ferrari, K.D.; Weber, B.; De Deyn, P.P.; et al. Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 2019, 103, 702–718.e5. [Google Scholar] [CrossRef]
- Totah, N.K.B.; Logothetis, N.K.; Eschenko, O. Noradrenergic ensemble-based modulation of cognition over multiple timescales. Brain Res. 2019, 1709, 50–66. [Google Scholar] [CrossRef]
- McBurney-Lin, J.; Sun, Y.; Tortorelli, L.S.; Nguyen, Q.A.; Haga-Yamanaka, S.; Yang, H. Bidirectional pharmacological perturbations of the noradrenergic system differentially affect tactile detection. Neuropharmacology 2020, 108151. [Google Scholar] [CrossRef]
- Li, L.; Feng, X.; Zhou, Z.; Zhang, H.; Shi, Q.; Lei, Z.; Shen, P.; Yang, Q.; Zhao, B.; Chen, S.; et al. Stress accelerates defensive responses to looming in mice and involves a locus coeruleus-superior colliculus projection. Curr. Biol. 2018, 28, 859–871.e5. [Google Scholar] [CrossRef]
- Bazzari, A.H.; Parri, H.R. Neuromodulators and long-term synaptic plasticity in learning and memory: A steered-glutamatergic perspective. Brain Sci. 2019, 9, 300. [Google Scholar] [CrossRef]
- Amieva, H.; Ouvrard, C.; Giulioli, C.; Meillon, C.; Rullier, L.; Dartigues, J. Self-Reported Hearing Loss, Hearing Aids, and Cognitive Decline in Elderly Adults: A 25-Year Study. J. Am. Geriatr. Soc. 2015, 63, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, A.; Benatti, A.; Velardita, C.; Favaro, D.; Padoan, E.; Severi, D.; Pagliaro, M.; Bovo, R.; Vallesi, A.; Gabelli, C.; et al. Aging, cognitive decline and hearing loss: Effects of auditory rehabilitation and training with hearing aids and cochlear implants on cognitive function and depression among older adults. Audiol. Neurootol. 2016, 21 (Suppl. 1), 21–28. [Google Scholar] [CrossRef]
- Maharani, A.; Dawes, P.; Nazroo, J.; Tampubolon, G.; Pendleton, N. SENSE-Cog WP1 group Longitudinal relationship between hearing aid use and cognitive function in older americans. J. Am. Geriatr. Soc. 2018, 66, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.-G.; Wang, Y.-J.; Zhou, H.; Li, X.-L.; Bai, F.; Ren, Q.-G.; Zhang, Z.-J. Citalopram ameliorates synaptic plasticity deficits in different cognition-associated brain regions induced by social isolation in middle-aged rats. Mol. Neurobiol. 2017, 54, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- Folmer, R.L.; Shi, Y.-B. SSRI use by tinnitus patients: Interactions between depression and tinnitus severity. Ear Nose Throat J. 2004, 83, 107–108, 110, 112 passim. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Kanzaki, S.; Shinden, S.; Saito, H.; Inoue, Y.; Ogawa, K. Effects of selective serotonin reuptake inhibitor on treating tinnitus in patients stratified for presence of depression or anxiety. Audiol. Neurootol. 2010, 15, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.K.; Viirre, E.S.; Bailey, K.A.; Gerke, M.A.; Harris, J.P.; Stein, M.B. Randomized placebo-controlled trial of a selective serotonin reuptake inhibitor in the treatment of nondepressed tinnitus subjects. Psychosom. Med. 2005, 67, 981–988. [Google Scholar] [CrossRef]
| Region | Reference | Receptor Type | Proposed Function of Serotonin |
|---|---|---|---|
| Cochlea | [105] | n/a | synaptically released |
| DCN | [56,61] | multiple | enhances multimodal pathways while dampening auditory pathways |
| [106] | 5-HT2, other | enhances excitability (5-HT2), decreases excitability (other) | |
| PVCN, AVCN, and DCN | [107] | likely multiple | inhibits and facilitates sound-evoked spiking |
| MNTB | [53] | 5-HT1B | presynaptically decreases glutamate release, developmentally regulated |
| LSO | [108] | 5-HT1, 5-HT2 | suppresses evoked excitatory postsynaptic currents, induces spontaneous inhibitory postsynaptic currents, developmentally regulated |
| [109] | n/a | promotes development of projections from LSO to IC | |
| IC | [110] | 5-HT1A, 5HT1B | 5-HT1A suppresses sound-evoked spiking, 5-HT1B increases sound-evoked spiking via GABAergic suppression |
| [93] | 5-HT2A | enhances spontaneous inhibitory postsynaptic potentials | |
| [111] | 5-HT3A | activity-dependent response gain adjustment | |
| [112] | 5-HT3A | response gain adjustment | |
| [94] | n/a | context-dependent alteration of immediate early gene expression | |
| MGB | [113] | n/a | reduces burst firing |
| Cortex | [114,115] | 5-HT1A, 5-HT2 | reduces excitatory and inhibitory postsynaptic currents |
| [116] | 5-HT3A | excites inhibitory interneurons that regulate critical period timing | |
| [117] | 5-HT2, 5-HT3 | regulates synaptic plasticity | |
| [118] | 5-HT2 | regulates plasticity in frequency tuning |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keesom, S.M.; Hurley, L.M. Silence, Solitude, and Serotonin: Neural Mechanisms Linking Hearing Loss and Social Isolation. Brain Sci. 2020, 10, 367. https://doi.org/10.3390/brainsci10060367
Keesom SM, Hurley LM. Silence, Solitude, and Serotonin: Neural Mechanisms Linking Hearing Loss and Social Isolation. Brain Sciences. 2020; 10(6):367. https://doi.org/10.3390/brainsci10060367
Chicago/Turabian StyleKeesom, Sarah M., and Laura M. Hurley. 2020. "Silence, Solitude, and Serotonin: Neural Mechanisms Linking Hearing Loss and Social Isolation" Brain Sciences 10, no. 6: 367. https://doi.org/10.3390/brainsci10060367
APA StyleKeesom, S. M., & Hurley, L. M. (2020). Silence, Solitude, and Serotonin: Neural Mechanisms Linking Hearing Loss and Social Isolation. Brain Sciences, 10(6), 367. https://doi.org/10.3390/brainsci10060367