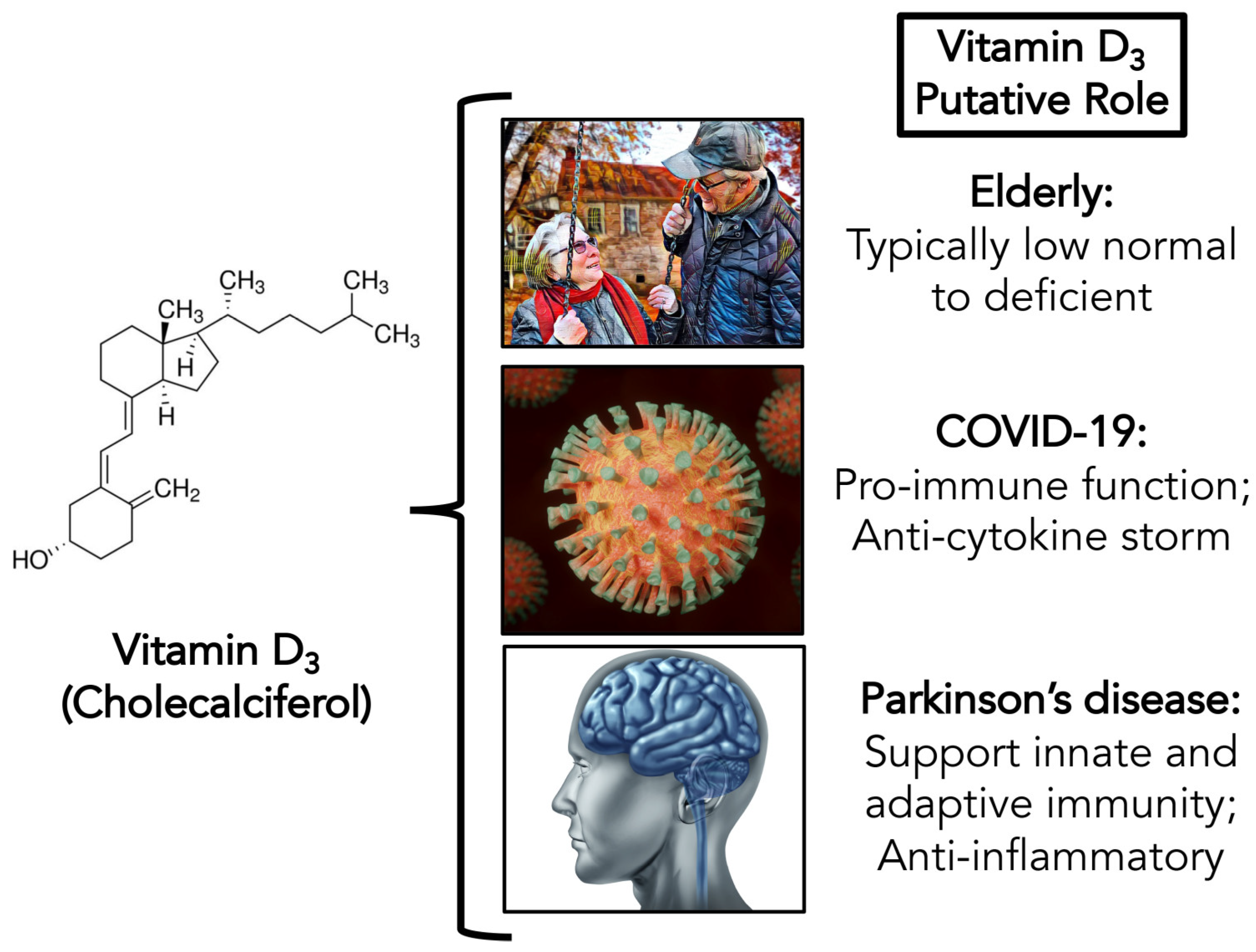
Potential Role of Vitamin D in the Elderly to Resist COVID-19 and to Slow Progression of Parkinson’s Disease
Abstract
1. Introduction
1.1. COVID-19
1.2. Vitamin D: Special Considerations in the Elderly
1.3. Parkinson’s Disease: The Intersection of COVID-19 and Vitamin D Deficiency
2. Advancing Our Understanding of Viral Diseases and Parkinson’s
2.1. Possible Antiviral Action of Vitamin D
2.2. Possible Slowing of PD Progression
2.3. Safety and Adverse Events of Vitamin D Supplementation
3. Fitting the Pieces of the Puzzle Together
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, P.; Lu, X.; Xu, C.; Sun, W.; Pan, B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef]
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Canna, S.W.; Behrens, E.M. Making sense of the cytokine storm: A conceptual framework for understanding, diagnosing, and treating hemophagocytic syndromes. Pediatr. Clin. 2012, 59, 329–344. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Derm. Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Boucher, B.J. The problems of vitamin d insufficiency in older people. Aging Dis. 2012, 3, 313. [Google Scholar]
- Meehan, M.; Penckofer, S. The role of vitamin D in the aging adult. J. Aging Gerontol. 2014, 2, 60. [Google Scholar] [CrossRef]
- Grant, W.B.; Holick, M.F. Benefits and requirements of vitamin D for optimal health: A review. Altern. Med. Rev. 2005, 10, 94–111. [Google Scholar]
- Ding, H.; Dhima, K.; Lockhart, K.C.; Locascio, J.J.; Hoesing, A.N.; Duong, K.; Trisini-Lipsanopoulos, A.; Hayes, M.T.; Sohur, U.S.; Wills, A.-M. Unrecognized vitamin D3 deficiency is common in Parkinson disease: Harvard Biomarker Study. Neurology 2013, 81, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.L.; Murchison, C.; Zabetian, C.; Leverenz, J.B.; Watson, G.; Montine, T.; Carney, N.; Bowman, G.L.; Edwards, K.; Quinn, J.F. Memory, mood, and vitamin D in persons with Parkinson’s disease. J. Parkinson’s Dis. 2013, 3, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.; Lang, A. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Ahlskog, J.E. The New Parkinson’s Disease Treatment Book: Partnering with Your Doctor to Get the Most from Your Medications; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Ahlskog, J.E. Cheaper, simpler, and better: Tips for treating seniors with Parkinson disease. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1211–1216. [Google Scholar]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef]
- Espay, A.J.; Lang, A.E. Common myths in the use of levodopa in Parkinson disease: When clinical trials misinform clinical practice. JAMA Neurol. 2017, 74, 633–634. [Google Scholar] [CrossRef]
- Spindler, M.A.; Tarsy, D. Initial Pharmacologic Treatment of Parkinson Disease. In UpToDate; 2019; Available online: https://www.uptodate.com/contents/initial-pharmacologic-treatment-of-parkinson-disease (accessed on 3 May 2020).
- Latt, M.D.; Lewis, S.; Zekry, O.; Fung, V.S. Factors to consider in the selection of dopamine agonists for older persons with Parkinson’s disease. Drugs Aging 2019, 36, 189–202. [Google Scholar] [CrossRef]
- Tosur, Z.; Green, D.; De Chavez, P.J.; Knutson, K.L.; Goldberger, J.J.; Zee, P.; Liu, K.; Kim, K.-Y.; Carnethon, M.R. The association between sleep characteristics and prothrombotic markers in a population-based sample: Chicago Area Sleep Study. Sleep Med. 2014, 15, 973–978. [Google Scholar] [CrossRef]
- Zesiewicz, T.A.; Bezchlibnyk, Y.; Dohse, N.; Ghanekar, S.D. Management of Early Parkinson Disease. Clin. Geriatr. Med. 2019, 35, 36–41. [Google Scholar] [CrossRef]
- Crowley, E.K.; Nolan, Y.M.; Sullivan, A.M. Exercise as therapy for Parkinson’s? Aging 2018, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Crowley, E.K.; Nolan, Y.M.; Sullivan, A.M. Exercise as a therapeutic intervention for motor and non-motor symptoms in Parkinson’s disease: Evidence from rodent models. Prog. Neurobiol. 2019, 172, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.; Rochester, L. Mobilizing Parkinson’s disease: The future of exercise. J. Parkinson’s Dis. 2018, 8, S95–S100. [Google Scholar] [CrossRef]
- Paillard, T.; Rolland, Y.; de Souto Barreto, P. Protective effects of physical exercise in Alzheimer’s disease and Parkinson’s disease: A narrative review. J. Clin. Neurol. 2015, 11, 212–219. [Google Scholar] [CrossRef]
- van der Kolk, N.M.; de Vries, N.M.; Kessels, R.P.; Joosten, H.; Zwinderman, A.H.; Post, B.; Bloem, B.R. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol. 2019, 18, 998–1008. [Google Scholar] [CrossRef]
- Hall, M.-F.E.; Church, F.C. Integrative Medicine and Health Therapy for Parkinson Disease. Top. Geriatr. Rehabil. 2020, in press. [Google Scholar]
- Kim, S.-N.; Wang, X.; Park, H.-J. Integrative Approach to Parkinson’s Disease. Front. Aging Neurosci. 2019, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef]
- Gruber-Bzura, B.M. Vitamin D and Influenza—Prevention or Therapy? Int. J. Mol. Sci. 2018, 19, 2419. [Google Scholar] [CrossRef] [PubMed]
- Bryson, K.; Nash, A.; Norval, M. Does vitamin D protect against respiratory viral infections? Epidemiol. Infect. 2014, 142, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Gal-Tanamy, M.; Bachmetov, L.; Ravid, A.; Koren, R.; Erman, A.; Tur-Kaspa, R.; Zemel, R. Vitamin D: An innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology 2011, 54, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, M.P.; Kumar, R.S.; Ratho, R.K. 25-Hydroxyvitamin D3 and 1, 25 dihydroxyvitamin D3 as an antiviral and immunomodulator against herpes simplex virus-1 infection in HeLa cells. Viral Immunol. 2018, 31, 589–593. [Google Scholar] [CrossRef]
- Sleeman, I.; Aspray, T.; Lawson, R.; Coleman, S.; Duncan, G.; Khoo, T.K.; Schoenmakers, I.; Rochester, L.; Burn, D.; Yarnall, A. The role of vitamin D in disease progression in early Parkinson’s disease. J. Parkinson’s Dis. 2017, 7, 669–675. [Google Scholar] [CrossRef]
- Telcian, A.G.; Zdrenghea, M.T.; Edwards, M.R.; Laza-Stanca, V.; Mallia, P.; Johnston, S.L.; Stanciu, L.A. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antivir. Res. 2017, 137, 93–101. [Google Scholar] [CrossRef]
- Teymoori-Rad, M.; Shokri, F.; Salimi, V.; Marashi, S.M. The interplay between vitamin D and viral infections. Rev. Med. Virol. 2019, 29, e2032. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Luo, B.-A.; Qin, L.-L. The association between vitamin D deficiency and community-acquired pneumonia: A meta-analysis of observational studies. Medicine 2019, 98, e17252. [Google Scholar] [CrossRef]
- Hong, M.; Xiong, T.; Huang, J.; Wu, Y.; Lin, L.; Zhang, Z.; Huang, L.; Gao, D.; Wang, H.; Kang, C. Association of vitamin D supplementation with respiratory tract infection in infants. Matern. Child Nutr. 2020, e12987. [Google Scholar] [CrossRef]
- Glinsky, G. Harnessing powers of genomics to build molecular maps of coronavirus targets in human cells: A guide for existing drug repurposing and experimental studies identifying candidate therapeutics to mitigate the pandemic COVID-19. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Glinsky, G.V. Genomics-guided tracing of SARS-CoV-2 targets in human cells identifies Vitamin D and Quercetin as candidate medicinal agents for mitigation of the severity of pandemic COVID-19. Available online: http://iem.ucsd.edu/people/profiles/guennadi-v-glinskii.html (accessed on 3 May 2020).
- Jakovac, H. COVID-19 and vitamin D—Is there a link and an opportunity for intervention? Am. J. Physiol. Endocrinol. Metab. 2020, 318, E589. [Google Scholar] [CrossRef] [PubMed]
- Arboleda, J.; Urcuqui-Inchima, S. Vitamin D supplementation: A potential approach for COVID-19 therapeutics? 2020. Available online: https://doi.org/10.31219/osf.io/cgd4t (accessed on 3 May 2020).
- Rashedi, J.; Poor, B.M.; Asgharzadeh, M. Vitamin D3 Administration to Patients with Confirmed COVID-19. Iran. J. Public Health 2020, 49, 141–142. [Google Scholar]
- Ghavideldarestani, M.; Honardoost, M.; Khamseh, M.E. Role of Vitamin D in Pathogenesis and Severity of COVID-19 Infection. 2020. Available online: https://www.preprints.org/manuscript/202004.0355/v1 (accessed on 3 May 2020).
- Berridge, M.J. Vitamin D: A Custodian of Cell Signa Lling Stability in Health and Disease; Portland Press Ltd.: London, UK, 2015. [Google Scholar]
- Baggerly, C.A.; Cuomo, R.E.; French, C.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Heaney, R.P.; Holick, M.F.; Hollis, B.W.; McDonnell, S.L. Sunlight and vitamin D: Necessary for public health. J. Am. Coll. Nutr. 2015, 34, 359–365. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hribar, C.A.; Cobbold, P.H.; Church, F.C. Potential Role of Vitamin D in the Elderly to Resist COVID-19 and to Slow Progression of Parkinson’s Disease. Brain Sci. 2020, 10, 284. https://doi.org/10.3390/brainsci10050284
Hribar CA, Cobbold PH, Church FC. Potential Role of Vitamin D in the Elderly to Resist COVID-19 and to Slow Progression of Parkinson’s Disease. Brain Sciences. 2020; 10(5):284. https://doi.org/10.3390/brainsci10050284
Chicago/Turabian StyleHribar, Casey A., Peter H. Cobbold, and Frank C. Church. 2020. "Potential Role of Vitamin D in the Elderly to Resist COVID-19 and to Slow Progression of Parkinson’s Disease" Brain Sciences 10, no. 5: 284. https://doi.org/10.3390/brainsci10050284
APA StyleHribar, C. A., Cobbold, P. H., & Church, F. C. (2020). Potential Role of Vitamin D in the Elderly to Resist COVID-19 and to Slow Progression of Parkinson’s Disease. Brain Sciences, 10(5), 284. https://doi.org/10.3390/brainsci10050284




