Effect of Rehabilitation with Extremely Low Frequency Electromagnetic Field on Molecular Mechanism of Apoptosis in Post-Stroke Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects Presentation and Blood Collection
2.2. Setting and Treatment of ELF-EMF
2.3. Isolation of RNA
2.4. Reverse Transcription
2.5. Real-Time PCR
2.6. Data Analysis
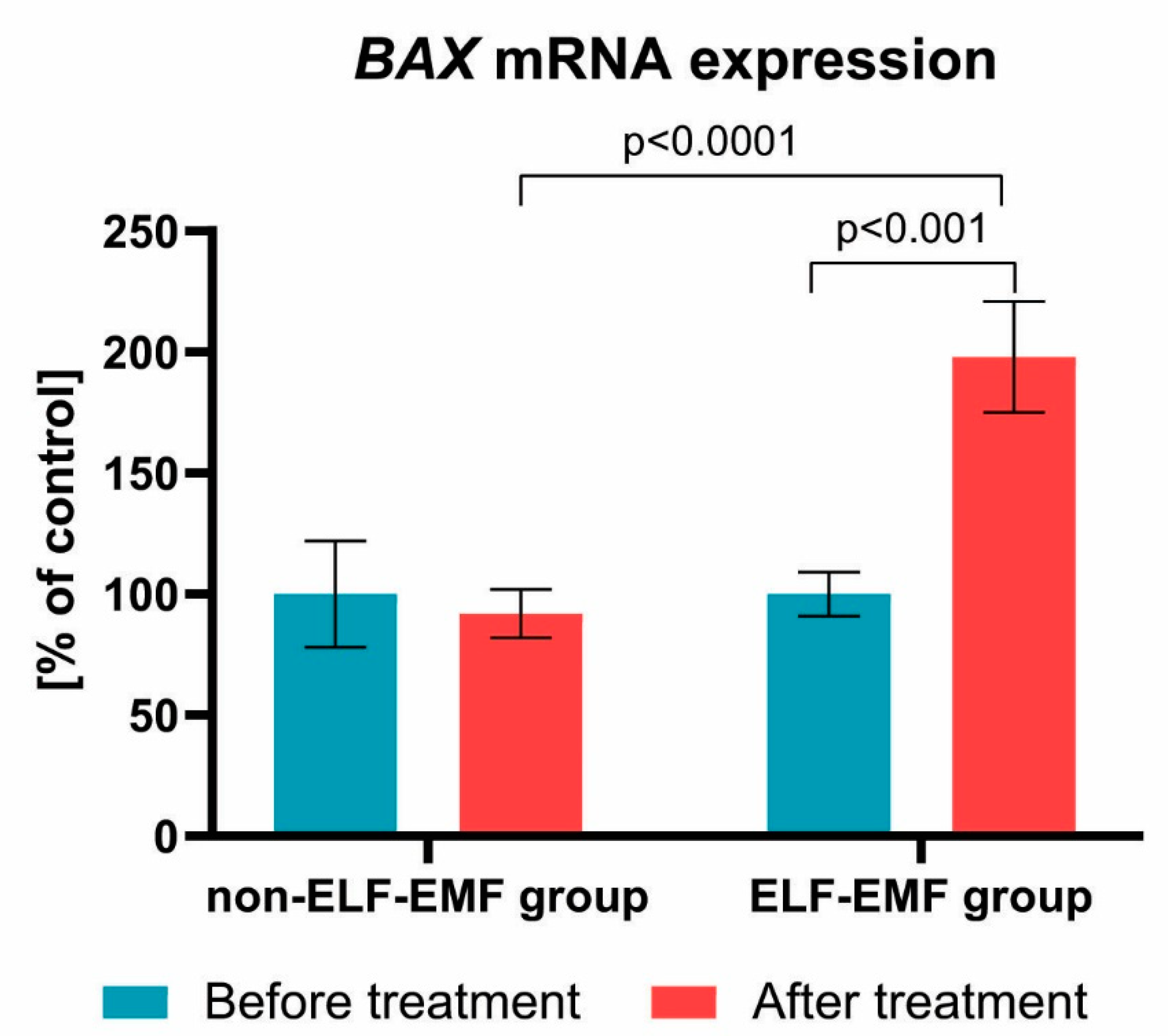
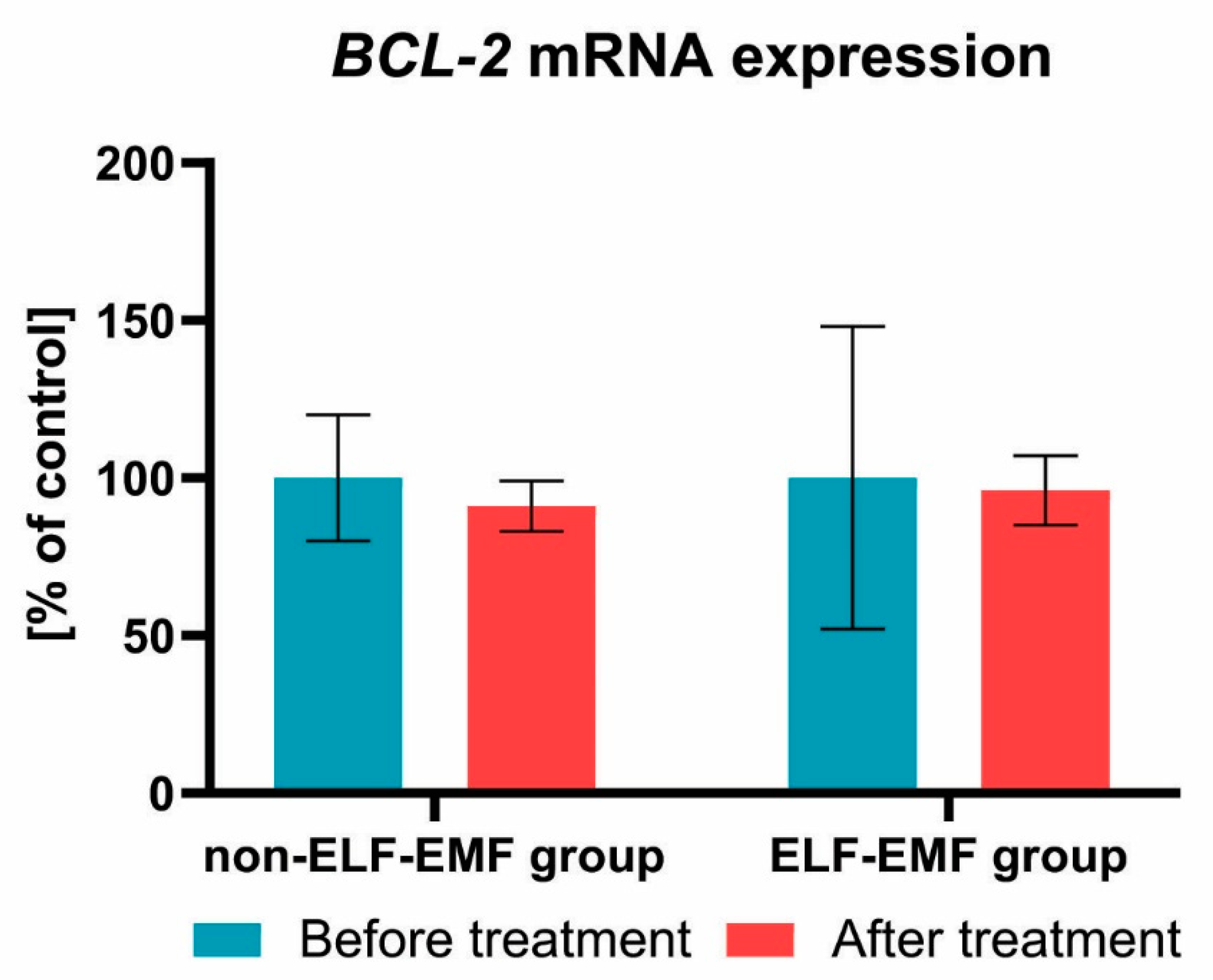
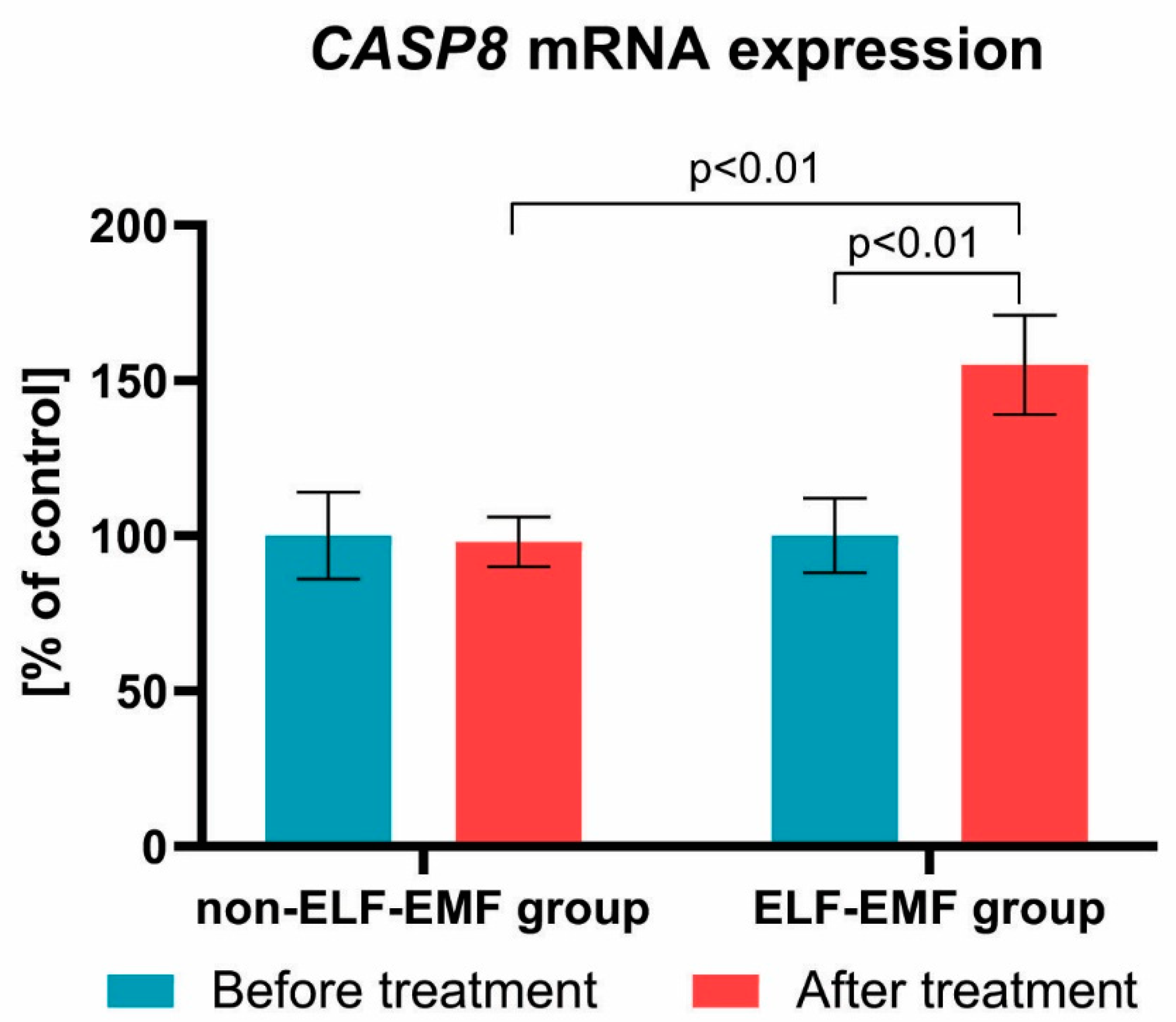
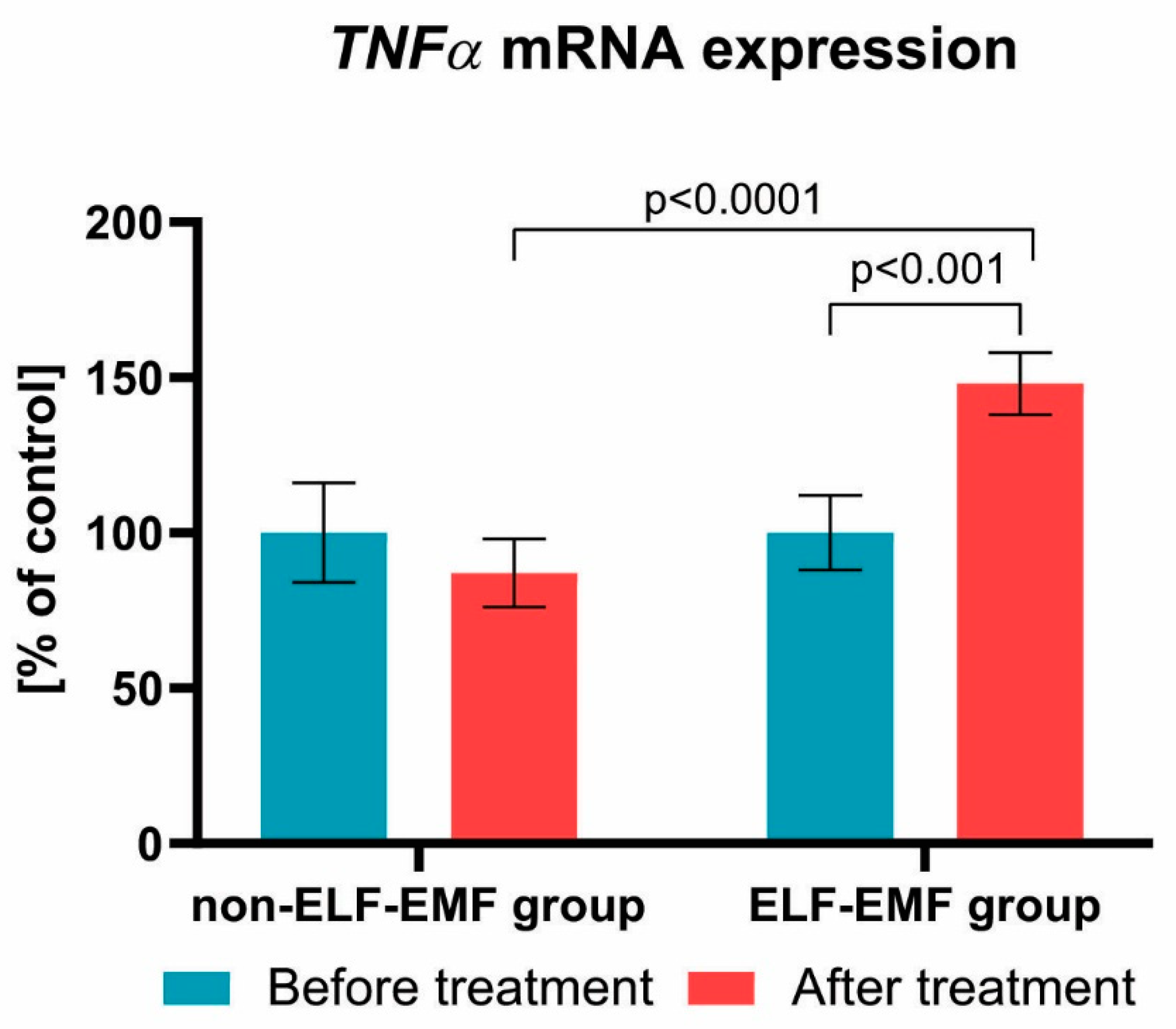
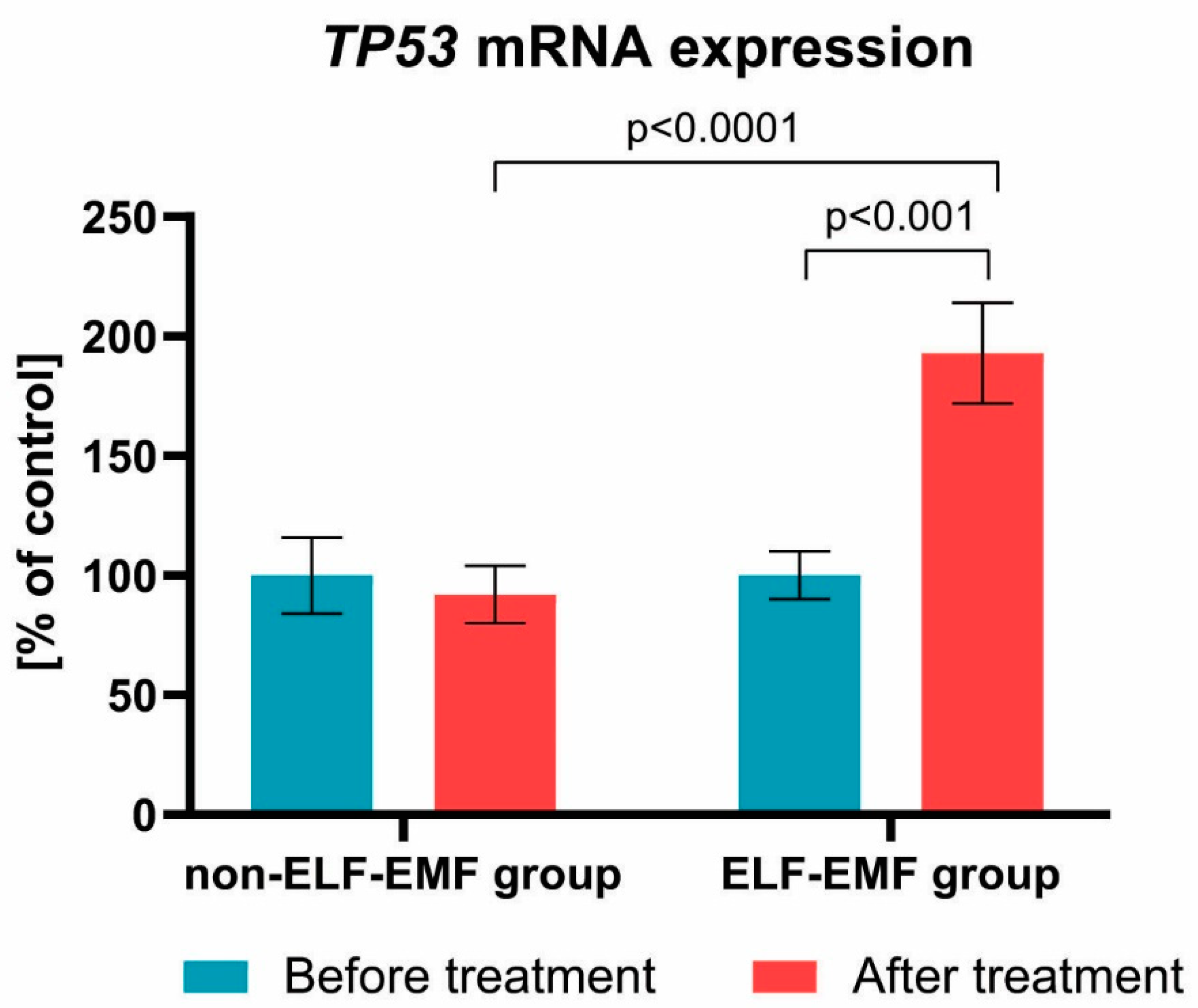
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Werner, C.; Engelhard, K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Novgorodov, S.A.; Voltin, J.R.; Gooz, M.A.; Li, L.; Lemasters, J.J.; Gudz, T.I. Acid sphingomyelinase promotes mitochondrial dysfunction due to glutamate-induced regulated necrosis. J. Lipid Res. 2018, 59, 312–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zhou, F.; Dou, Y.; Tian, X.; Liu, C.; Li, H.; Shen, H.; Chen, G. Melatonin alleviates intracerebral hemorrhage-induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Transl. Stroke Res. 2018, 9, 74–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.W.; Zhang, Y.Y.; Su, J.; Liu, A.F.; Wang, K.; Li, C.; Liu, Y.E.; Zhang, Y.Q.; Lv, J.; Jiang, W.J. Ischemia reperfusion injury after gradual versus rapid flow restoration for middle cerebral artery occlusion rats. Sci. Rep. 2018, 8, 1638. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gan, Y.; Han, P.; Yin, J.; Liu, Q.; Ghanian, S.; Gao, F.; Gong, G.; Tang, Z. Ischemia-induced Neuronal Cell Death Is Mediated by Chemokine Receptor CX3CR1. Sci. Rep. 2018, 8, 556. [Google Scholar] [CrossRef] [Green Version]
- Neto-Neves, E.M.; Frump, A.L.; Vayl, A.; Kline, J.A.; Lahm, T. Isolated heart model demonstrates evidence of contractile and diastolic dysfunction in right ventricles from rats with sugen/hypoxia-induced pulmonary hypertension. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef]
- Wafa, A.; Moassass, F.; Liehr, T.; Bhatt, S.; Aljapawe, A.; Al Achkar, W. A high complex karyotype involving eleven chromosomes including three novel chromosomal aberrations and monoallelic loss of. Mol. Cytogenet. 2016, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Walensky, L.D. BCL-2 in the crosshairs: Tipping the balance of life and death. Cell Death Differ. 2006, 13, 1339–1350. [Google Scholar] [CrossRef]
- Kruidering, M.; Evan, G.I. Caspase-8 in apoptosis: The beginning of “the end”? IUBMB Life 2000, 50, 85–90. [Google Scholar] [CrossRef]
- Chen, J.; Magavi, S.S.; Macklis, J.D. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc. Natl. Acad. Sci. USA 2004, 101, 16357–16362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magavi, S.S.; Leavitt, B.R.; Macklis, J.D. Induction of neurogenesis in the neocortex of adult mice. Nature 2000, 405, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Balbinot, G.; Schuch, C.P. Compensatory Relearning Following Stroke: Cellular and Plasticity Mechanisms in Rodents. Front. Neurosci. 2018, 12, 1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelentsova-Levytskyi, K.; Talmi, Z.; Abboud-Jarrous, G.; Capucha, T.; Sapir, T.; Burstyn-Cohen, T. Protein S Negatively Regulates Neural Stem Cell Self-Renewal through Bmi-1 Signaling. Front. Mol. Neurosci. 2017, 10, 124. [Google Scholar] [CrossRef]
- Aimone, J.B.; Li, Y.; Lee, S.W.; Clemenson, G.D.; Deng, W.; Gage, F.H. Regulation and function of adult neurogenesis: From genes to cognition. Physiol. Rev. 2014, 94, 991–1026. [Google Scholar] [CrossRef] [Green Version]
- Patruno, A.; Ferrone, A.; Costantini, E.; Franceschelli, S.; Pesce, M.; Speranza, L.; Amerio, P.; D’Angelo, C.; Felaco, M.; Grilli, A.; et al. Extremely low-frequency electromagnetic fields accelerates wound healing modulating MMP-9 and inflammatory cytokines. Cell Prolif. 2018, 51, e12432. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Dey, S.; Jain, S. Extremely low-frequency electromagnetic fields: A possible non-invasive therapeutic tool for spinal cord injury rehabilitation. Electromagn. Biol. Med. 2017, 36, 88–101. [Google Scholar] [CrossRef]
- Cichon, N.; Olejnik, A.; Miller, E.; Saluk, J. The multipotent action of magnetic fields. Biologia 2016, 71, 1103–1110. [Google Scholar] [CrossRef]
- Mattsson, M.O.; Simkó, M. Emerging medical applications based on non-ionizing electromagnetic fields from 0 Hz to 10 THz. Med. Devices 2019, 12, 347–368. [Google Scholar] [CrossRef] [Green Version]
- Radak, D.; Katsiki, N.; Resanovic, I.; Jovanovic, A.; Sudar-Milovanovic, E.; Zafirovic, S.; Mousad, S.A.; Isenovic, E.R. Apoptosis and acute brain ischemia in ischemic stroke. Curr. Vasc. Pharmacol. 2017, 15, 115–122. [Google Scholar] [CrossRef]
- Cichon, N.; Bijak, M.; Synowiec, E.; Miller, E.; Sliwinski, T.; Saluk-Bijak, J. Modulation of antioxidant enzyme gene expression by extremely low frequency electromagnetic field in post-stroke patients. Scand. J. Clin. Lab. Investig. 2018, 78, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Cichon, N.; Saluk-Bijak, J.; Miller, E.; Sliwinski, T.; Synowiec, E.; Wigner, P.; Bijak, M. Evaluation of the effects of extremely low frequency electromagnetic field on the levels of some inflammatory cytokines in post-stroke patients. J. Rehabil. Med. 2019, 51, 854–860. [Google Scholar] [CrossRef]
- Cichoń, N.; Czarny, P.; Bijak, M.; Miller, E.; Śliwiński, T.; Szemraj, J.; Saluk-Bijak, J. Benign effect of extremely low-frequency electromagnetic field on brain plasticity assessed by nitric oxide metabolism during poststroke rehabilitation. Oxid. Med. Cell. Longev. 2017, 2017, 2181942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cichoń, N.; Bijak, M.; Miller, E.; Saluk, J. Extremely low frequency electromagnetic field (ELF-EMF) reduces oxidative stress and improves functional and psychological status in ischemic stroke patients. Bioelectromagnetics 2017, 38, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Cichoń, N.; Bijak, M.; Czarny, P.; Miller, E.; Synowiec, E.; Sliwinski, T.; Saluk-Bijak, J. Increase in blood levels of growth factors involved in the neuroplasticity process by using an extremely low frequency electromagnetic field in post-stroke patients. Front. Aging Neurosci. 2018, 10, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorberboym, M.; Blankenberg, F.G.; Sadeh, M.; Lampl, Y. In vivo imaging of apoptosis in patients with acute stroke: Correlation with blood-brain barrier permeability. Brain Res. 2006, 1103, 13–19. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Pérez-Cejas, A.; González-Rivero, A.F.; Sabatel, R.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; et al. Non-Survivor Ischemic Stroke Patients Maintain High Serum Caspase-Cleaved Cytokeratin-18 Levels. Brain Sci. 2020, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Olsen, T.S.; Larsen, B.; Herning, M.; Skriver, E.B.; Lassen, N.A. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke 1983, 14, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Balduini, W.; Carloni, S.; Buonocore, G. Autophagy in hypoxia-ischemia induced brain injury. J. Matern. Fetal Neonatal Med. 2012, 25 (Suppl. 1), 30–34. [Google Scholar] [CrossRef]
- Deng, Y.H.; He, H.Y.; Yang, L.Q.; Zhang, P.Y. Dynamic changes in neuronal autophagy and apoptosis in the ischemic penumbra following permanent ischemic stroke. Neural. Regen. Res. 2016, 11, 1108–1114. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Yang, Y.; Zhai, M.; Shao, X.; Yan, Z.; Zhang, X.; Wu, Y.; Cao, L.; Sui, B.; et al. Differential intensity-dependent effects of pulsed electromagnetic fields on RANKL-induced osteoclast formation, apoptosis, and bone resorbing ability in RAW264.7 cells. Bioelectromagnetics 2017, 38, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Tenuzzo, B.; Vergallo, C.; Dini, L. Effect of 6mT static magnetic field on the bcl-2, bax, p53 and hsp70 expression in freshly isolated and in vitro aged human lymphocytes. Tissue Cell 2009, 41, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.R.; Nakahara, T.; Hirose, H.; Koyama, S.; Takashima, Y.; Miyakoshi, J. Extremely low frequency magnetic fields and the promotion of H2O2-induced cell death in HL-60 cells. Int. J. Radiat. Biol. 2004, 80, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhou, J.; Luo, Q.L.; Liu, H.F.; He, C.Q. Pulsed electromagnetic field therapy inhibits chondrocyte apoptosis in rabbits with osteoarthritis. Sichuan Da Xue Xue Bao Yi Xue Ban 2014, 45, 107–110. [Google Scholar] [PubMed]
- Vincenzi, F.; Targa, M.; Corciulo, C.; Gessi, S.; Merighi, S.; Setti, S.; Cadossi, R.; Borea, P.A.; Varani, K. The anti-tumor effect of A3 adenosine receptors is potentiated by pulsed electromagnetic fields in cultured neural cancer cells. PLoS ONE 2012, 7, e39317. [Google Scholar] [CrossRef]
- Ma, Q.; Deng, P.; Zhu, G.; Liu, C.; Zhang, L.; Zhou, Z.; Luo, X.; Li, M.; Zhong, M.; Yu, Z.; et al. Extremely low-frequency electromagnetic fields affect transcript levels of neuronal differentiation-related genes in embryonic neural stem cells. PLoS ONE 2014, 9, e90041. [Google Scholar] [CrossRef]
- Grassi, C.; D’Ascenzo, M.; Torsello, A.; Martinotti, G.; Wolf, F.; Cittadini, A.; Azzena, G.B. Effects of 50 Hz electromagnetic fields on voltage-gated Ca2+ channels and their role in modulation of neuroendocrine cell proliferation and death. Cell Calcium 2004, 35, 307–315. [Google Scholar] [CrossRef]
- Morgado-Valle, C.; Verdugo-Díaz, L.; García, D.E.; Morales-Orozco, C.; Drucker-Colín, R. The role of voltage-gated Ca2+ channels in neurite growth of cultured chromaffin cells induced by extremely low frequency (ELF) magnetic field stimulation. Cell Tissue Res. 1998, 291, 217–230. [Google Scholar] [CrossRef]
- Pall, M.L. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell. Mol. Med. 2013, 17, 958–965. [Google Scholar] [CrossRef]
- Sun, Z.C.; Ge, J.L.; Guo, B.; Guo, J.; Hao, M.; Wu, Y.C.; Lin, Y.A.; La, T.; Yao, P.T.; Mei, Y.A.; et al. Extremely low frequency electromagnetic fields facilitate vesicle endocytosis by increasing presynaptic calcium channel expression at a central synapse. Sci. Rep. 2016, 6, 21774. [Google Scholar] [CrossRef] [Green Version]
- Martinou, J.C.; Green, D.R. Breaking the mitochondrial barrier. Nat. Rev. Mol. Cell Biol. 2001, 2, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Harwood, S.M.; Yaqoob, M.M.; Allen, D.A. Caspase and calpain function in cell death: Bridging the gap between apoptosis and necrosis. Ann. Clin. Biochem. 2005, 42, 415–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morabito, C.; Guarnieri, S.; Fanò, G.; Mariggiò, M.A. Effects of acute and chronic low frequency electromagnetic field exposure on PC12 cells during neuronal differentiation. Cell. Physiol. Biochem. 2010, 26, 947–958. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cichon, N.; Synowiec, E.; Miller, E.; Sliwinski, T.; Ceremuga, M.; Saluk-Bijak, J.; Bijak, M. Effect of Rehabilitation with Extremely Low Frequency Electromagnetic Field on Molecular Mechanism of Apoptosis in Post-Stroke Patients. Brain Sci. 2020, 10, 266. https://doi.org/10.3390/brainsci10050266
Cichon N, Synowiec E, Miller E, Sliwinski T, Ceremuga M, Saluk-Bijak J, Bijak M. Effect of Rehabilitation with Extremely Low Frequency Electromagnetic Field on Molecular Mechanism of Apoptosis in Post-Stroke Patients. Brain Sciences. 2020; 10(5):266. https://doi.org/10.3390/brainsci10050266
Chicago/Turabian StyleCichon, Natalia, Ewelina Synowiec, Elzbieta Miller, Tomasz Sliwinski, Michal Ceremuga, Joanna Saluk-Bijak, and Michal Bijak. 2020. "Effect of Rehabilitation with Extremely Low Frequency Electromagnetic Field on Molecular Mechanism of Apoptosis in Post-Stroke Patients" Brain Sciences 10, no. 5: 266. https://doi.org/10.3390/brainsci10050266
APA StyleCichon, N., Synowiec, E., Miller, E., Sliwinski, T., Ceremuga, M., Saluk-Bijak, J., & Bijak, M. (2020). Effect of Rehabilitation with Extremely Low Frequency Electromagnetic Field on Molecular Mechanism of Apoptosis in Post-Stroke Patients. Brain Sciences, 10(5), 266. https://doi.org/10.3390/brainsci10050266






