The Effect of Mental Fatigue on Neuromuscular Function is Similar in Young and Older Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Questionnaires
2.3. Neuromuscular Measures
2.4. Mental Fatigue Task
2.5. Statistical Analyses
3. Results
3.1. Participant Characteristics
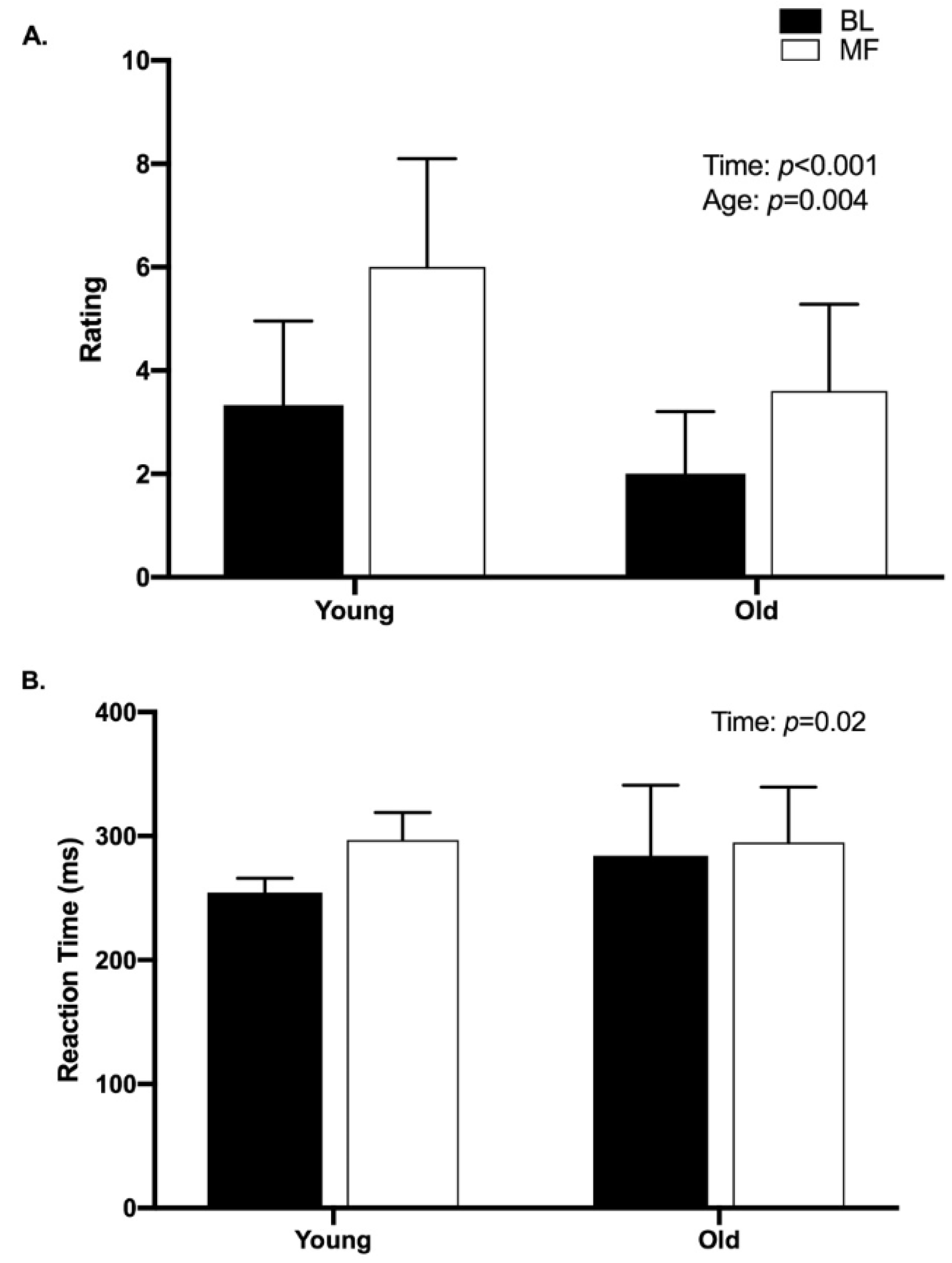
3.2. Mental Fatigue
3.3. Force
3.4. Neuromuscular Measurements
4. Discussion
4.1. Baseline Measurements
4.2. Mental Fatigue and Force Output
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boksem, M.A.; Tops, M. Mental fatigue: Costs and benefits. Brain Res. Rev. 2008, 59, 125–139. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, D.; Massar, S.A.; Schellekens, A.F.; Ellenbroek, B.A.; Verkes, R.J. Disrupted sensorimotor gating due to mental fatigue: Preliminary evidence. Int. J. Psychophysiol. 2006, 62, 168–174. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, D.; Frese, M.; Meijman, T.F. Mental fatigue and the control of cognitive processes: Effects on perseveration and planning. Acta Psychol. (Amst) 2003, 113, 45–65. [Google Scholar] [CrossRef]
- Marcora, S.M.; Staiano, W.; Manning, V. Mental fatigue impairs physical performance in humans. J. Appl. Physiol. (1985) 2009, 106, 857–864. [Google Scholar] [CrossRef]
- Zering, J.C.; Brown, D.M.Y.; Graham, J.D.; Bray, S.R. Cognitive control exertion leads to reductions in peak power output and [Formula: See text] as well as increased perceived exertion on a graded exercise test to exhaustion. J. Sports Sci. 2017, 35, 1–9. [Google Scholar] [CrossRef]
- Smith, M.R.; Marcora, S.M.; Coutts, A.J. Mental Fatigue Impairs Intermittent Running Performance. Med. Sci. Sports Exerc. 2015, 47, 1682–1690. [Google Scholar] [CrossRef]
- Smith, M.R.; Coutts, A.J.; Merlini, M.; Deprez, D.; Lenoir, M.; Marcora, S.M. Mental Fatigue Impairs Soccer-Specific Physical and Technical Performance. Med. Sci. Sports Exerc. 2016, 48, 267–276. [Google Scholar] [CrossRef]
- Bray, S.R.; Graham, J.D.; Martin Ginis, K.A.; Hicks, A.L. Cognitive task performance causes impaired maximum force production in human hand flexor muscles. Biol. Psychol. 2012, 89, 195–200. [Google Scholar] [CrossRef]
- Pageaux, B.; Marcora, S.M.; Lepers, R. Prolonged mental exertion does not alter neuromuscular function of the knee extensors. Med. Sci. Sports Exerc. 2013, 45, 2254–2264. [Google Scholar] [CrossRef]
- Le Mansec, Y.; Pageaux, B.; Nordez, A.; Dorel, S.; Jubeau, M. Mental fatigue alters the speed and the accuracy of the ball in table tennis. J. Sports Sci. 2018, 36, 2751–2759. [Google Scholar] [CrossRef]
- Pageaux, B.; Marcora, S.M.; Rozand, V.; Lepers, R. Mental fatigue induced by prolonged self-regulation does not exacerbate central fatigue during subsequent whole-body endurance exercise. Front. Hum. Neurosci. 2015, 9, 67. [Google Scholar] [CrossRef]
- Budini, F.; Lowery, M.; Durbaba, R.; De Vito, G. Effect of mental fatigue on induced tremor in human knee extensors. J. Electromyogr. Kinesiol. 2014, 24, 412–418. [Google Scholar] [CrossRef]
- Pageaux, B.; Lepers, R. The effects of mental fatigue on sport-related performance. Prog. Brain Res. 2018, 240, 291–315. [Google Scholar]
- Avlund, K. Fatigue in older adults: An early indicator of the aging process? Aging Clin. Exp. Res. 2010, 22, 100–115. [Google Scholar] [CrossRef]
- Chen, M.K. The epidemiology of self-perceived fatigue among adults. Prev. Med. 1986, 15, 74–81. [Google Scholar] [CrossRef]
- Lin, J.M.; Brimmer, D.J.; Maloney, E.M.; Nyarko, E.; Belue, R.; Reeves, W.C. Further validation of the Multidimensional Fatigue Inventory in a US adult population sample. Popul. Health. Metr. 2009, 7, 18. [Google Scholar] [CrossRef]
- Loge, J.H.; Ekeberg, O.; Kaasa, S. Fatigue in the general Norwegian population: Normative data and associations. J. Psychosom. Res. 1998, 45, 53–65. [Google Scholar] [CrossRef]
- Hardy, S.E.; Studenski, S.A. Fatigue predicts mortality in older adults. J. Am. Geriatr. Soc. 2008, 56, 1910–1914. [Google Scholar] [CrossRef]
- Rankin, J.K.; Woollacott, M.H.; Shumway-Cook, A.; Brown, L.A. Cognitive influence on postural stability: A neuromuscular analysis in young and older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M112-9. [Google Scholar] [CrossRef]
- Manierre, M.; Jansen, E.; Boolani, A. Sleep quality and sex modify the relationships between trait energy and fatigue on state energy and fatigue. PLoS ONE 2020, 15, e0227511. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Orth, M.; Rothwell, J.C. The cortical silent period: Intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin. Neurophysiol. 2004, 115, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.; Fling, B.; Crews, R.T.; Mulwitz, L.A.; Kamen, G. Reliability of motor-evoked potentials in the ADM muscle of older adults. J. Neurosci. Methods 2007, 164, 320–324. [Google Scholar] [CrossRef]
- Basner, M.; Mollicone, D.; Dinges, D.F. Validity and Sensitivity of a Brief Psychomotor Vigilance Test (PVT-B) to Total and Partial Sleep Deprivation. Acta Astronaut. 2011, 69, 949–959. [Google Scholar] [CrossRef]
- Lee, I.S.; Bardwell, W.A.; Ancoli-Israel, S.; Dimsdale, J.E. Number of lapses during the psychomotor vigilance task as an objective measure of fatigue. J. Clin. Sleep Med. 2010, 6, 163–168. [Google Scholar] [CrossRef]
- Lim, J.; Ebstein, R.; Tse, C.Y.; Monakhov, M.; Lai, P.S.; Dinges, D.F.; Kwok, K. Dopaminergic polymorphisms associated with time-on-task declines and fatigue in the Psychomotor Vigilance Test. PLoS ONE 2012, 7, e33767. [Google Scholar] [CrossRef]
- van der Linden, D.; Eling, P. Mental fatigue disturbs local processing more than global processing. Psychol. Res. 2006, 70, 395–402. [Google Scholar] [CrossRef]
- Lim, J.; Wu, W.C.; Wang, J.; Detre, J.A.; Dinges, D.F.; Rao, H. Imaging brain fatigue from sustained mental workload: An ASL perfusion study of the time-on-task effect. Neuroimage 2010, 49, 3426–3435. [Google Scholar] [CrossRef]
- Schwarz, R.; Krauss, O.; Hinz, A. Fatigue in the general population. Onkologie 2003, 26, 140–144. [Google Scholar] [CrossRef]
- Woollacott, M.H.; Tang, P.F. Balance control during walking in the older adult: Research and its implications. Phys. Ther. 1997, 77, 646–660. [Google Scholar] [CrossRef]
- Kent-Braun, J.A.; Ng, A.V. Specific strength and voluntary muscle activation in young and elderly women and men. J. Appl. Physiol. (1985) 1999, 87, 22–29. [Google Scholar] [CrossRef]
- Lanza, I.R.; Russ, D.W.; Kent-Braun, J.A. Age-related enhancement of fatigue resistance is evident in men during both isometric and dynamic tasks. J. Appl. Physiol. (1985) 2004, 97, 967–975. [Google Scholar] [CrossRef]
- McNeil, C.J.; Vandervoort, A.A.; Rice, C.L. Peripheral impairments cause a progressive age-related loss of strength and velocity-dependent power in the dorsiflexors. J. Appl. Physiol. (1985) 2007, 102, 1962–1968. [Google Scholar] [CrossRef]
- McNeil, C.J.; Doherty, T.J.; Stashuk, D.W.; Rice, C.L. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 2005, 31, 461–467. [Google Scholar] [CrossRef]
- Johnson, M.A.; Polgar, J.; Weightman, D.; Appleton, D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J. Neurol. Sci. 1973, 18, 111–129. [Google Scholar] [CrossRef]
- Winegard, K.J.; Hicks, A.L.; Sale, D.G.; Vandervoort, A.A. A 12-year follow-up study of ankle muscle function in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 1996, 51, B202–B207. [Google Scholar] [CrossRef]
- Larsson, L.; Sjodin, B.; Karlsson, J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol. Scand. 1978, 103, 31–39. [Google Scholar] [CrossRef]
- Klass, M.; Baudry, S.; Duchateau, J. Aging does not affect voluntary activation of the ankle dorsiflexors during isometric, concentric, and eccentric contractions. J. Appl. Physiol. (1985) 2005, 99, 31–38. [Google Scholar] [CrossRef]
- Fling, B.W.; Knight, C.A.; Kamen, G. Relationships between motor unit size and recruitment threshold in older adults: Implications for size principle. Exp. Brain Res. 2009, 197, 125–133. [Google Scholar] [CrossRef]
- Roos, M.R.; Rice, C.L.; Vandervoort, A.A. Age-related changes in motor unit function. Muscle Nerve 1997, 20, 679–690. [Google Scholar] [CrossRef]
- McGinley, M.; Hoffman, R.L.; Russ, D.W.; Thomas, J.S.; Clark, B.C. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp. Gerontol. 2010, 45, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.A.; Seidler, R.D. Evidence for motor cortex dedifferentiation in older adults. Neurobiol. Aging 2012, 33, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Park, D.C.; Polk, T.A.; Mikels, J.A.; Taylor, S.F.; Marshuetz, C. Cerebral aging: Integration of brain and behavioral models of cognitive function. Dialogues Clin. Neurosci. 2001, 3, 151–165. [Google Scholar] [PubMed]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- De Beaumont, L.; Lassonde, M.; Leclerc, S.; Theoret, H. Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery 2007, 61, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Priori, A.; Berardelli, A.; Inghilleri, M.; Accornero, N.; Manfredi, M. Motor cortical inhibition and the dopaminergic system. Pharmacological changes in the silent period after transcranial brain stimulation in normal subjects, patients with Parkinson’s disease and drug-induced parkinsonism. Brain 1994, 117, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Aalto, S.; Bruck, A.; Laine, M.; Nagren, K.; Rinne, J.O. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: A positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J. Neurosci. 2005, 25, 2471–2477. [Google Scholar] [CrossRef]
- Martin, K.; Meeusen, R.; Thompson, K.G.; Keegan, R.; Rattray, B. Mental Fatigue Impairs Endurance Performance: A Physiological Explanation. Sports Med. 2018, 48, 2041–2051. [Google Scholar] [CrossRef]
- Christie, M.A.; Bolortuya, Y.; Chen, L.C.; McKenna, J.T.; McCarley, R.W.; Strecker, R.E. Microdialysis elevation of adenosine in the basal forebrain produces vigilance impairments in the rat psychomotor vigilance task. Sleep 2008, 31, 1393–1398. [Google Scholar]
- Davis, J.M.; Zhao, Z.; Stock, H.S.; Mehl, K.A.; Buggy, J.; Hand, G.A. Central nervous system effects of caffeine and adenosine on fatigue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R399–R404. [Google Scholar] [CrossRef]
- Doherty, M.; Smith, P.M. Effects of caffeine ingestion on rating of perceived exertion during and after exercise: A meta-analysis. Scand. J. Med. Sci. Sports 2005, 15, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Lovatt, D.; Xu, Q.; Liu, W.; Takano, T.; Smith, N.A.; Schnermann, J.; Tieu, K.; Nedergaard, M. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc. Natl. Acad. Sci. USA 2012, 109, 6265–6270. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.F.; Stewart, K.; Arseneault, R.; Moineddin, R.; Cellarius, V.; Librach, S.L.; Dudgeon, D. Women experience higher levels of fatigue than men at the end of life: A longitudinal home palliative care study. J. Pain Symptom Manag. 2007, 33, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Kiers, L.; Cros, D.; Chiappa, K.H.; Fang, J. Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 1993, 89, 415–423. [Google Scholar] [CrossRef]
- Harden, C.L. Transcranial magnetic stimulation across the menstrual cycle: What do hormones have to do with it? Epilepsy Curr. 2014, 14, 17–18. [Google Scholar] [CrossRef][Green Version]


| YW (n = 9) | OW (n = 16) | ES (d) | |
|---|---|---|---|
| Age, year * | 22.4 ± 2.9 | 74.1 ± 6.3 | 8.5 |
| Height, in | 65.4 ± 3.4 | 64.1 ± 2.1 | 0.29 |
| Weight, kg | 56.2 ± 12.8 | 60.5 ± 8.8 | 0.25 |
| MFI * | 48.1 ± 25.5 | 55.0 ± 14.9 | 0.21 |
| PSQI | 5.3 ± 2.8 | 4.5 ± 2.1 | 0.21 |
| SFR | 3.3 ± 1.6 | 2.0 ± 1.2 | 0.59 |
| YF (n = 9) | OF (n = 16) | |||||
|---|---|---|---|---|---|---|
| Baseline | Mental Fatigue | Baseline | Mental Fatigue | ES (d) Age | ES (d) Time | |
| PTF, N | 13.7 ± 5.5 | 12.0 ± 2.6 | 16.5 ± 2.2 | 13.5 ± 5.0 | 0.56 | 0.61 |
| TTP, ms | 86.4 ± 16.8 | 88.8 ± 13.5 | 94.9± 7.7 | 84.8 ± 12.0 | 0.18 | 0.31 |
| HRT, ms | 98.5 ± 10.9 | 89.0 ± 14.8 | 105.9 ± 23.0 | 91.8 ± 23.7 | 0.28 | 0.65 |
| MMax, mv * | 6.0 ± 1.2 | 5.6 ± 1.3 | 4.0 ± 0.8 | 3.5 ± 1.2 | 1.8 | 0.40 |
| CSP, ms | 74.4 ± 6.3 | 81.4 ± 9.7 | 101.4 ± 27.0 | 114.7 ± 30.2 | 1.6 | 0.56 |
| MEP, %MMax | 27.8 ± 8.0 | 23.0 ± 7.9 | 42.9 ± 15.2 | 45.8 ± 17.1 | 1.6 | 0.08 |
| YF (n = 9) | OF (n = 16) | |||||
|---|---|---|---|---|---|---|
| Baseline | Mental Fatigue | Baseline | Mental Fatigue | ES (d) Time | ES (d) Age | |
| False Starts * † | 0.38 ± 0.52 | 0.25 ± 0.46 | 2.13 ± 1.5 | 0.75 ± 1.18 | 1.2 | 0.76 |
| Lapses | 0.13 ± 0.35 | 0.63 ± 1.06 | 1.44 ± 1.5 | 0.63 ± 1.36 | 0.61 | 0.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morris, A.J.; Christie, A.D. The Effect of Mental Fatigue on Neuromuscular Function is Similar in Young and Older Women. Brain Sci. 2020, 10, 191. https://doi.org/10.3390/brainsci10040191
Morris AJ, Christie AD. The Effect of Mental Fatigue on Neuromuscular Function is Similar in Young and Older Women. Brain Sciences. 2020; 10(4):191. https://doi.org/10.3390/brainsci10040191
Chicago/Turabian StyleMorris, Amanda J., and Anita D. Christie. 2020. "The Effect of Mental Fatigue on Neuromuscular Function is Similar in Young and Older Women" Brain Sciences 10, no. 4: 191. https://doi.org/10.3390/brainsci10040191
APA StyleMorris, A. J., & Christie, A. D. (2020). The Effect of Mental Fatigue on Neuromuscular Function is Similar in Young and Older Women. Brain Sciences, 10(4), 191. https://doi.org/10.3390/brainsci10040191




