Evaluating Preschool Visual Attentional Selective-Set: Preliminary ERP Modeling and Simulation of Target Enhancement Homology
Abstract
1. Introduction
2. Materials and Methods
2.1. Part 1: Reanalysis of Children’s ERP Data
2.1.1. Participants
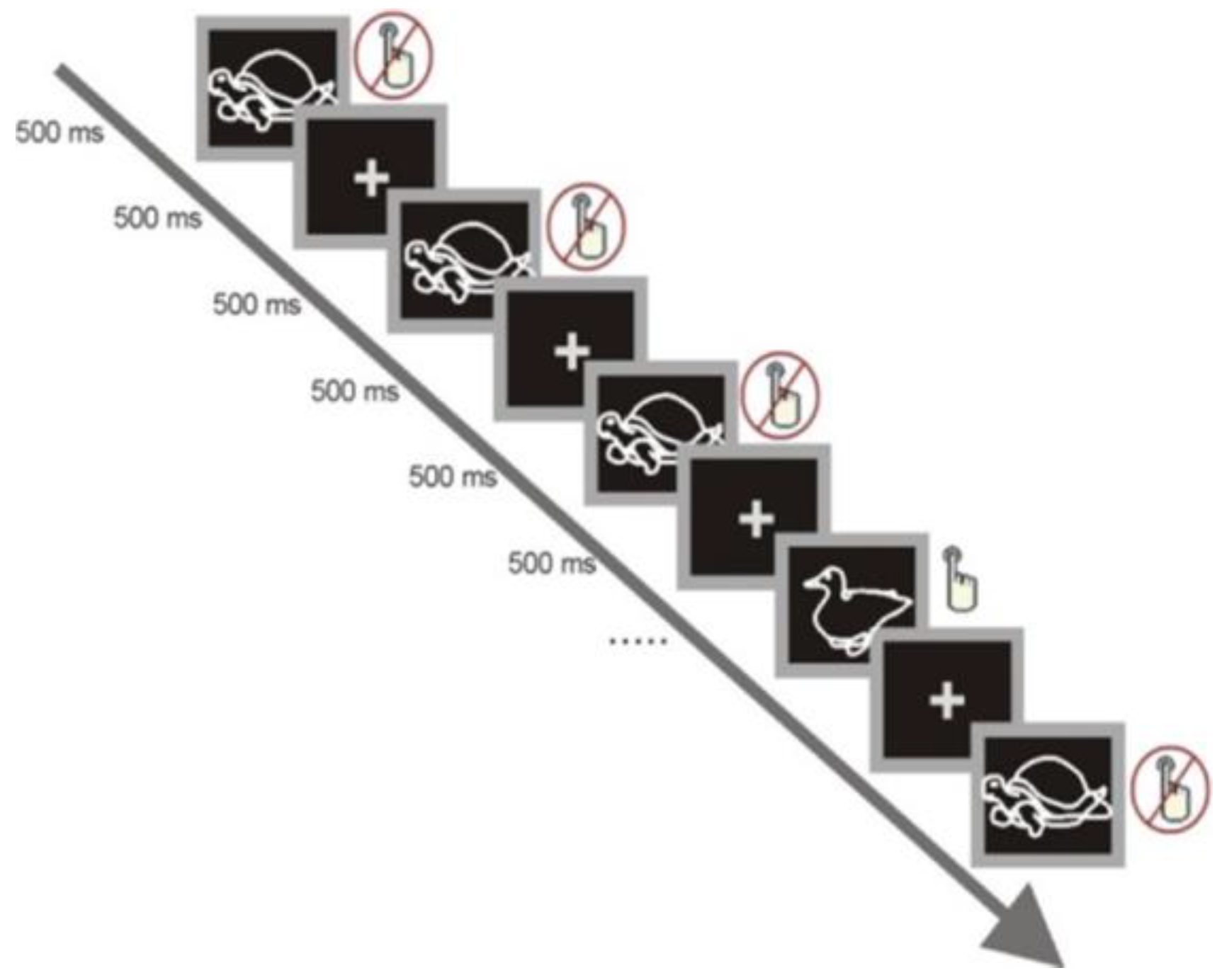
2.1.2. Visual Sustained Selective-set Attention Task (VSSAT)
2.1.3. Procedure
2.1.4. Data Collection and Processing
2.1.5. ERP Data Analysis
2.1.6. ERP Activity Paths
2.2. Part 2: Modeling of Children’s ERP Data
2.2.1. Independent Component Analysis (Step 1) and Topographic Mapping (Step 2)
2.2.2. Dipole Analysis (Step 3)
2.2.3. Translation to Adult MRI Template (Step 4)
2.3. Part 3: Simulation of Adult ERP Data
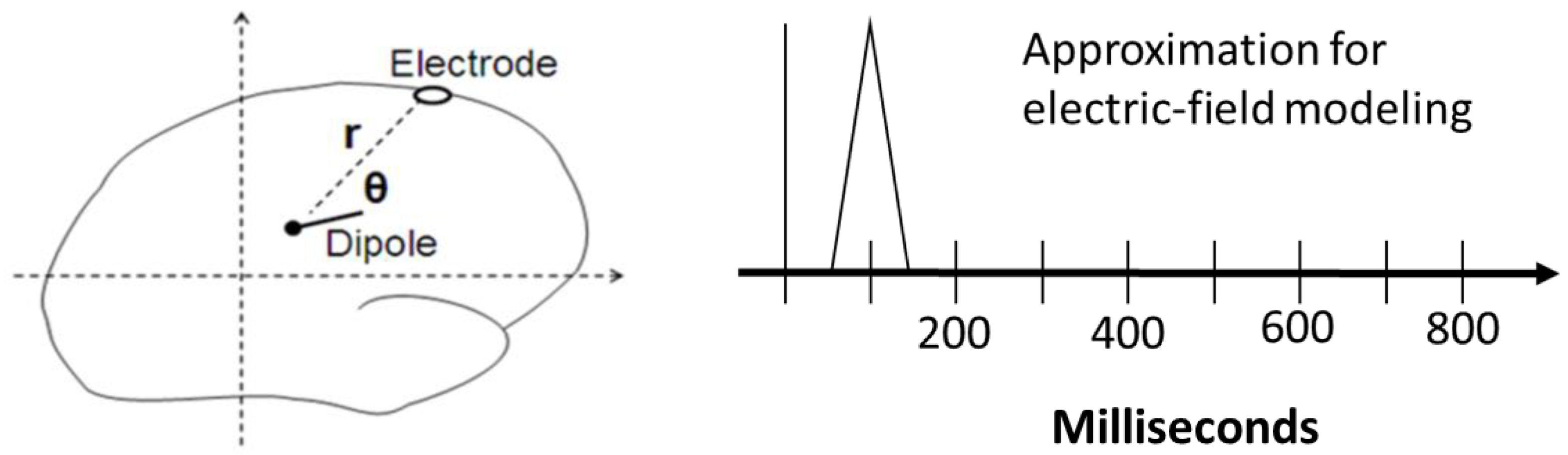
2.3.1. Electric-Field Spiking Modeling (Step 5)
2.3.2. ACT–R Simulation (Step 6)
2.3.3. Adult ACT–R Simulated Dipole Mapping (Step 7)
2.3.4. Adult ACT–R Simulated Spike Series (Polyspiking) (Step 8)
2.3.5. Adult ACT–R Simulated ERP Topographical Mapping (Step 9)
2.3.6. Actual vs. Simulated Data Comparisons: Homology Tests
MRI-mappings and Structural Test
Topographical Mappings and Functional Test
3. Results
3.1. Behavioral Performance
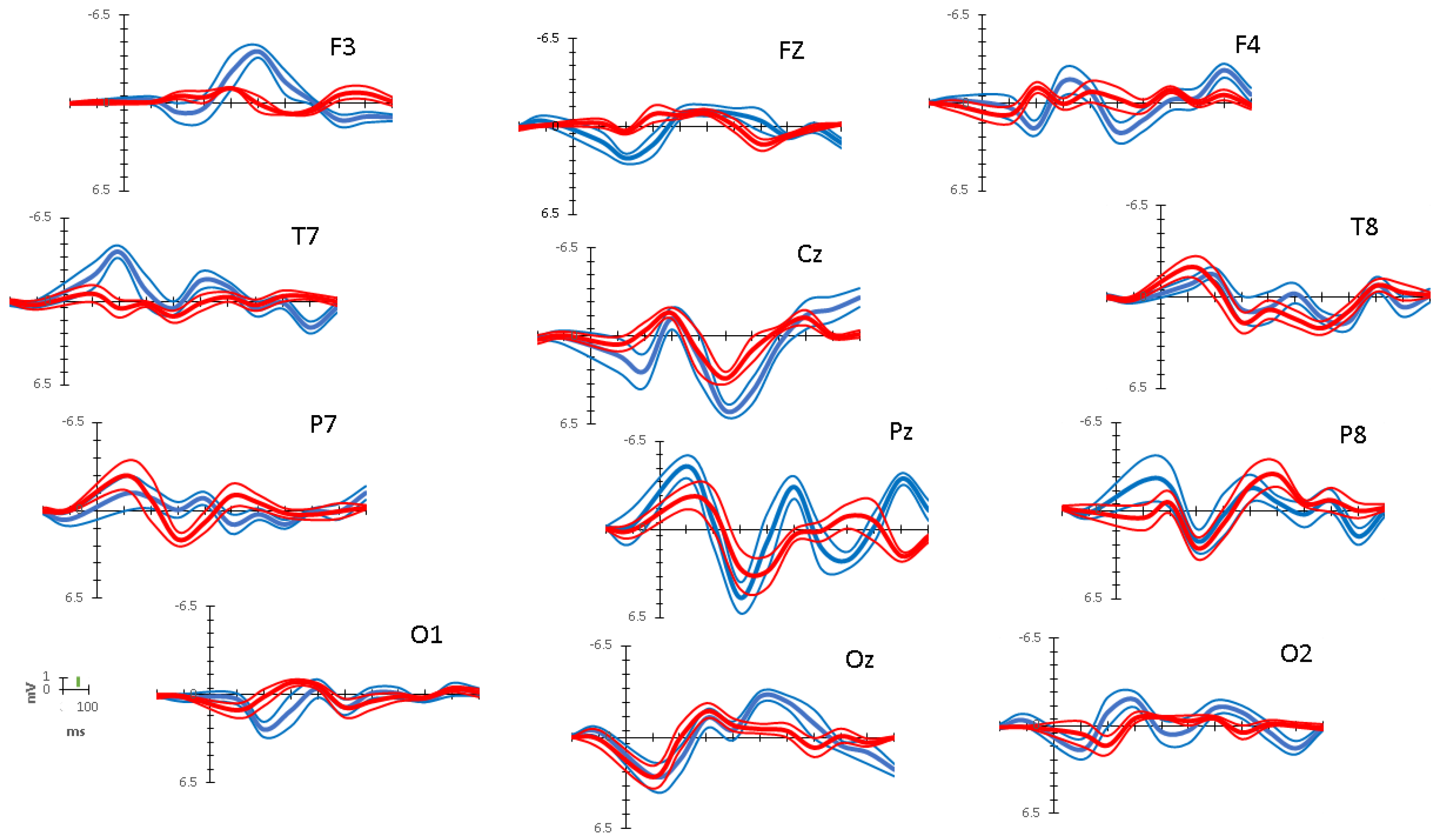
3.2. ERP Data
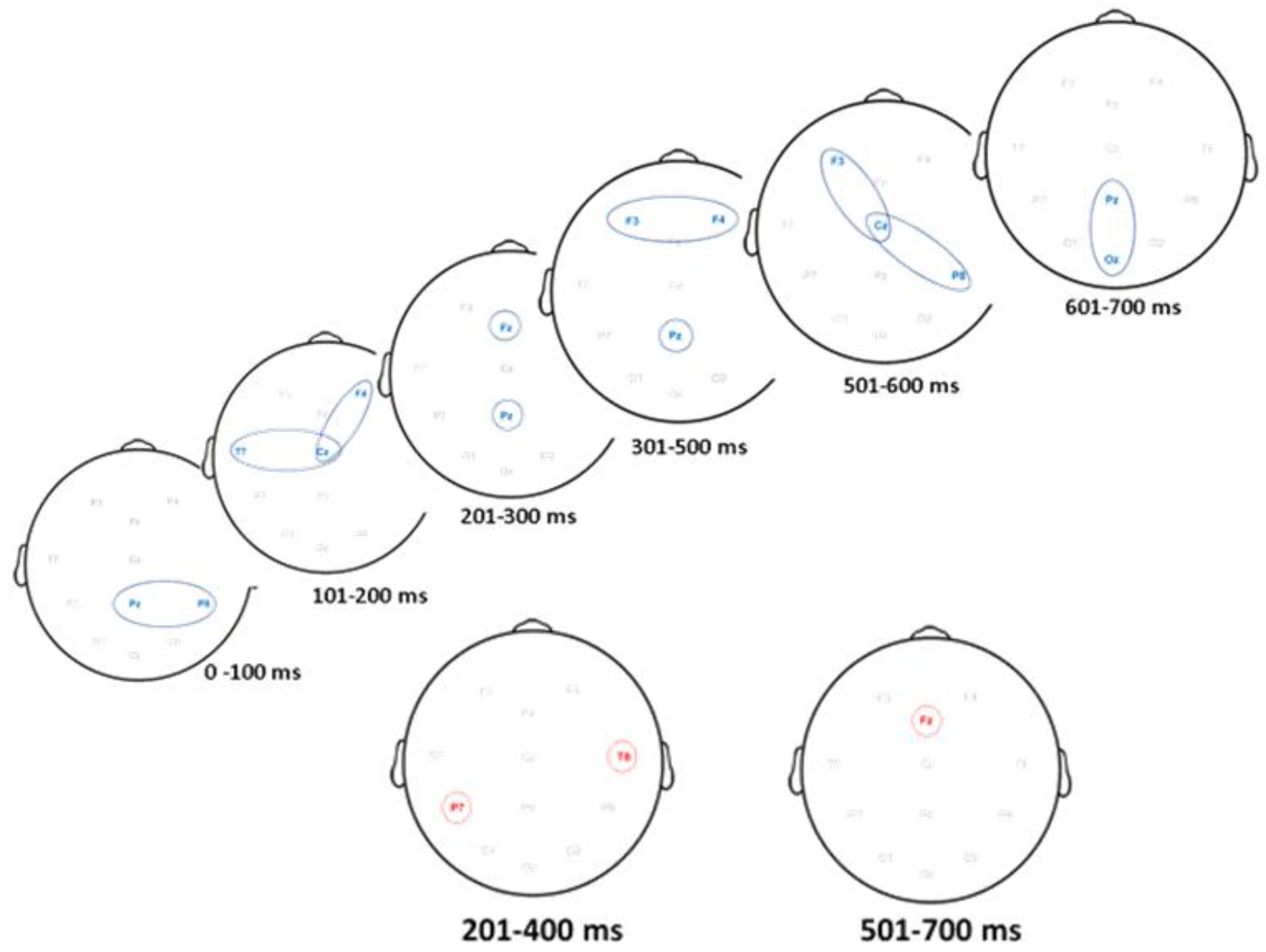
3.3. ERP Activity Paths
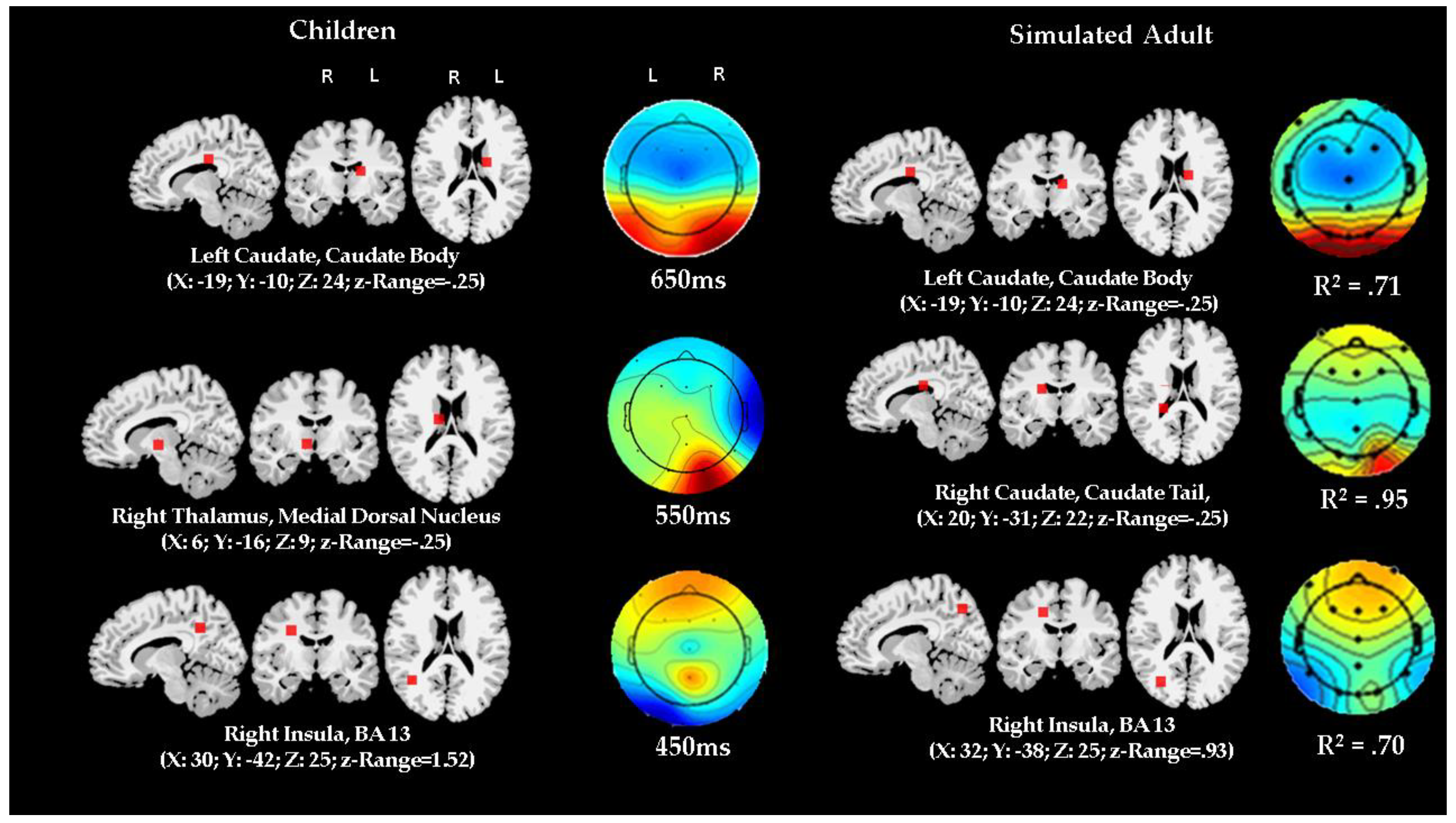
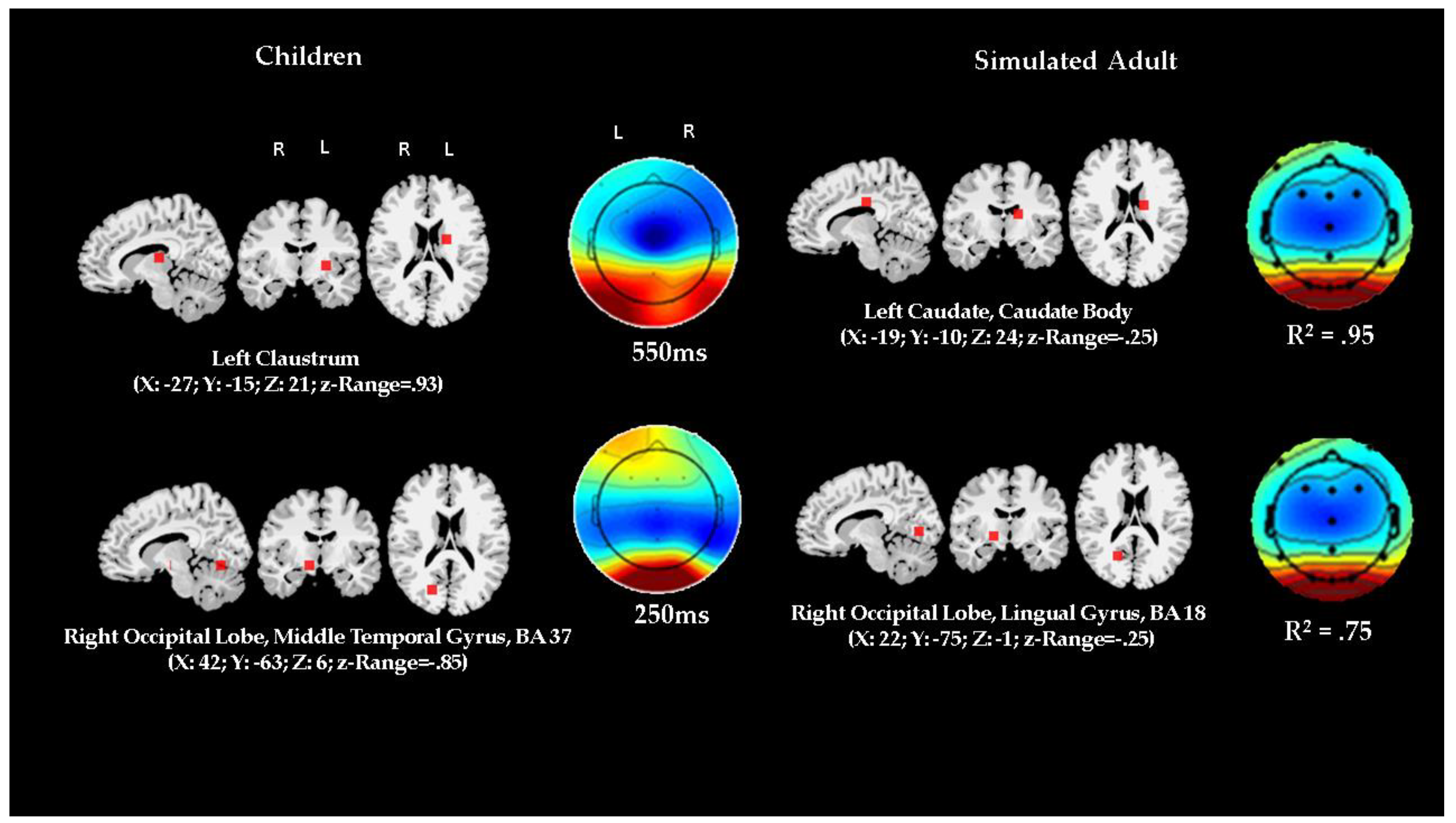
3.4. Comparison of ERP Activity and Localization: Preschool Data vs. Adult Simulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kahneman, D.; Treisman, A. Changing views of attention and automaticity. In Varieties of Attention; Parasuraman, R., Davies, D.R., Eds.; Academic Press: New York, NY, USA, 1984; pp. 29–61. [Google Scholar]
- Fisher, A.V.; Thiessen, E.; Godwin, K.; Kloos, H.; Dickerson, J. Assessing selective sustained attention in 3- to 5-year-old children: Evidence from a new paradigm. J. Exp. Child Psychol. 2012, 114, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.; Rothbart, M.K.; Voelker, P. Developing brain networks of attention. Curr. Opin. Pediatr. 2016, 28, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.; Rothbart, M.K.; Sheese, B.E.; Voelker, P. Developing Attention: Behavioral and Brain Mechanisms. Adv. Neurosci. 2014, 2014, 405094. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Rothbart, M.K.; Posner, M. Development of executive attention in preschool children. Dev. Sci. 2003, 6, 498–504. [Google Scholar] [CrossRef]
- Jodo, E.; Kayama, Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalogr. Clin. Neurophysiol. 1992, 82, 477–482. [Google Scholar] [CrossRef]
- Johnstone, S.J.; Pleffer, C.B.; Barry, R.J.; Clarke, A.R.; Smith, J.L. Development of inhibitory processing during the go/nogo task. J. Psychophysiol. 2005, 19, 11–23. [Google Scholar] [CrossRef]
- Lahat, A.; Todd, R.M.; Mahy, C.E.V.; Lau, K.; Zelazo, P.D. Neurophysiological Correlates of Executive Function: A Comparison of European-Canadian and Chinese-Canadian 5-Year-Old Children. Front. Hum. Neurosci. 2010, 3, 72. [Google Scholar] [CrossRef]
- Lamm, C.; Zelazo, P.D.; Lewis, M.D. Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychology 2006, 44, 2139–2148. [Google Scholar] [CrossRef]
- Jonkman, L.M. The development of preparation, conflict monitoring and inhibition from early childhood to young adulthood; a Go/Nogo ERP study. Brain Res. 2006, 1097, 181–193. [Google Scholar] [CrossRef]
- Kaganovich, N.; Ancel, E. Different neural processes underlie visual speech perception in school-age children and adults: An event-related potentials study. J. Exp. Child Psychol. 2019, 184, 98–122. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, J.; Luo, Y.; Zhao, D.; Yang, J. Intelligence and information processing during a visual search task in children: An event-related potential study. NeuroReport 2006, 17, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhao, J.; Weng, X.; Li, S. Event-related potential evidence in Chinese children. Int. J. Behav. Dev. 2017, 42, 311–320. [Google Scholar] [CrossRef]
- Kim, S.H.; Buzzell, G.; Faja, S.; Choi, Y.B.; Thomas, H.R.; Brito, N.H.; Shuffrey, L.C.; Fifer, W.P.; Morrison, F.D.; Lord, C.; et al. Neural dynamics of executive function in cognitively able kindergarteners with autism spectrum disorders as predictors of concurrent academic achievement. Autism 2019, 23. [Google Scholar] [CrossRef] [PubMed]
- Grammer, J.K.; Carrasco, M.; Gehring, W.J.; Morrison, F.J. Age-related changes in error processing in young children: A school-based investigation. Dev. Cogn. Neurosci. 2014, 9, 93–105. [Google Scholar] [CrossRef]
- Kim, M.H.; Grammer, J.K.; Marulis, L.M.; Carrasco, M.; Morrison, F.J.; Gehring, W.J. Early math and reading achievement are associated with the error positivity. Dev. Cogn. Neurosci. 2016, 22, 18–26. [Google Scholar] [CrossRef]
- Ridderinkhof, K.R.; Van Der Stelt, O. Attention and selection in the growing child: Views derived from developmental psychophysiology. Boil. Psychol. 2000, 54, 55–106. [Google Scholar] [CrossRef]
- Courchesne, E. Chronology of postnatal human brain development: Event-related potential, positron emission tomography, myelinogenesis, and synaptogenesis studies. In Related Brain Potentials: Basic Issues and Applications; Rohrbaugh, J.W., Parasuraman, R., Johnson, R., Eds.; Oxford University Press: New York, NY, USA, 1990; pp. 210–241. [Google Scholar]
- Lickliter, R.; Bahrick, L.E. The concept of homology as a basis for evaluating developmental mechanisms: Exploring selective attention across the life-span. Dev. Psychobiol. 2012, 55, 76–83. [Google Scholar] [CrossRef]
- Ruff, H.A.; Rothbart, M.K. Attention in early development. In Themes and Variations; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Rueda, M.R.; Posner, M.I.; Rothbart, M.K. Attentional control and self-regulation. In Handbook of Self-Regulation: Research, Theory, and Applications, 2nd ed.; Baumeister, R.F., Vohs, K.D., Eds.; Guilford Press: New York, NY, USA, 2011; pp. 284–299. [Google Scholar]
- Casey, B.; Tottenham, N.; Liston, C.; Durston, S. Imaging the developing brain: What have we learned about cognitive development? Trends Cogn. Sci. 2005, 9, 104–110. [Google Scholar] [CrossRef]
- Tsujimoto, S. The Prefrontal Cortex: Functional Neural Development during Early Childhood. Neuroscientist 2008, 14, 345–358. [Google Scholar] [CrossRef]
- Brown, T.T.; Jernigan, T.L. Brain development during the preschool years. Neuropsychol. Rev. 2012, 22, 313–333. [Google Scholar] [CrossRef]
- Chevalier, N.; Kurth, S.; Doucette, M.R.; Wiseheart, M.; Deoni, S.C.L.; Dean, D.C.; O’Muircheartaigh, J.; Blackwell, K.A.; Munakata, Y.; LeBourgeois, M.K. Myelination Is Associated with Processing Speed in Early Childhood: Preliminary Insights. PLoS ONE 2015, 10, e0139897. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C.; O’Muircheartaigh, J.; Elison, J.T.; Walker, L.; Doernberg, E.; Waskiewicz, N.; Dirks, H.; Piryatinsky, I.; Dean, D.C.; Jumbé, N.L. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct. Funct. 2014, 221, 1189–1203. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.D. Change in the Brain’s white matter: The role of the brain’s white matter in active learning and memory may be underestimated. Science 2010, 330, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Dehaene-Lambertz, G.; Soarès, C.; Cointepas, Y.; Le Bihan, D.; Hertz-Pannier, L. Microstructural Correlates of Infant Functional Development: Example of the Visual Pathways. J. Neurosci. 2008, 28, 1943–1948. [Google Scholar] [CrossRef]
- Jolles, D.; Wassermann, D.; Chokhani, R.; Richardson, J.; Tenison, C.; Bammer, R.; Fuchs, L.; Supekar, K.; Menon, V. Plasticity of left perisylvian white-matter tracts is associated with individual differences in math learning. Brain Struct. Funct. 2015, 221, 1337–1351. [Google Scholar] [CrossRef]
- Nagy, Z.; Westerberg, H.; Klingberg, T. Maturation of White Matter is Associated with the Development of Cognitive Functions during Childhood. J. Cogn. Neurosci. 2004, 16, 1227–1233. [Google Scholar] [CrossRef]
- O’Muircheartaigh, J.; Dean, D.C.; Ginestet, C.E.; Walker, L.; Waskiewicz, N.; Lehman, K.; Dirks, H.; Piryatinsky, I.; Deoni, S.C.; Iii, D.C.D. White matter development and early cognition in babies and toddlers. Hum. Brain Mapp. 2014, 35, 4475–4487. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Fields, R.D.; Johansen-Berg, H. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012, 15, 528–536. [Google Scholar] [CrossRef]
- Heinen, F.; Glocker, F.-X.; Fietzek, U.; Meyer, B.-U.; Lücking, C.-H.; Korinthenberg, R. Absence of transcallosal inhibition following focal mangnetic stimulation in preschool children. Ann. Neurol. 1998, 43, 608–612. [Google Scholar] [CrossRef]
- Dai, X.; Hadjipantelis, P.; Wang, J.-L.; Deoni, S.C.L.; Müller, H.-G. Longitudinal associations between white matter maturation and cognitive development across early childhood. Hum. Brain Mapp. 2019, 40, 4130–4145. [Google Scholar] [CrossRef]
- Schafer, R.; Moore, T. Selective Attention from Voluntary Control of Neurons in Prefrontal Cortex. Science 2011, 332, 1568–1571. [Google Scholar] [CrossRef] [PubMed]
- Hopfinger, J.; Luck, S.; Hillyard, S. Selective attention: Electrophysiological and neuromagnetic studies. In The Cognitive Neurosciences III; Gazzaniga, M., Ed.; MIT Press: Cambridge, MA, USA, 2004; pp. 561–574. [Google Scholar]
- Casteau, S.; Smith, D. Associations and Dissociations between Oculomotor Readiness and Covert Attention. Vision 2019, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Akshoomoff, N.A. Selective Attention and Active Engagement in Young Children. Dev. Neuropsychol. 2002, 22, 625–642. [Google Scholar] [CrossRef] [PubMed]
- D’Angiulli, A.; Warburton, W.; Dahinten, V.S.; Hertzman, C. Population-Level Associations between Preschool Vulnerability and Grade-Four Basic Skills. PLoS ONE 2009, 4, e7692. [Google Scholar] [CrossRef]
- D’Angiulli, A.; Griffiths, G.; Marmolejo-Ramos, F. Neural correlates of visualizations of concrete and abstract words in preschool children: A developmental embodied approach. Front. Psychol. 2015, 6, 1–17. [Google Scholar] [CrossRef]
- Gioia, G.A.; Espy, K.A.; Isquith, P.K. Behavior Rating Inventory of Executive Function®–Preschool Version (BRIEF®–P); Psychological Assessment Resources: Odessa, FL, USA, 2003. [Google Scholar]
- Achenbach, T.M. Achenbach System of Empirically Based Assessment (ASEBA): Development, Findings, Theory, and Applications; University of Vermont, Research Center of Children, Youth & Families: Burlington, VT, USA, 2009. [Google Scholar]
- D’Angiulli, A.; Devenyi, P. Retooling computational techniques for EEG-based neurocognitive modeling of children’s data, validity and prospects for learning and education. Front. Comput. Neurosci. 2019, 13, 4. [Google Scholar] [CrossRef]
- Griffiths, G.; West, R.; D’Angiulli, A. Cognitive modeling of Event-Related Potentials. In Proceedings of the 33th Cognitive Science Society Annual Meeting, Boston, MA, USA, 20–23 July 2011. [Google Scholar]
- D’Angiulli, A.; Yeh, W.-H.; Griffiths, G. Correlation between parent-reported executive functions and EEG response during selective-attention and language tasks in preschool children: An event-related and spectral measurement study. Front. Hum. Neurosci. 2010, 82. [Google Scholar] [CrossRef]
- Yeh, W.-H.; D’Angiulli, A. Compensatory Mechanism and Circadian Preference Related Frontal Cortex Activation in Adolescent: The Relationship to Sleep Pattern and Testing Time. Front. Hum. Neurosci. 2010, 4. [Google Scholar] [CrossRef]
- Van Roon, P.; D’Angiulli, A. Preschooler’s ERPs of online/offline visualizations and embodiment theory. In Proceedings of the 36th Annual Conference of the Cognitive Science Society, Quebec City, QC, Canada, 23–26 July 2014. [Google Scholar]
- Ille, N.; Berg, P.; Scherg, M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. J. Clin. Neurophysiol. 2002, 19, 113–124. [Google Scholar] [CrossRef]
- Berg, P.; Scherg, M. A multiple source approach to the correction of eye artifacts. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 229–241. [Google Scholar] [CrossRef]
- Luck, S. An Introduction to the Event-Related Potential Technique; MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Filipović, S.; Andreassi, J.L. Psychophysiology: Human Behavior and Physiological Response. Psychophysiology 2001, 15, 210–212. [Google Scholar] [CrossRef]
- Berman, S.; Friedman, D. The Development of Selective Attention as Reflected by Event-Related Brain Potentials. J. Exp. Child. Psychol. 1995, 59, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, N.; Schibli, K.; D’Angiulli, A. El Sistema-inspired ensemble music training is associated with changes in children’s neurocognitive functional integration: Preliminary ERP evidence. Neurocase 2016, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Vincent, S.B. The Functions of the Vibrissae in the Behavior of the White Rat. Behavior Monographs, 1; University of Chicago: Chicago, IL, USA, 1912. [Google Scholar]
- Hilgard, E.R. Methods and procedures in the study of learning. In Handbook of Experimental Psychology; Stevens, S.S., Ed.; Wiley: New York, NY, USA, 1951; pp. 517–567. [Google Scholar]
- Genest, C. Vincentization Revisited. Ann. Stat. 1992, 20, 1137–1142. [Google Scholar] [CrossRef]
- Rosenthal, R.; Rosnow, R.L.; Rubin, D.B. Contrasts and Effect Sizes in Behavioral Research: A Correlational Approach; Cambridge University Press: New York, NY, USA, 2000. [Google Scholar]
- Cherniak, C. Neural wiring optimization. Prog. Brain Res. 2012, 195, 361–371. [Google Scholar]
- Jung, T.-P.; Makeig, S.; Westerfield, M.; Townsend, J.; Courchesne, E.; Sejnowski, T.J. Analysis and visualization of single-trial event-related potentials. Hum. Brain Mapp. 2001, 14, 166–185. [Google Scholar] [CrossRef]
- Hyvärinen, A.; Oja, E. A Fast Fixed-Point Algorithm for Independent Component Analysis. Neural Comput. 1997, 9, 1483–1492. [Google Scholar] [CrossRef]
- Lancaster, J.; Fox, P. Talairach Client Version 2.4.3; Research Imaging Institute, University of Texas Health Science Center: San Antonio, TX, USA, 2015. [Google Scholar]
- Papademetris, X.; Scheinost, D. Yale Bioimage Suite Web. Dept. of Radiology and Biomedical Imaging, Yale School of Medicine. Available online: https://bioimagesuiteweb.github.io/webapp/ (accessed on 22 February 2020).
- Anderson, J.R.; Lebiere, C. The Atomic Components of Thought; Psychology Press: New York, NY, USA, 2012. [Google Scholar]
- Stewart, T.C.; Eliasmith, C. Neural Symbolic Decision Making: A Scalable and Realistic Foundation for Cognitive Architectures. In Biologically Inspired Cognitive Architectures; Series in Frontiers in Artificial Intelligence and Applications, volume 221; Samsonovich, A.V., Jóhannsdóttir, K.R., Chella, A., Goertzel, B., Eds.; IOS Press: Clifton, VA, USA, 2010; pp. 147–152. [Google Scholar]
- Stewart, T.C.; Eliasmith, C. Spiking neurons and central executive control: The origin of the 50-millisecond cognitive cycle. In Proceedings of the 9th International Conference on Cognitive Modelling 2009; University of Manchester: Manchester, UK, 2009; Volume 122, pp. 130–131. [Google Scholar]
- Brasoveanu, A. Introduction to (Python) ACT-R; semantics seminar: Computing dynamic meanings. 2015. Available online: https://people.ucsc.edu/~abrsvn/Intro_to_Python_ACT-R.pdf (accessed on 20 February 2020).
- Liu, Q.; Zhu, X.; Ziegler, A.; Shi, J. The effects of inhibitory control training for preschoolers on reasoning ability and neural activity. Sci. Rep. 2015, 5, 14200. [Google Scholar] [CrossRef]
- Kim, H. Involvement of the dorsal and ventral attention networks in oddball stimulus processing: A meta-analysis. Hum. Brain Mapp. 2013, 35, 2265–2284. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Vossel, S.; Geng, J.J.; Fink, G.R. Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscience 2014, 20, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.F.; Pessoa, L.; DeSimone, R.; Ungerleider, L. The prefrontal cortex and the executive control of attention. Exp. Brain Res. 2008, 192, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, D.; Levitin, D.J.; Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. USA 2008, 105, 12569–12574. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Chen, T.; Ryali, S.; Kochalka, J.; Li, C.-S.R.; Menon, V. Causal Interactions Within a Frontal-Cingulate-Parietal Network During Cognitive Control: Convergent Evidence from a Multisite-Multitask Investigation. Cereb. Cortex 2015, 26, 2140–2153. [Google Scholar] [CrossRef] [PubMed]
- Pergola, G.; Danet, L.; Pitel, A.-L.; Carlesimo, G.A.; Segobin, S.; Pariente, J.; Suchan, B.; Mitchell, A.S.; Barbeau, E.J. The Regulatory Role of the Human Mediodorsal Thalamus. Trends Cogn. Sci. 2018, 22, 1011–1025. [Google Scholar] [CrossRef]
- Robinson, J.L.; Laird, A.R.; Glahn, D.C.; Blangero, J.; Sanghera, M.K.; Pessoa, L.; Uecker, A.; Friehs, G.; Young, K.; Griffin, J.L.; et al. The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. NeuroImage 2011, 60, 117–129. [Google Scholar] [CrossRef]
- Grahn, J.A.; Parkinson, J.A.; Owen, A.M. The cognitive functions of the caudate nucleus. Prog. Neurobiol. 2008, 86, 141–155. [Google Scholar] [CrossRef]
- Hedden, T.; Gabrieli, J.D.E. Shared and selective neural correlates of inhibition, facilitation, and shifting processes during executive control. NeuroImage 2010, 51, 421–431. [Google Scholar] [CrossRef]
- Ardila, A.; Bernal, B.; Rosselli, M. Language and Visual Perception Associations: Meta-Analytic Connectivity Modeling of Brodmann Area 37. Behav. Neurol. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Brown, S.P.; Mathur, B.; Olsen, S.R.; Luppi, P.-H.; Bickford, M.E.; Citri, A. New Breakthroughs in Understanding the Role of Functional Interactions between the Neocortex and the Claustrum. J. Neurosci. 2017, 37, 10877–10881. [Google Scholar] [CrossRef] [PubMed]
- Leisman, G.; Melillo, R. The basal ganglia: Motor and cognitive relationships in a clinical neurobehavioral context. Rev. Neurosci. 2013, 24. [Google Scholar] [CrossRef] [PubMed]
- Tipper, S.P.; Weaver, B.; Houghton, G. Behavioural Goals Determine Inhibitory Mechanisms of Selective Attention. Q. J. Exp. Psychol. Sect. A 1994, 47, 809–840. [Google Scholar] [CrossRef]
- Gevins, A. The future of electroencephalography in assessing neurocognitive functioning. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 165–172. [Google Scholar] [CrossRef]
- Cassenti, D.N. ACT-R Model of EEG Latency Data. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting, Belfast, UK, 3–5 September 2007; Volume 51, pp. 812–816. [Google Scholar]
- Cassenti, D.N.; Kerick, S.; McDowell, K. Observing and modeling cognitive events through event-related potentials and ACT-R. Cogn. Syst. Res. 2011, 12, 56–65. [Google Scholar] [CrossRef]



| Function | Brain Region | Time (ms) |
|---|---|---|
| Visual processing | Occipital | 150 |
| Spatial attention | Parietal | 250 |
| Declarative | Temporal | 350 |
| Executive | Frontal | 450 |
| Procedural | Basal ganglia | 550 |
| Manual | Parietal | 650 |
| Interval Bins | |||||||
| (0–100 ms) | (101–200 ms) | (201–300 ms) | (301–400 ms) | (401–500 ms) | (501–600 ms) | (601–700 ms) | |
| Mean Differences in Peak Amplitudes (Target – Distractor) in μV | |||||||
| Frontal Network | |||||||
| Left (F3) | −0.142 | 1.280 T | 0.809 T | −1.198 T | −3.855 T | −2.266 T | −0.686 |
| Midline (Fz) | 1.298 T | 1.997 T | 2.275 T | −0.101 | −0.109 | −0.876 D | −1.781 D |
| Right (F4) | −0.550 | 2.962 T | −1.617 T | −0.024 | 2.493 T | 1.017 T | 0.800 T |
| Centro-Temporal Network | |||||||
| Left (T7) | −1.644 T | −4.249 T | 0.095 | −1.375 T | −0.568 | 1.067 T | −0.782 |
| Midline (Cz) | 0.805 T | 2.976 T | 0.436 | 0.371 | 2.356 T | 3.061 T | 1.074 T |
| Right (T8) | 1.240 T | −0.646 | −0.946 D | −0.148 | −1.806 T | −0.749 | 0.753 |
| Parietal Network | |||||||
| Left (P7) | 1.366 T | −0.106 | −2.172 D | −1.772 D | 2.118 T | 0.870 T | 0.822 T |
| Midline (Pz) | −2.256 T | 0.993 T | 2.139 T | −2.181 T | −3.538 T | 1.241 T | 3.268 T |
| Right (P8) | −2.720 T | −1.200 T | −0.526 | −0.620 | 0.293 | 2.100 T | 0.846 T |
| Occipital Network | |||||||
| Left (O1) | −0.882 T | 2.402 T | 2.020 T | 0.147 | −0.059 | −0.677 | −0.314 |
| Midline (Oz) | −0.045 | 0.060 | 0.561 | 0.388 | −2.287 T | −2.029 T | −1.690 T |
| Right (O2) | 1.196 T | −2.431 T | −1.358 T | 1.062 T | 0.729 | −0.742 | −1.466 T |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D'Angiulli, A.; Pham, D.A.T.; Leisman, G.; Goldfield, G. Evaluating Preschool Visual Attentional Selective-Set: Preliminary ERP Modeling and Simulation of Target Enhancement Homology. Brain Sci. 2020, 10, 124. https://doi.org/10.3390/brainsci10020124
D'Angiulli A, Pham DAT, Leisman G, Goldfield G. Evaluating Preschool Visual Attentional Selective-Set: Preliminary ERP Modeling and Simulation of Target Enhancement Homology. Brain Sciences. 2020; 10(2):124. https://doi.org/10.3390/brainsci10020124
Chicago/Turabian StyleD'Angiulli, Amedeo, Dao Anh Thu Pham, Gerry Leisman, and Gary Goldfield. 2020. "Evaluating Preschool Visual Attentional Selective-Set: Preliminary ERP Modeling and Simulation of Target Enhancement Homology" Brain Sciences 10, no. 2: 124. https://doi.org/10.3390/brainsci10020124
APA StyleD'Angiulli, A., Pham, D. A. T., Leisman, G., & Goldfield, G. (2020). Evaluating Preschool Visual Attentional Selective-Set: Preliminary ERP Modeling and Simulation of Target Enhancement Homology. Brain Sciences, 10(2), 124. https://doi.org/10.3390/brainsci10020124






