Efficacy of Intravenous Hydrocortisone Treatment in Refractory Neonatal Seizures: A Report on Three Cases
Abstract
1. Introduction
2. Materials and Methods
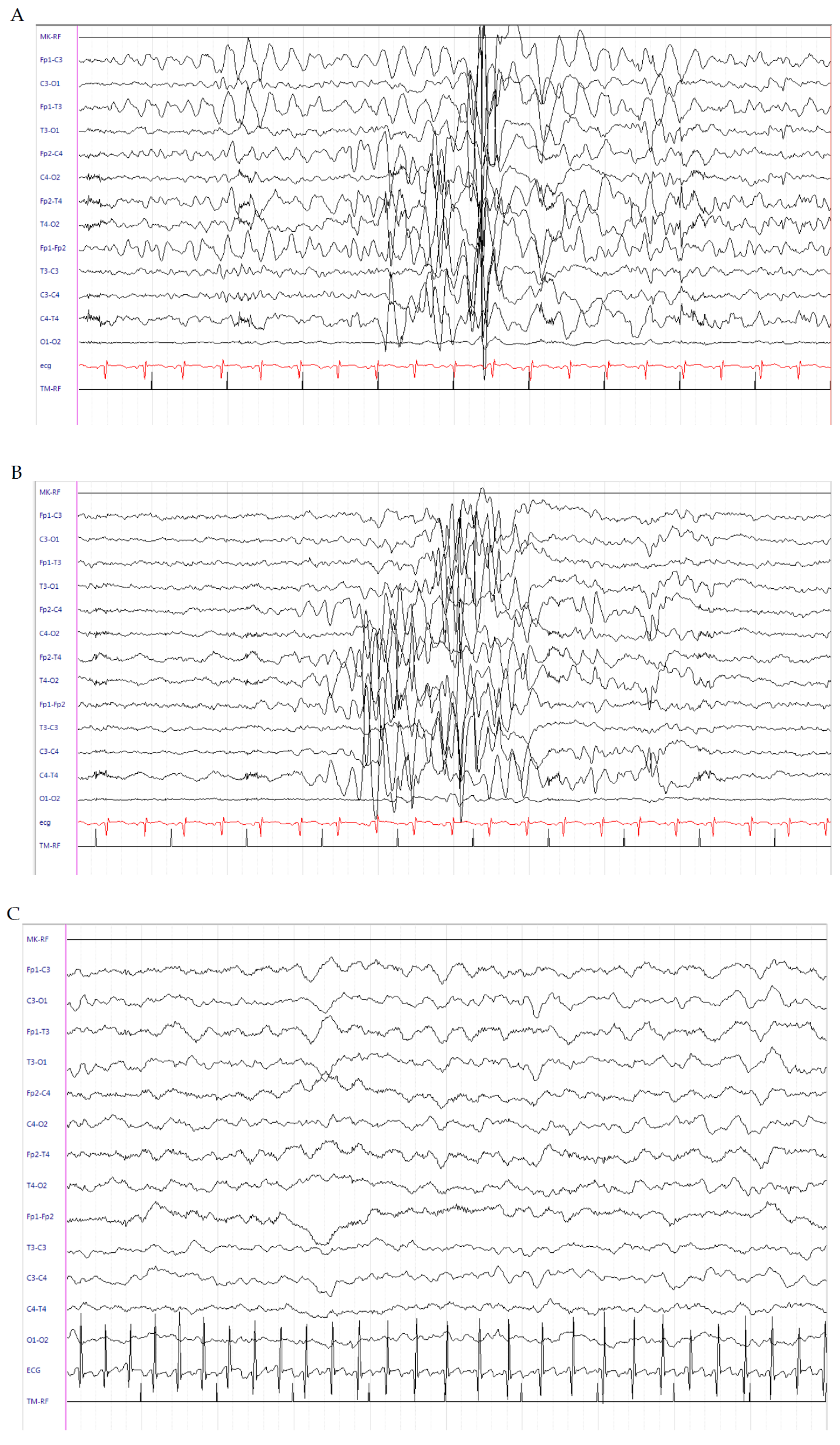
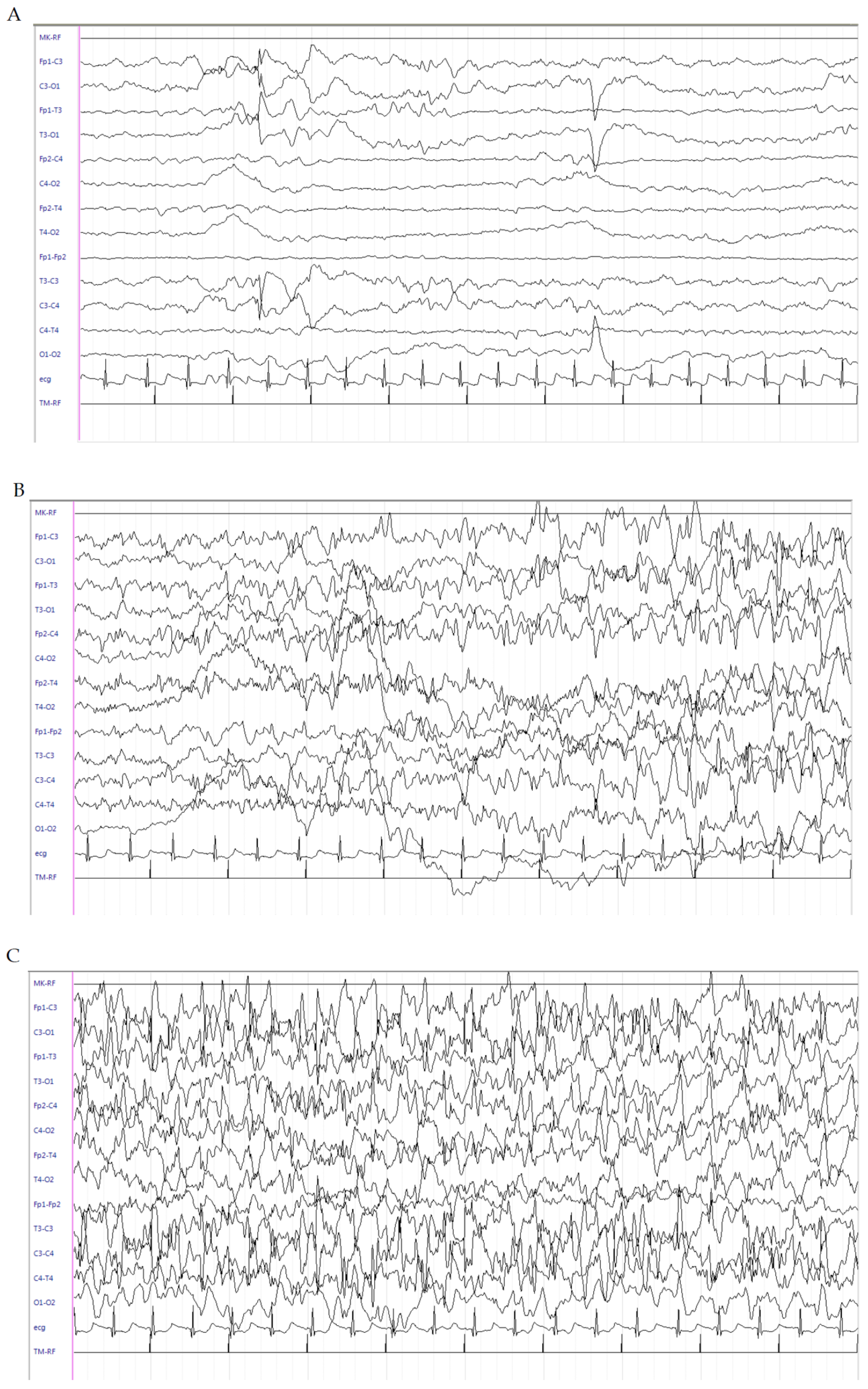
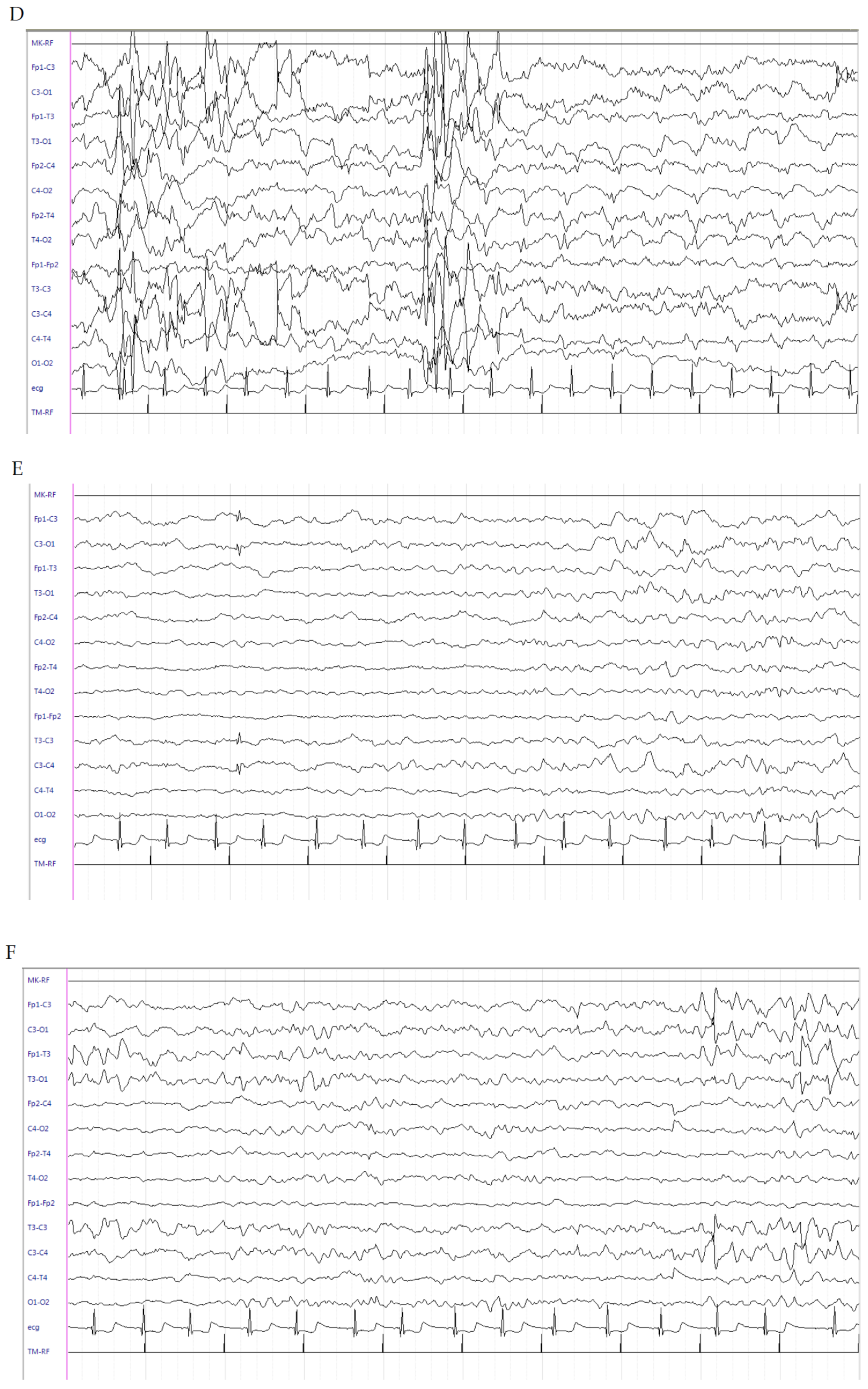
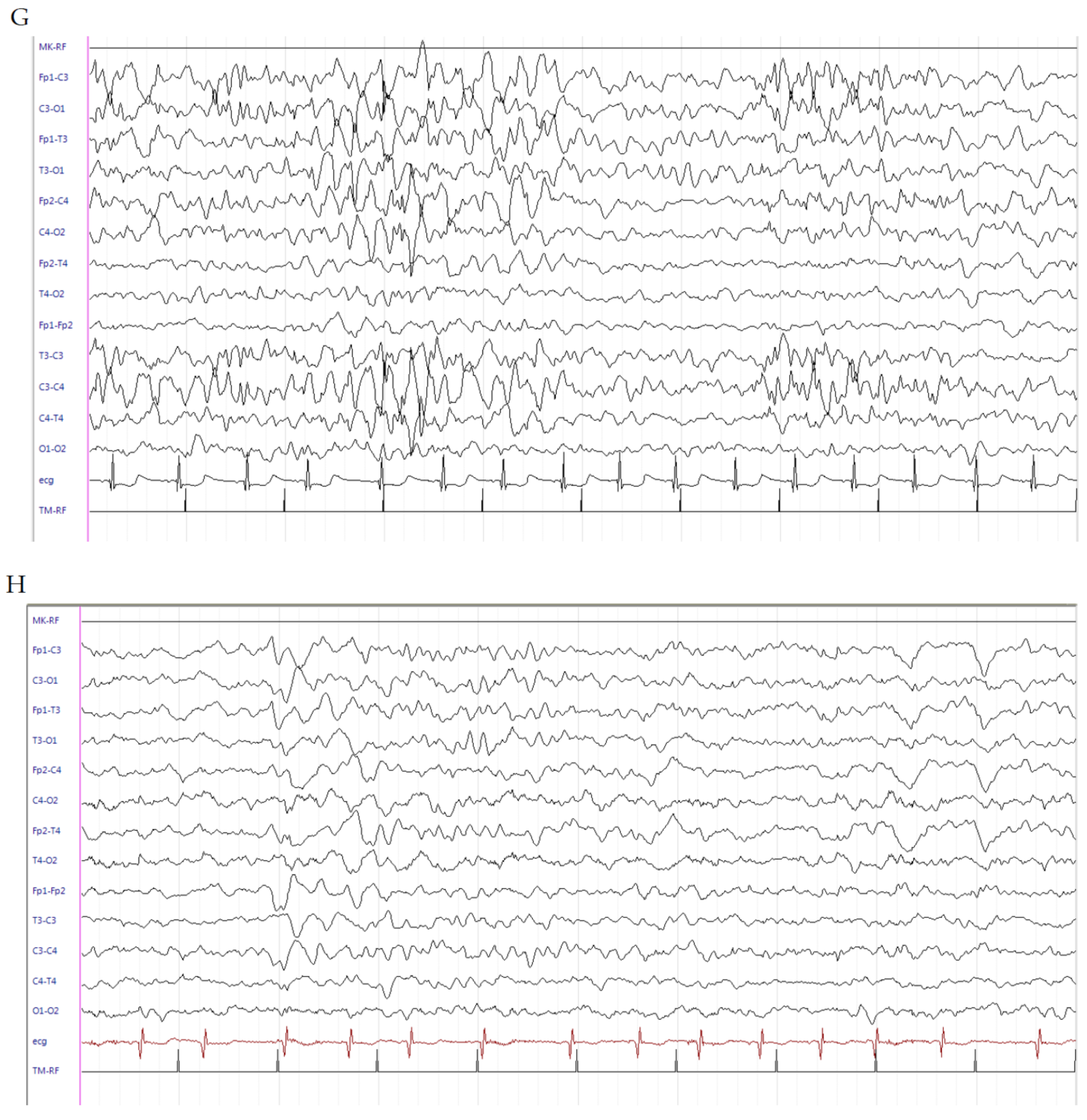
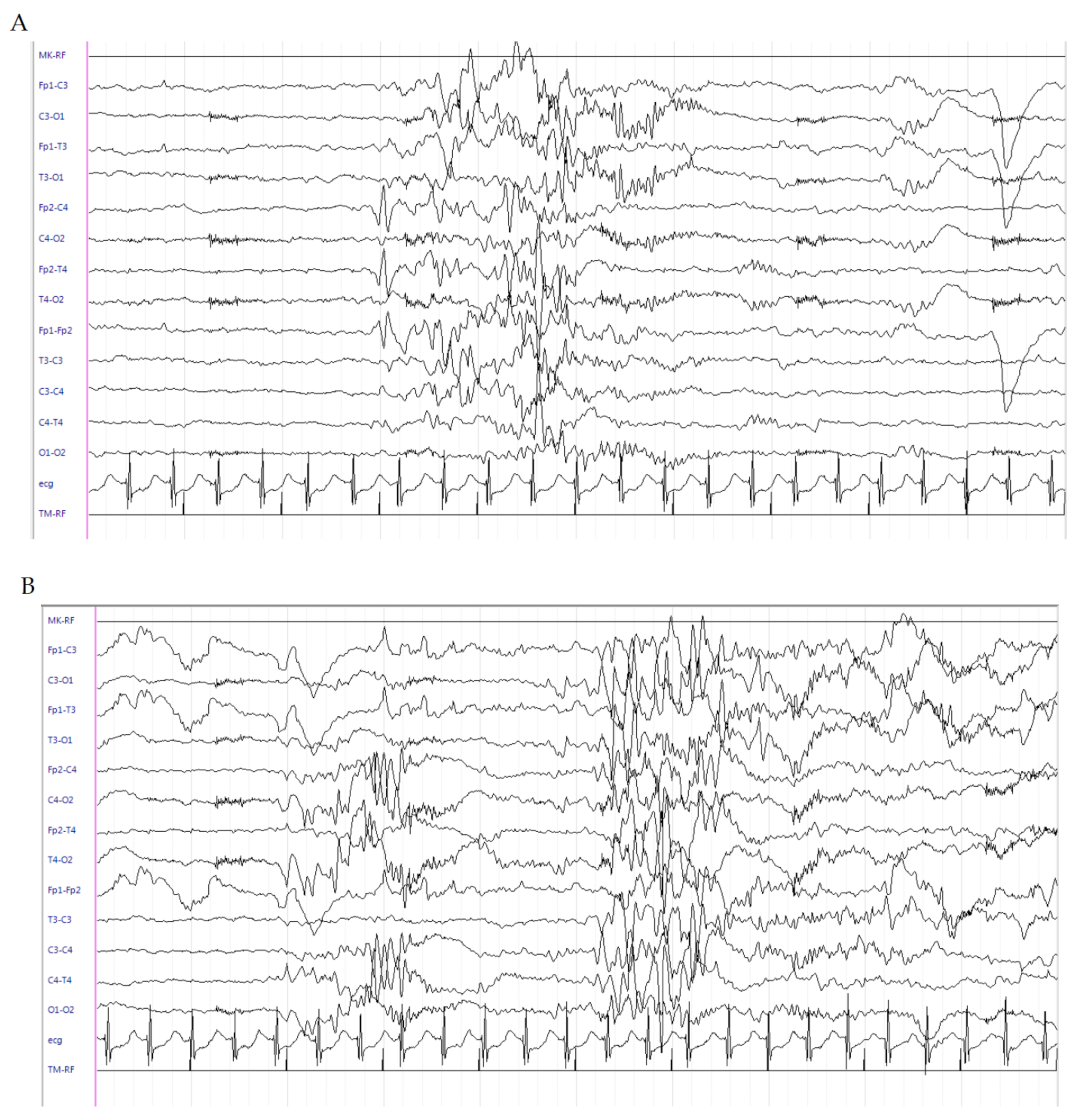
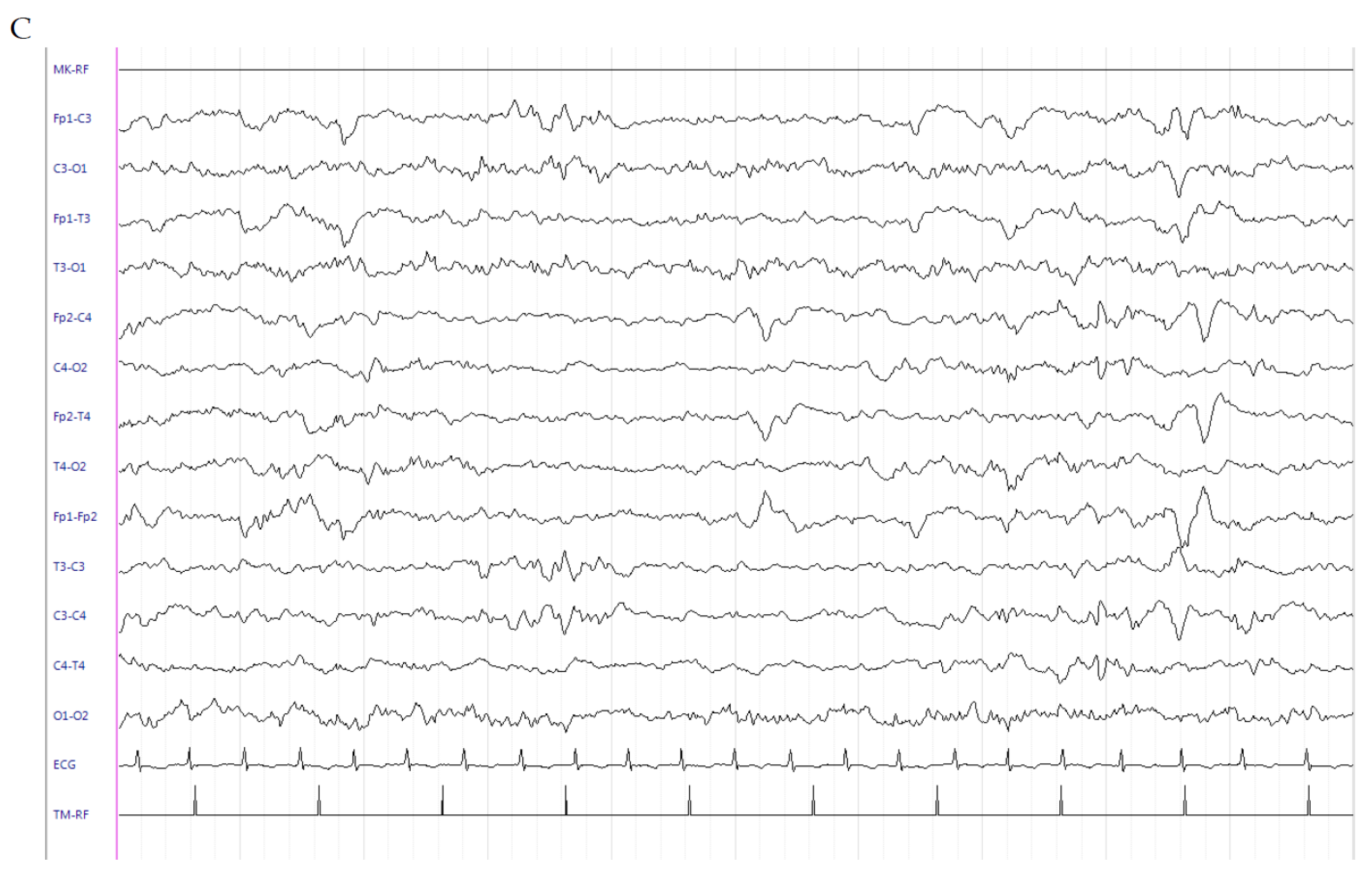
2.1. Patient 1
2.2. Patient 2
2.3. Patient 3
3. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramantani, G.; Schmitt, B.; Plecko, B.; Pressler, R.M.; Wohlrab, G.; Klebermass-Schrehof, K.; Hagmann, C.; Pisani, F.; Boylan, G.B. Neonatal Seizures-Are We there Yet? Neuropediatrics 2019, 50, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, E.; Spagnoli, C.; Pelosi, A.; Mazzotta, S.; Pisani, F. Neonatal status epilepticus: Differences between preterm and term newborns. Eur. J. Paediatr. Neurol. 2015, 19, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Pustorino, G.; Spano, M.; Sgro, D.L.; Di Rosa, G.; Tricomi, G.; Bellantone, D.; Tortorella, G. Status gelasticus associated with levetiracetam as add-on treatment. Epileptic Disord. 2007, 9, 186–189. [Google Scholar] [CrossRef]
- Vezzani, A.; Maroso, M.; Balosso, S.; Sanchez, M.A.; Bartfai, T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav. Immun. 2011, 25, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Suppiej, A.; Gentilomo, C.; Saracco, P.; Sartori, S.; Agostini, M.; Bagna, R.; Bassi, B.; Giordano, P.; Grassi, M.; Guzzetta, A.; et al. Paediatric arterial ischaemic stroke and cerebral sinovenous thrombosis. First report from the Italian Registry of Pediatric Thrombosis (R. I. T. I., Registro Italiano Trombosi Infantili). Thromb. Haemost. 2015, 113, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, G.; Deodato, F.; Loupatty, F.J.; Rizzo, C.; Carrozzo, R.; Santorelli, F.M.; Boenzi, S.; D’Amico, A.; Tozzi, G.; Bertini, E.; et al. Hypertrophic cardiomyopathy, cataract, developmental delay, lactic acidosis: A novel subtype of 3-methylglutaconic aciduria. J. Inherit. Metab. Dis. 2006, 29, 546–550. [Google Scholar] [CrossRef]
- Johnson, P.J. Hydrocortisone for Treatment of Hypotension in the Newborn. Neonatal Netw. 2015, 34, 46–51. [Google Scholar] [CrossRef]
- Doyle, L.W.; Cheong, J.L.; Ehrenkranz, R.A.; Halliday, H.L. Early (<8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017, 10, CD001146. [Google Scholar] [CrossRef]
- Baud, O.; Trousson, C.; Biran, V.; Leroy, E.; Mohamed, D.; Alberti, C. Association Between Early Low-Dose Hydrocortisone Therapy in Extremely Preterm Neonates and Neurodevelopmental Outcomes at 2 Years of Age. JAMA 2017, 317, 1329–1337. [Google Scholar] [CrossRef]
- Vezzani, A.; Viviani, B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 2015, 96 Pt A, 70–82. [Google Scholar] [CrossRef]
- Bergamo, S.; Parata, F.; Nosadini, M.; Boniver, C.; Toldo, I.; Suppiej, A.; Vecchi, M.; Amigoni, A.; Da Dalt, L.; Zanconato, S.; et al. Children with convulsive epileptic seizures presenting to padua pediatric emergency department: The first retrospective population-based descriptive study in an Italian Health District. J. Child Neurol. 2015, 30, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.; Polli, R.; Bettella, E.; Rossato, S.; Andreoli, W.; Vecchi, M.; Giordano, L.; Accorsi, P.; Di Rosa, G.; Toldo, I.; et al. Pathogenic role of the X-linked cyclin-dependent kinase-like 5 and aristaless-related homeobox genes in epileptic encephalopathy of unknown etiology with onset in the first year of life. J. Child Neurol. 2011, 26, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Brigo, F.; Sartori, S. The new definition and classification of status epilepticus: What are the implications for children? Epilepsia 2016, 57, 1942–1943. [Google Scholar] [CrossRef] [PubMed]
- Pisani, F.; Pavlidis, E. What Is New: Talk About Status Epilepticus in the Neonatal Period. Eur. J. Paediatr. Neurol. 2018, 22, 757. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; D’Angelo, G.; Granese, R.; Falsaperla, R.; Reiter, R.J.; Corsello, G.; Gitto, E. Role of oxidative stress in neonatal respiratory distress syndrome. Free Radic. Biol. Med. 2019, 142, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, L.; Rulli, I.; Falsaperla, R.; Corsello, G.; Gitto, E. Ventilation, oxidative stress and risk of brain injury in preterm newborn. Ital. J. Paediatr. 2020, 46, 100. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Pineda, E.; Auvin, S.; Shin, D.; Mazarati, A.; Sankar, R. Neuroprotective and antiepileptogenic effects of combination of anti-inflammatory drugs in the immature brain. J. Neuroinflamm. 2013, 10, 30. [Google Scholar] [CrossRef]
- Ravizza, T.; Rizzi, M.; Perego, C.; Richichi, C.; Velískǒvá, J.; Moshé, S.L.; De Simoni, M.G.; Vezzani, A. Inflammatory response and glia activation in developing rat hippocampus after status epilepticus. Epilepsia 2005, 46 (Suppl. S5), 113–117. [Google Scholar] [CrossRef]
- Haut, S.R.; Veliskova, J.; Moshe, S.L. Susceptibility of immature and adult brains to seizure effects. Lancet Neurol. 2004, 3, 608–617. [Google Scholar] [CrossRef]
- Galanopoulou, A.S.; Moshe, S.L. The epileptic hypothesis: Developmentally related arguments based on animal models. Epilepsia 2009, 50 (Suppl. S7), 37–42. [Google Scholar] [CrossRef]
- Auvin, S.; Mazarati, A.; Shin, D. Inflammation enhances epileptogenesis in the developing rat brain. Neurobiol. Dis. 2010, 40, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, H.; Boon, P.; Buyse, G.; Ceulemans, B.; D’Hooghe, M.; Meirleir, L.D.; Hasaerts, D.; Jansen, A.; Lagae, L.; Meurs, A.; et al. Steroids in intractable childhood epilepsy: Clinical experience and review of the literature. Seizure 2005, 14, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Ishii, A.; Shibata, M.; Ihara, Y.; Cooper, E.C.; Hirose, S. Characteristics of KCNQ2 variants causing either benign neonatal epilepsy or developmental and epileptic encephalopathy. Epilepsia 2019, 60, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Rosa, G.; Dicanio, D.; Nicotera, A.G.; Mondello, P.; Cannavò, L.; Gitto, E. Efficacy of Intravenous Hydrocortisone Treatment in Refractory Neonatal Seizures: A Report on Three Cases. Brain Sci. 2020, 10, 885. https://doi.org/10.3390/brainsci10110885
Di Rosa G, Dicanio D, Nicotera AG, Mondello P, Cannavò L, Gitto E. Efficacy of Intravenous Hydrocortisone Treatment in Refractory Neonatal Seizures: A Report on Three Cases. Brain Sciences. 2020; 10(11):885. https://doi.org/10.3390/brainsci10110885
Chicago/Turabian StyleDi Rosa, Gabriella, Daniela Dicanio, Antonio Gennaro Nicotera, Patrizia Mondello, Laura Cannavò, and Eloisa Gitto. 2020. "Efficacy of Intravenous Hydrocortisone Treatment in Refractory Neonatal Seizures: A Report on Three Cases" Brain Sciences 10, no. 11: 885. https://doi.org/10.3390/brainsci10110885
APA StyleDi Rosa, G., Dicanio, D., Nicotera, A. G., Mondello, P., Cannavò, L., & Gitto, E. (2020). Efficacy of Intravenous Hydrocortisone Treatment in Refractory Neonatal Seizures: A Report on Three Cases. Brain Sciences, 10(11), 885. https://doi.org/10.3390/brainsci10110885