TMS Correlates of Pyramidal Tract Signs and Clinical Motor Status in Patients with Cervical Spondylotic Myelopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Assessment
2.2. Transcranial Magnetic Stimulation
2.3. Statistical Analysis
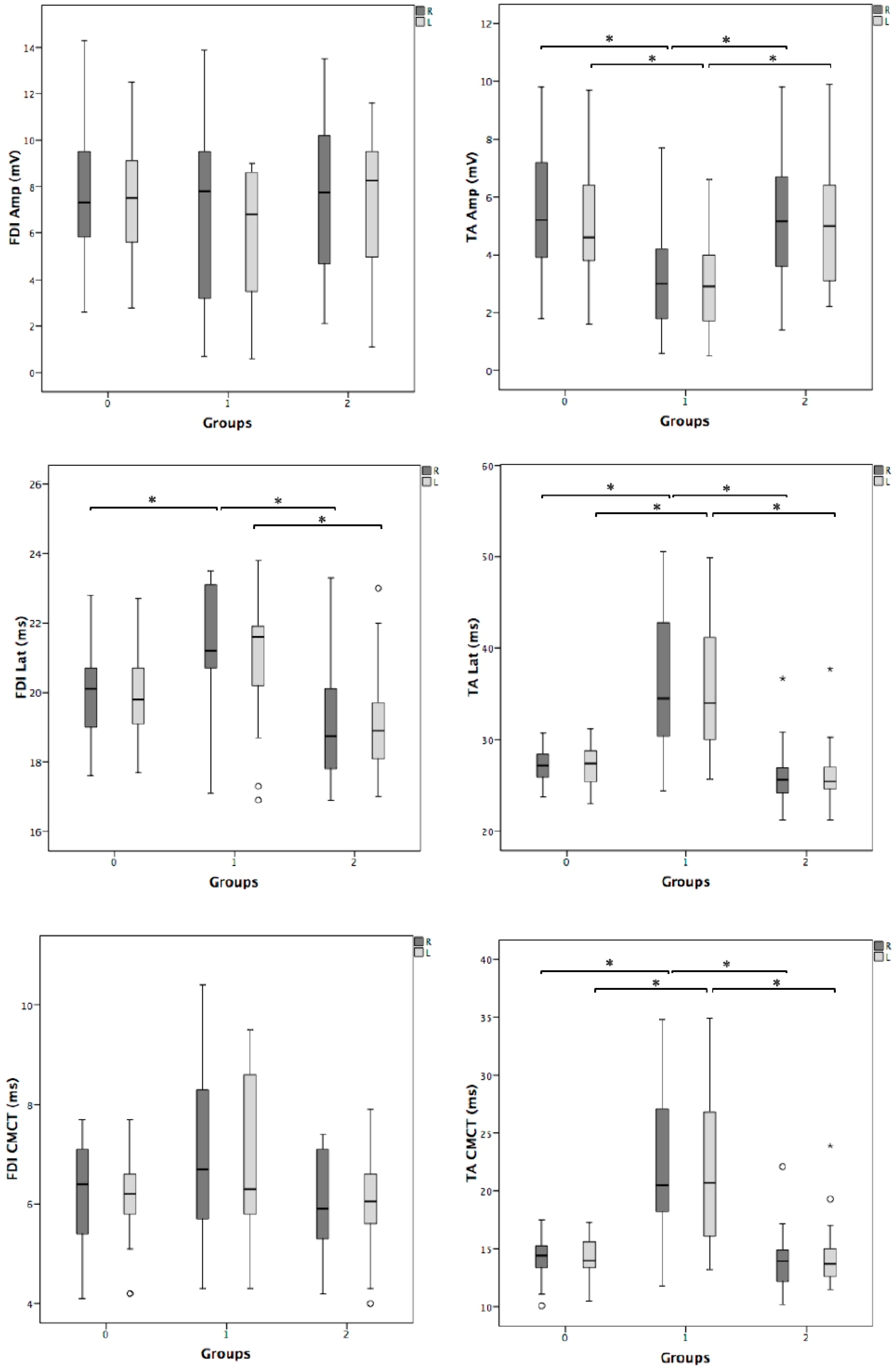
3. Results
4. Discussion
4.1. Main Findings
4.2. Clinical Implications
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hallett, M. Transcranial magnetic stimulation and the human brain. Nature 2000, 406, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Ziemann, U. The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. Front. Neural Circuits 2013, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Bella, R.; Giuffrida, S.; Cantone, M.; Pennisi, G.; Spampinato, C.; Giordano, D.; Malaguarnera, G.; Raggi, A.; Pennisi, M. Preserved transcallosal inhibition to transcranial magnetic stimulation in nondemented elderly patients with leukoaraiosis. BioMed Res. Int. 2013, 2013, 351680. [Google Scholar] [CrossRef]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet Lond. Engl. 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- Pennisi, M.; Bramanti, A.; Cantone, M.; Pennisi, G.; Bella, R.; Lanza, G. Neurophysiology of the “Celiac Brain”: Disentangling Gut-Brain Connections. Front. Neurosci. 2017, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Pascual-Leone, A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003, 2, 145–156. [Google Scholar] [CrossRef]
- Bella, R.; Ferri, R.; Cantone, M.; Pennisi, M.; Lanza, G.; Malaguarnera, G.; Spampinato, C.; Giordano, D.; Raggi, A.; Pennisi, G. Motor cortex excitability in vascular depression. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2011, 82, 248–253. [Google Scholar] [CrossRef]
- Pennisi, G.; Lanza, G.; Giuffrida, S.; Vinciguerra, L.; Puglisi, V.; Cantone, M.; Pennisi, M.; D’Agate, C.C.; Naso, P.; Aprile, G.; et al. Excitability of the Motor Cortex in De Novo Patients with Celiac Disease. PLoS ONE 2014, 9, e102790. [Google Scholar] [CrossRef]
- Bella, R.; Cantone, M.; Lanza, G.; Ferri, R.; Vinciguerra, L.; Puglisi, V.; Pennisi, M.; Ricceri, R.; Di Lazzaro, V.; Pennisi, G. Cholinergic circuitry functioning in patients with vascular cognitive impairment—No dementia. Brain Stimulat. 2016, 9, 225–233. [Google Scholar] [CrossRef]
- Pennisi, M.; Lanza, G.; Cantone, M.; Ricceri, R.; Ferri, R.; D’Agate, C.C.; Pennisi, G.; Di Lazzaro, V.; Bella, R. Cortical involvement in celiac disease before and after long-term gluten-free diet: A Transcranial Magnetic Stimulation study. PLoS ONE 2017, 12, e0177560. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Bella, R.; Lanza, G. Motor cortex plasticity in subcortical ischemic vascular dementia: What can TMS say? Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2015, 126, 851–852. [Google Scholar] [CrossRef] [PubMed]
- Bordet, R.; Ihl, R.; Korczyn, A.D.; Lanza, G.; Jansa, J.; Hoerr, R.; Guekht, A. Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: A consensus report. BMC Med. 2017, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Cantone, M.; Aricò, D.; Lanuzza, B.; Cosentino, F.I.I.; Paci, D.; Papotto, M.; Pennisi, M.; Bella, R.; Pennisi, G.; et al. Clinical and electrophysiological impact of repetitive low-frequency transcranial magnetic stimulation on the sensory–motor network in patients with restless legs syndrome. Ther. Adv. Neurol. Disord. 2018, 11, 175628641875997. [Google Scholar] [CrossRef]
- Lanza, G.; Lanuzza, B.; Aricò, D.; Cantone, M.; Cosentino, F.I.I.; Bella, R.; Pennisi, G.; Ferri, R.; Pennisi, M. Impaired short-term plasticity in restless legs syndrome: A pilot rTMS study. Sleep Med. 2018, 46, 1–4. [Google Scholar] [CrossRef]
- Fisicaro, F.; Lanza, G.; Grasso, A.A.; Pennisi, G.; Bella, R.; Paulus, W.; Pennisi, M. Repetitive transcranial magnetic stimulation in stroke rehabilitation: Review of the current evidence and pitfalls. Ther. Adv. Neurol. Disord. 2019, 12, 175628641987831. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Profice, P.; Ferrara, L.; Saturno, E.; Pilato, F.; Tonali, P. The diagnostic value of motor evoked potentials. Clin. Neurophysiol. 1999, 110, 1297–1307. [Google Scholar] [CrossRef]
- Lanza, G.; Kosac, A.; Trajkovic, G.; Whittaker, R.G. Nerve Conduction Studies as a Measure of Disease Progression: Objectivity or Illusion? J. Neuromuscul. Dis. 2017, 4, 209–215. [Google Scholar] [CrossRef]
- Cantone, M.; Lanza, G.; Vinciguerra, L.; Puglisi, V.; Ricceri, R.; Fisicaro, F.; Vagli, C.; Bella, R.; Ferri, R.; Pennisi, G.; et al. Age, Height, and Sex on Motor Evoked Potentials: Translational Data From a Large Italian Cohort in a Clinical Environment. Front. Hum. Neurosci. 2019, 13, 185. [Google Scholar] [CrossRef]
- Van der Kamp, W.; Maertens de Noordhout, A.; Thompson, P.D.; Rothwell, J.C.; Day, B.L.; Marsden, C.D. Correlation of phasic muscle strength and corticomotoneuron conduction time in multiple sclerosis. Ann. Neurol. 1991, 29, 6–12. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Tchen, P.H.; Chen, J.D. The relation between motor evoked potential and clinical motor status in stroke patients. Electromyogr. Clin. Neurophysiol. 1992, 32, 615–620. [Google Scholar] [PubMed]
- Sangari, S.; Perez, M.A. Imbalanced Corticospinal and Reticulospinal Contributions to Spasticity in Humans with Spinal Cord Injury. J. Neurosci. 2019, 39, 7872–7881. [Google Scholar] [CrossRef]
- Iyer, A.; Azad, T.D.; Tharin, S. Cervical Spondylotic Myelopathy. Clin. Spine Surg. 2016, 29, 408–414. [Google Scholar] [CrossRef]
- Nardone, R.; Höller, Y.; Brigo, F.; Frey, V.N.; Lochner, P.; Leis, S.; Golaszewski, S.; Trinka, E. The contribution of neurophysiology in the diagnosis and management of cervical spondylotic myelopathy: A review. Spinal Cord 2016, 54, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Cantone, M.; Lanza, G.; Le Pira, A.; Barone, R.; Pennisi, G.; Bella, R.; Pennisi, M.; Fiumara, A. Adjunct Diagnostic Value of Transcranial Magnetic Stimulation in Mucopolysaccharidosis-Related Cervical Myelopathy: A Pilot Study. Brain Sci. 2019, 9, 200. [Google Scholar] [CrossRef]
- Paulus, W.; Classen, J.; Cohen, L.G.; Large, C.H.; Di Lazzaro, V.; Nitsche, M.; Pascual-Leone, A.; Rosenow, F.; Rothwell, J.C.; Ziemann, U. State of the art: Pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimulat. 2008, 1, 151–163. [Google Scholar] [CrossRef]
- Ziemann, U. Pharmaco-transcranial magnetic stimulation studies of motor excitability. Handb. Clin. Neurol. 2013, 116, 387–397. [Google Scholar] [CrossRef]
- Ziemann, U.; Reis, J.; Schwenkreis, P.; Rosanova, M.; Strafella, A.; Badawy, R.; Müller-Dahlhaus, F. TMS and drugs revisited 2014. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2015, 126, 1847–1868. [Google Scholar] [CrossRef]
- O’Brien, M. Aids to the Examination of the Peripheral Nervous System, 5th ed.; Saunders: Nottingham, UK, 2010; ISBN 978-0-7020-3447-3. [Google Scholar]
- Groppa, S.; Oliviero, A.; Eisen, A.; Quartarone, A.; Cohen, L.G.; Mall, V.; Kaelin-Lang, A.; Mima, T.; Rossi, S.; Thickbroom, G.W.; et al. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin. Neurophysiol. 2012, 123, 858–882. [Google Scholar] [CrossRef]
- Wassermann, E.; Epstein, C.; Ziemann, U.; Walsh, V. Oxford Handbook of Transcranial Stimulation; Oxford University Press: Oxford, UK, 2008; ISBN 978-0-19-174401-3. [Google Scholar]
- Amassian, V.E.; Cracco, R.Q.; Maccabee, P.J. Focal stimulation of human cerebral cortex with the magnetic coil: A comparison with electrical stimulation. Electroencephalogr. Clin. Neurophysiol. 1989, 74, 401–416. [Google Scholar] [CrossRef]
- Alexeeva, N.; Broton, J.G.; Calancie, B. Latency of changes in spinal motoneuron excitability evoked by transcranial magnetic brain stimulation in spinal cord injured individuals. Electroencephalogr. Clin. Neurophysiol. 1998, 109, 297–303. [Google Scholar] [CrossRef]
- Garry, M.I.; Kamen, G.; Nordstrom, M.A. Hemispheric differences in the relationship between corticomotor excitability changes following a fine-motor task and motor learning. J. Neurophysiol. 2004, 91, 1570–1578. [Google Scholar] [CrossRef]
- Udupa, K.; Chen, R. Central motor conduction time. Handb. Clin. Neurol. 2013, 116, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Tobimatsu, S.; Sun, S.-J.; Fukui, R.; Kato, M. Effects of sex, height and age on motor evoked potentials with magnetic stimulation. J. Neurol. 1998, 245, 256–261. [Google Scholar] [CrossRef]
- Coupar, F.; Pollock, A.; Rowe, P.; Weir, C.; Langhorne, P. Predictors of upper limb recovery after stroke: A systematic review and meta-analysis. Clin. Rehabil. 2012, 26, 291–313. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Pilato, F.; Saturno, E.; Dileone, M.; Mazzone, P.; Insola, A.; Tonali, P.A.; Rothwell, J.C. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2004, 115, 255–266. [Google Scholar] [CrossRef]
- Thompson, P.D.; Day, B.L.; Rothwell, J.C.; Dick, J.P.; Cowan, J.M.; Asselman, P.; Griffin, G.B.; Sheehy, M.P.; Marsden, C.D. The interpretation of electromyographic responses to electrical stimulation of the motor cortex in diseases of the upper motor neurone. J. Neurol. Sci. 1987, 80, 91–110. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Rothwell, J.C. Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex. J. Physiol. 2014, 592, 4115–4128. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Oliviero, A.; Pilato, F.; Mazzone, P.; Insola, A.; Ranieri, F.; Tonali, P.A. Corticospinal volleys evoked by transcranial stimulation of the brain in conscious humans. Neurol. Res. 2003, 25, 143–150. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Profice, P.; Saturno, E.; Pilato, F.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J.C. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr. Clin. Neurophysiol. 1998, 109, 397–401. [Google Scholar] [CrossRef]
- Lanza, G.; Cantone, M.; Puglisi, V.; Vinciguerra, L.; Fisicaro, F.; Vagli, C.; Bella, R.; Pennisi, G.; Di Lazzaro, V.; Pennisi, M. “Mute” plantar response: Does the cortico-spinal tract “speak”? Brain Stimulat. 2019, 12, 1579–1580. [Google Scholar] [CrossRef]
- Curt, A.; Dietz, V. Electrophysiological recordings in patients with spinal cord injury: Significance for predicting outcome. Spinal Cord 1999, 37, 157–165. [Google Scholar] [CrossRef]
- Iseli, E.; Cavigelli, A.; Dietz, V.; Curt, A. Prognosis and recovery in ischaemic and traumatic spinal cord injury: Clinical and electrophysiological evaluation. J. Neurol. Neurosurg. Psychiatry 1999, 67, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Cortes, M.; Thickbroom, G.W.; Elder, J.; Rykman, A.; Valls-Sole, J.; Pascual-Leone, A.; Edwards, D.J. The corticomotor projection to liminally-contractable forearm muscles in chronic spinal cord injury: A transcranial magnetic stimulation study. Spinal Cord 2017, 55, 362–366. [Google Scholar] [CrossRef]
- Edwards, D.J.; Cortes, M.; Thickbroom, G.W.; Rykman, A.; Pascual-Leone, A.; Volpe, B.T. Preserved corticospinal conduction without voluntary movement after spinal cord injury. Spinal Cord 2013, 51, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.J.; Cortes, M.; Thickbroom, G.W.; Rykman, A.; Pascual-Leone, A.; Volpe, B.T. Reply: Evidence against volume conduction to explain normal MEPs in muscles with low motor power in SCI. Spinal Cord 2014, 52, 718. [Google Scholar] [CrossRef]
- Bunge, R.P.; Puckett, W.R.; Hiester, E.D. Observations on the pathology of several types of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv. Neurol. 1997, 72, 305–315. [Google Scholar]
- Kakulas, B.A. Neuropathology: The foundation for new treatments in spinal cord injury. Spinal Cord 2004, 42, 549–563. [Google Scholar] [CrossRef]
- Tansey, K.E.; McKay, W.B.; Kakulas, B.A. Restorative neurology: Consideration of the new anatomy and physiology of the injured nervous system. Clin. Neurol. Neurosurg. 2012, 114, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Restuccia, D.; Colosimo, C.; Tonali, P. The contribution of magnetic stimulation of the motor cortex to the diagnosis of cervical spondylotic myelopathy. Correlation of central motor conduction to distal and proximal upper limb muscles with clinical and MRI findings. Electroencephalogr. Clin. Neurophysiol. 1992, 85, 311–320. [Google Scholar] [CrossRef]
- Rapisarda, G.; Bastings, E.; de Noordhout, A.M.; Pennisi, G.; Delwaide, P.J. Can motor recovery in stroke patients be predicted by early transcranial magnetic stimulation? Stroke 1996, 27, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Cortes, M.; Black-Schaffer, R.M.; Edwards, D.J. Transcranial magnetic stimulation as an investigative tool for motor dysfunction and recovery in stroke: An overview for neurorehabilitation clinicians. Neuromodul. J. Int. Neuromodul. Soc. 2012, 15, 316–325. [Google Scholar] [CrossRef]
- Bembenek, J.P.; Kurczych, K.; Karliński, M.; Członkowska, A. The prognostic value of motor-evoked potentials in motor recovery and functional outcome after stroke—A systematic review of the literature. Funct. Neurol. 2012, 27, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Lüdemann-Podubecká, J.; Nowak, D.A. Mapping cortical hand motor representation using TMS: A method to assess brain plasticity and a surrogate marker for recovery of function after stroke? Neurosci. Biobehav. Rev. 2016, 69, 239–251. [Google Scholar] [CrossRef]
- Beaulieu, L.-D.; Milot, M.-H. Changes in transcranial magnetic stimulation outcome measures in response to upper-limb physical training in stroke: A systematic review of randomized controlled trials. Ann. Phys. Rehabil. Med. 2018, 61, 224–234. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, Y.C. Magnetic Motor Evoked Potentials in Motor Pathway Lesions. J. Korean Neurol. Assoc. 1992, 10, 59–71. [Google Scholar]
- Funaba, M.; Kanchiku, T.; Imajo, Y.; Suzuki, H.; Yoshida, Y.; Nishida, N.; Fujimoto, K.; Taguchi, T. Characteristics of C6–7 myelopathy: Assessment of clinical symptoms and electrophysiological findings. Spinal Cord 2016, 54, 798–803. [Google Scholar] [CrossRef]
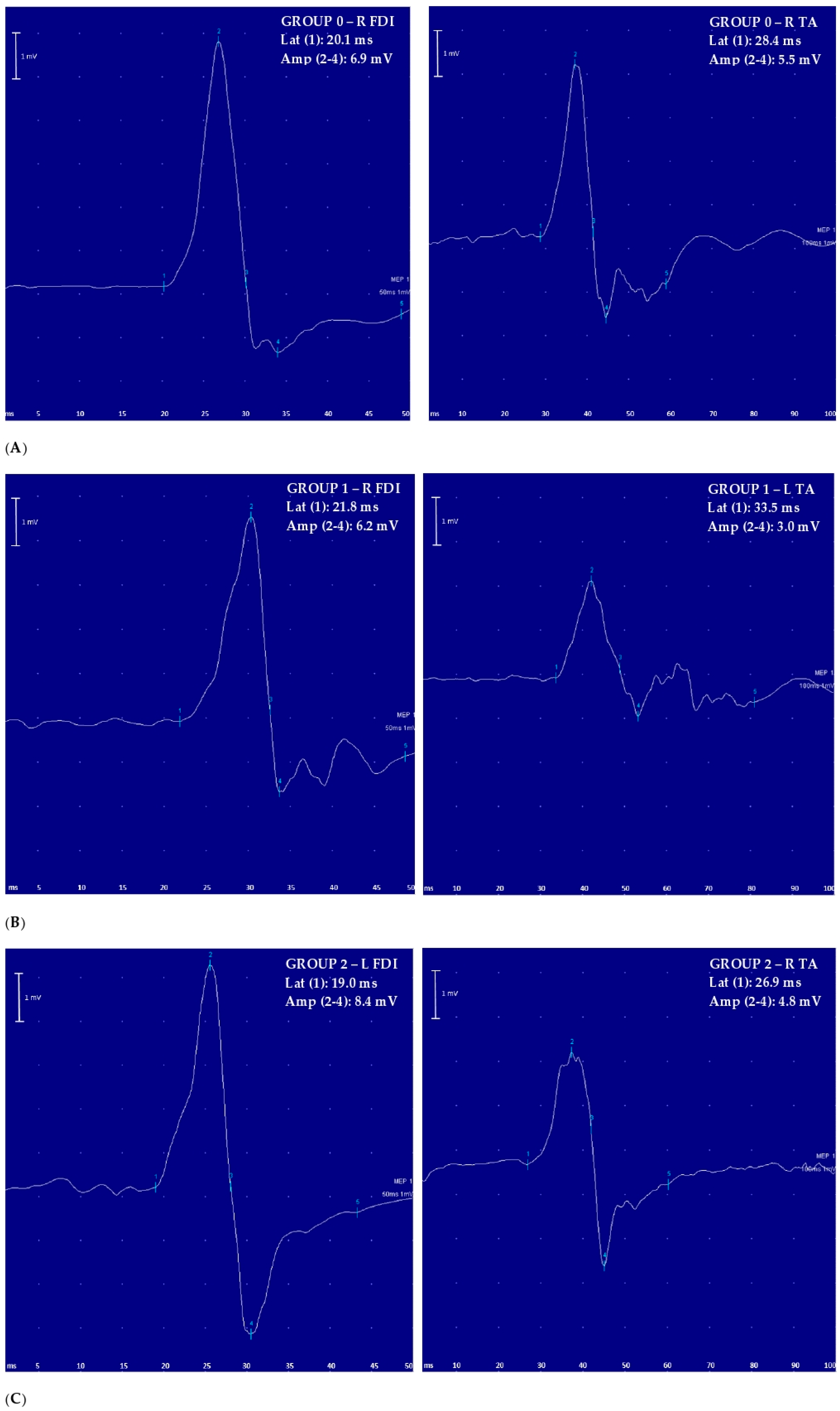
| Variable, Unit | Group 0 (n = 33) | Group 1 (n = 21) | Group 2 (n = 22) | p Value | Post Hoc Analysis | |
|---|---|---|---|---|---|---|
| Difference | p Value | |||||
| Age, years | 54.00 (22.50) | 57.00 (14.00) | 49.50 (21.80) | 0.290 * | NS | NS |
| Sex, male (%) female (%) | 18.00 (54.50) 15.00 (45.50) | 14.00 (66.70) 7.00 (33.30) | 8.00 (36.40) 14.00 (63.60) | 0.130 † | NS | NS |
| Height, cm | 163.00 (7.00) | 166.00 (10.50) | 163.00 (7.80) | 0.260 * | NS | NS |
| Disease duration, months | - | 19.5 (8.7) | 21.0 (9.0) | 0.981 ‡ | - | - |
| ASIA UER | - | 20.0 (1.0) | 25.0 (0.0) | <0.001 ‡ | - | - |
| ASIA UEL | - | 20.0 (1.0) | 25.0 (0.0) | <0.001 ‡ | - | - |
| ASIA LER | - | 18.0 (5.0) | 25.0 (0.0) | <0.001 ‡ | - | - |
| ASIA LEL | - | 19.0 (3.0) | 25.0 (0.0) | <0.001 ‡ | - | - |
| Variable, Unit | Group 0 (n = 33), p | Group 1 (n = 21), p | Group 2 (n = 22), p |
|---|---|---|---|
| FDI amplitude, mV | 0.91 | 0.20 | 0.75 |
| FDI latency, ms | 0.67 | 0.73 | 0.78 |
| FDI CMCT, ms | 0.39 | 0.96 | 0.95 |
| TA amplitude, mV | 0.56 | 0.75 | 0.83 |
| TA latency, ms | 0.95 | 1.00 | 0.69 |
| TA CMCT, ms | 0.81 | 0.74 | 0.73 |
| (A) Dependent Variable | Predictor | Std Beta | p | Adjusted R2 |
| Right FDI MEP amplitude | Pyramidal signs | −0.04 | 0.770 | 0.02 |
| Motor deficit | −0.05 | 0.690 | ||
| Right FDI MEP latency | Pyramidal signs | −0.18 | 0.130 | 0.21 |
| Motor deficit | 0.56 | <0.001 | ||
| Right FDI CMCT | Pyramidal signs | −0.07 | 0.550 | 0.10 |
| Motor deficit | 0.39 | 0.003 | ||
| Left FDI MEP amplitude | Pyramidal signs | −0.06 | 0.630 | 0.32 |
| Motor deficit | −0.19 | 0.146 | ||
| Left FDI MEP latency | Pyramidal signs | −0.16 | 0.199 | 0.18 |
| Motor deficit | 0.51 | <0.001 | ||
| Left FDI CMCT | Pyramidal signs | 0.56 | 0.741 | 0.10 |
| Motor deficit | 0.62 | 0.005 | ||
| Right TA MEP amplitude | Pyramidal signs | −0.06 | 0.610 | 0.10 |
| Motor deficit | −0.32 | 0.015 | ||
| Right TA MEP latency | Pyramidal signs | −0.06 | 0.530 | 0.37 |
| Motor deficit | 0.65 | <0.001 | ||
| Right TA CMCT | Pyramidal signs | 0.02 | 0.850 | 0.30 |
| Motor deficit | 0.58 | <0.001 | ||
| Left TA MEP amplitude | Pyramidal signs | 0.35 | 0.790 | 0.09 |
| Motor deficit | −0.38 | 0.006 | ||
| Left TA MEP latency | Pyramidal signs | −0.04 | 0.670 | 0.34 |
| Motor deficit | 0.63 | <0.001 | ||
| Left TA CMCT | Pyramidal signs | 0.006 | 0.959 | 0.30 |
| Motor deficit | 0.56 | <0.001 | ||
| (B) Dependent Variable | Predictor | Std Beta | p | Adjusted R2 |
| Right FDI MEP amplitude | Age | −0.26 | 0.040 | 0.07 |
| Right FDI MEP latency | Motor deficit | 0.50 | <0.001 | 0.22 |
| Right FDI CMCT | Motor deficit | 0.41 | 0.003 | 0.07 |
| Left FDI MEP amplitude | Motor deficit | −0.23 | 0.090 | 0.10 |
| Age | −0.23 | 0.050 | ||
| Left FDI MEP latency | Motor deficit | 0.43 | 0.001 | 0.21 |
| Left FDI CMCT | Motor deficit | 0.40 | 0.004 | 0.08 |
| Right TA MEP amplitude | Sex | −0.32 | 0.017 | 0.18 |
| Motor deficit | −0.37 | 0.005 | ||
| Right TA MEP latency | Motor deficit | 0.66 | <0.001 | 0.39 |
| Sex | 0.23 | 0.042 | ||
| Right TA CMCT | Motor deficit | 0.61 | <0.001 | 0.31 |
| Left TA MEP amplitude | Motor deficit | −0.38 | 0.006 | 0.12 |
| Left TA MEP latency | Motor deficit | 0.63 | <0.001 | 0.36 |
| Left TA CMCT | Motor deficit | 0.58 | <0.001 | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanza, G.; Puglisi, V.; Vinciguerra, L.; Fisicaro, F.; Vagli, C.; Cantone, M.; Pennisi, G.; Pennisi, M.; Bella, R. TMS Correlates of Pyramidal Tract Signs and Clinical Motor Status in Patients with Cervical Spondylotic Myelopathy. Brain Sci. 2020, 10, 806. https://doi.org/10.3390/brainsci10110806
Lanza G, Puglisi V, Vinciguerra L, Fisicaro F, Vagli C, Cantone M, Pennisi G, Pennisi M, Bella R. TMS Correlates of Pyramidal Tract Signs and Clinical Motor Status in Patients with Cervical Spondylotic Myelopathy. Brain Sciences. 2020; 10(11):806. https://doi.org/10.3390/brainsci10110806
Chicago/Turabian StyleLanza, Giuseppe, Valentina Puglisi, Luisa Vinciguerra, Francesco Fisicaro, Carla Vagli, Mariagiovanna Cantone, Giovanni Pennisi, Manuela Pennisi, and Rita Bella. 2020. "TMS Correlates of Pyramidal Tract Signs and Clinical Motor Status in Patients with Cervical Spondylotic Myelopathy" Brain Sciences 10, no. 11: 806. https://doi.org/10.3390/brainsci10110806
APA StyleLanza, G., Puglisi, V., Vinciguerra, L., Fisicaro, F., Vagli, C., Cantone, M., Pennisi, G., Pennisi, M., & Bella, R. (2020). TMS Correlates of Pyramidal Tract Signs and Clinical Motor Status in Patients with Cervical Spondylotic Myelopathy. Brain Sciences, 10(11), 806. https://doi.org/10.3390/brainsci10110806







