Effect of Treadmill Exercise and Trans-Cinnamaldehyde against d-Galactose- and Aluminum Chloride-Induced Cognitive Dysfunction in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
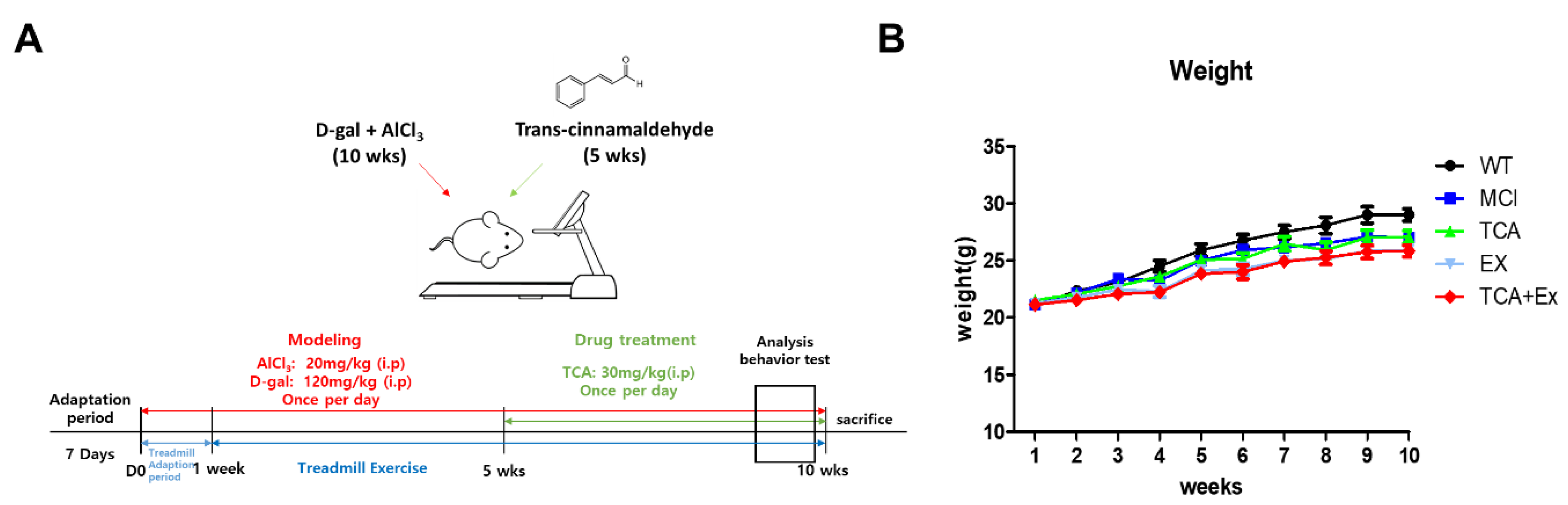
2.2. Establishment of the Mild Cognitive Impairment Model
2.3. Trans-Cinnamaldehyde (TCA) Treatment
2.4. Treadmill Exercise Protocol
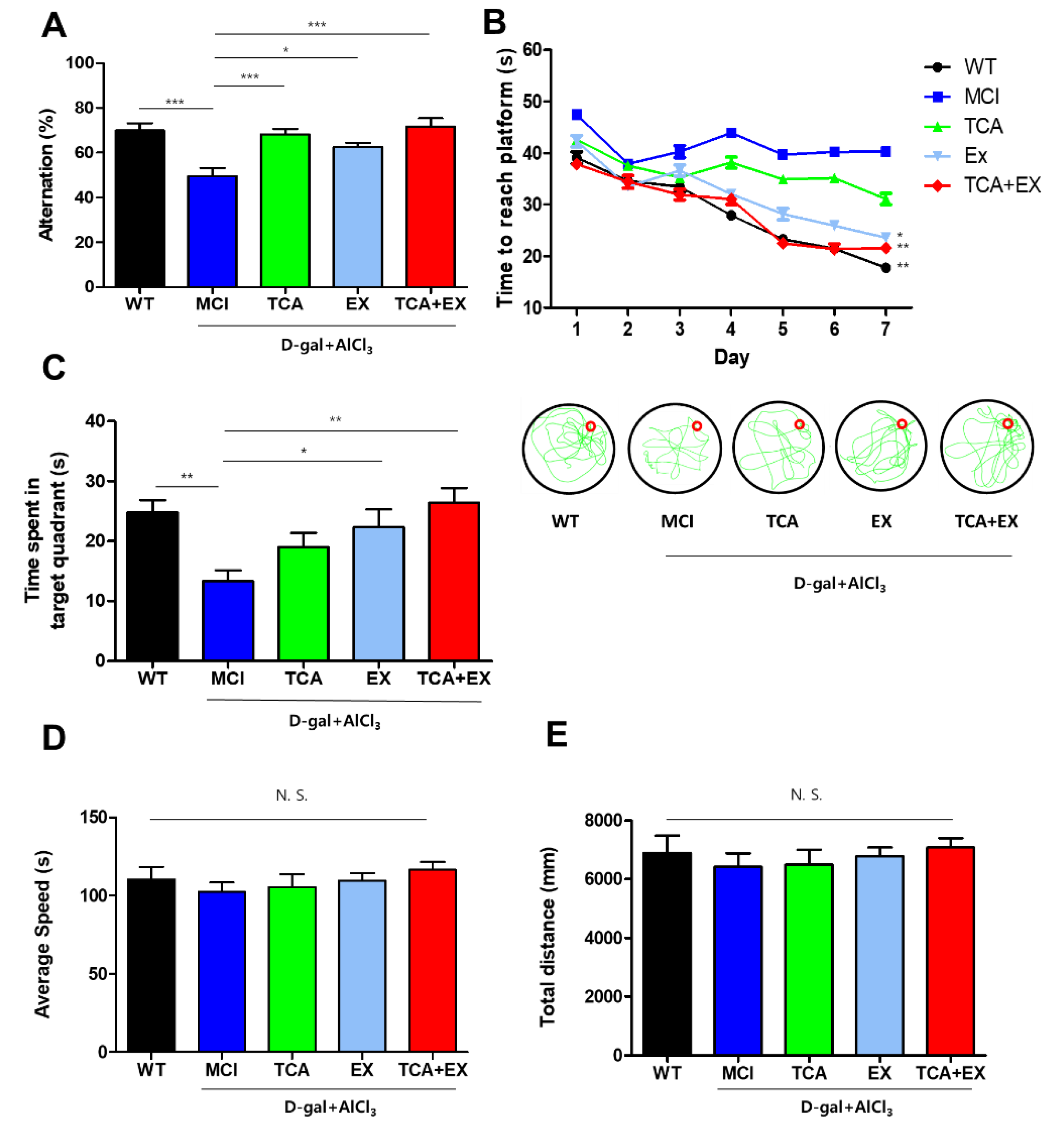
2.5. Y-Maze
2.6. Morris Water Maze (MWM)
2.7. Immunoblotting
2.8. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. TCA and Treadmill Exercise Improved Cognitive Function in Mice with a d-gal- and AlCl3-Induced Cognitive Deficiencies
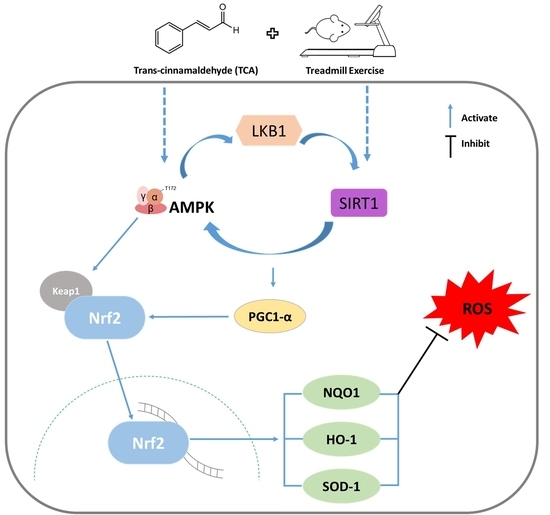
3.2. Effects of TCA and Exercise on the Nrf2 Signaling Pathway in the Brain of d-gal- and AlCl3-Treated Mice
3.3. TCA and Treadmill Exercise Triggered LKB1/AMPK and SIRT1/PGC1-α Expression in d-gal- and AlCl3-Treated Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Singer, J.; Trollor, J.N.; Baune, B.T.; Sachdev, P.S.; Smith, E. Arterial stiffness, the brain and cognition: A systematic review. Ageing Res. Rev. 2014, 15, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Moller, J.T.; Cluitmans, P.; Rasmussen, L.S.; Houx, P.; Rasmussen, H.; Canet, J.; Rabbitt, P.; Jolles, J.; Larsen, K.; Hanning, C.D.; et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998, 351, 857–861. [Google Scholar] [CrossRef]
- Hovens, I.B.; Schoemaker, R.G.; van der Zee, E.A.; Heineman, E.; Izaks, G.J.; van Leeuwen, B.L. Thinking through postoperative cognitive dysfunction: How to bridge the gap between clinical and pre-clinical perspectives. Brain Behav. Immun. 2012, 26, 1169–1179. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative stress and β-amyloid protein in Alzheimer’s disease. Neuromolecular Med. 2011, 13, 223–250. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Lovell, M.A.; Markesbery, W.R. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res. 2007, 35, 7497–7504. [Google Scholar] [CrossRef]
- Bartley, M.G.; Marquardt, K.; Kirchhof, D.; Wilkins, H.M.; Patterson, D.; Linseman, D.A. Overexpression of amyloid-β protein precursor induces mitochondrial oxidative stress and activates the intrinsic apoptotic cascade. J. Alzheimers Dis. 2012, 28, 855–868. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Chen, Z.B.; Jiang, H.; Li, L.L.; Li, E.G.; Xu, Y. Alteration of Aβ metabolism-related molecules in predementia induced by AlCl3 and D-galactose. Age 2009, 31, 277–284. [Google Scholar] [CrossRef]
- Peng, X.M.; Gao, L.; Huo, S.X.; Liu, X.M.; Yan, M. The Mechanism of Memory Enhancement of Acteoside (Verbascoside) in the Senescent Mouse Model Induced by a Combination of d-gal and AlCl3. Phytother. Res. 2015, 29, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, X.G.; Zhang, X.Y.; Hou, J.D.; Lin, L.F.; Gao, Q.; Luo, H.M. Combined administration of D-galactose and aluminium induces Alzheimer-like lesions in brain. Neurosci. Bull. 2011, 27, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, R.; You, X.; Luo, F.; He, H.; Chang, X.; Zhu, L.; Ding, X.; Yan, T. Salidroside suppresses inflammation in a D-galactose-induced rat model of Alzheimer’s disease via SIRT1/NF-κB pathway. Metab. Brain Dis. 2016, 31, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Jayant, S.; Sharma, B.; Sharma, B. Protective effect of transient receptor potential vanilloid subtype 1 (TRPV1) modulator, against behavioral, biochemical and structural damage in experimental models of Alzheimer’s disease. Brain Res. 2016, 1642, 397–408. [Google Scholar] [CrossRef]
- Chan, L.; Chin, L.M.K.; Kennedy, M.; Woolstenhulme, J.G.; Nathan, S.D.; Weinstein, A.A.; Connors, G.; Weir, N.A.; Drinkard, B.; Lamberti, J.; et al. Benefits of intensive treadmill exercise training on cardiorespiratory function and quality of life in patients with pulmonary hypertension. Chest 2013, 143, 333–343. [Google Scholar] [CrossRef]
- Daniele, T.M.; de Bruin, V.M.; e Forte, A.C.; de Oliveira, D.S.; Pompeu, C.M.; de Bruin, P.F. The relationship between physical activity, restless legs syndrome, and health-related quality of life in type 2 diabetes. Endocrine 2013, 44, 125–131. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Volek, J.S.; Clark, K.L.; Gordon, S.E.; Incledon, T.; Puhl, S.M.; Triplett-McBride, N.T.; McBride, J.M.; Putukian, M.; Sebastianelli, W.J. Physiological adaptations to a weight-loss dietary regimen and exercise programs in women. J. Appl. Physiol. 1997, 83, 270–279. [Google Scholar] [CrossRef]
- Vadstrup, E.S.; Frølich, A.; Perrild, H.; Borg, E.; Røder, M. Lifestyle intervention for type 2 diabetes patients: Trial protocol of The Copenhagen Type 2 Diabetes Rehabilitation Project. BMC Public Health 2009, 9, 166. [Google Scholar] [CrossRef]
- Fratiglioni, L.; Paillard-Borg, S.; Winblad, B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004, 3, 343–353. [Google Scholar] [CrossRef]
- Um, H.S.; Kang, E.B.; Koo, J.H.; Kim, H.T.; Jin, L.; Kim, E.J.; Yang, C.H.; An, G.Y.; Cho, I.H.; Cho, J.Y. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease. Neurosci. Res. 2011, 69, 161–173. [Google Scholar] [CrossRef]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Trigiani, L.J.; Lacalle-Aurioles, M.; Bourourou, M.; Li, L.; Greenhalgh, A.D.; Zarruk, J.G.; David, S.; Fehlings, M.G.; Hamel, E. Benefits of physical exercise on cognition and glial white matter pathology in a mouse model of vascular cognitive impairment and dementia. Glia 2020, 68, 1925–1940. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.X.; Liu, G.C.; Chen, H.L.; Lu, M.N.; Chen, B.; Hu, T.; Zhang, L.; Mao, R.; Li, S.; Mei, R.; et al. Exercise-Induced Cognitive Improvement Is Associated with Sodium Channel-Mediated Excitability in APP/PS1 Mice. Neural Plast. 2020, 2020, 9132720. [Google Scholar] [CrossRef] [PubMed]
- Gowder, S.J.; Devaraj, H. Effect of the food flavour cinnamaldehyde on the antioxidant status of rat kidney. Basic. Clin. Pharmacol. Toxicol. 2006, 99, 379–382. [Google Scholar] [CrossRef]
- Tanaka, Y.; Uchi, H.; Furue, M. Antioxidant cinnamaldehyde attenuates UVB-induced photoaging. J. Dermatol. Sci. 2019, 96, 151–158. [Google Scholar] [CrossRef]
- Kim, S.H.; Hyun, S.H.; Choung, S.Y. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J. Ethnopharmacol. 2006, 104, 119–123. [Google Scholar] [CrossRef]
- Schink, A.; Naumoska, K.; Kitanovski, Z.; Kampf, C.J.; Fröhlich-Nowoisky, J.; Thines, E.; Pöschl, U.; Schuppan, D.; Lucas, K. Anti-inflammatory effects of cinnamon extract and identification of active compounds influencing the TLR2 and TLR4 signaling pathways. Food Funct. 2018, 9, 5950–5964. [Google Scholar] [CrossRef]
- Mustafa, H.N. Neuro-amelioration of cinnamaldehyde in aluminum-induced Alzheimer’s disease rat model. J. Histotechnol. 2020, 43, 11–20. [Google Scholar] [CrossRef]
- Do, J.; Kim, N.; Jeon, S.H.; Gee, M.S.; Ju, Y.J.; Kim, J.H.; Oh, M.S.; Lee, J.K. Trans-Cinnamaldehyde Alleviates Amyloid-Beta Pathogenesis via the SIRT1-PGC1α-PPARγ Pathway in 5XFAD Transgenic Mice. Int. J. Mol. Sci. 2020, 21, 4492. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Lu, W.; Zhang, S.; Guan, X.; Li, Z.; Wang, D. Anti-Oxidative Stress Activity Is Essential for Amanita caesarea Mediated Neuroprotection on Glutamate-Induced Apoptotic HT22 Cells and an Alzheimer’s Disease Mouse Model. Int. J. Mol. Sci. 2017, 18, 1623. [Google Scholar] [CrossRef]
- Gao, L.; Peng, X.M.; Huo, S.X.; Liu, X.M.; Yan, M. Memory Enhancement of Acteoside (Verbascoside) in a Senescent Mice Model Induced by a Combination of d-gal and AlCl3. Phytother. Res. 2015, 29, 1131–1136. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, D.; Zheng, Y.; Li, H.; Hao, C.; Ouyang, W. Protective effects of kinetin against aluminum chloride and D-galactose induced cognitive impairment and oxidative damage in mouse. Brain Res. Bull. 2017, 134, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, Q.; Huang, T.; Zhao, N.; Liang, F.; Xu, B.; Chen, X.; Li, T.; Bi, J. Treadmill Exercise Decreases Aβ Deposition and Counteracts Cognitive Decline in APP/PS1 Mice, Possibly via Hippocampal Microglia Modifications. Front. Aging Neurosci. 2019, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Kang, E.B.; Oh, Y.S.; Yang, D.S.; Cho, J.Y. Treadmill exercise decreases amyloid-β burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer’s disease. Exp. Neurol. 2017, 288, 142–152. [Google Scholar] [CrossRef]
- Plucińska, K.; Crouch, B.; Koss, D.; Robinson, L.; Siebrecht, M.; Riedel, G.; Platt, B. Knock-in of human BACE1 cleaves murine APP and reiterates Alzheimer-like phenotypes. J. Neurosci. 2014, 34, 10710–10728. [Google Scholar] [CrossRef]
- Crouch, B.; Yeap, J.M.; Pais, B.; Riedel, G.; Platt, B. Of mice and motion: Behavioural-EEG phenotyping of Alzheimer’s disease mouse models. J. Neurosci. Methods 2019, 319, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Crouch, B.; Sommerlade, L.; Veselcic, P.; Riedel, G.; Schelter, B.; Platt, B. Detection of time-, frequency- and direction-resolved communication within brain networks. Sci. Rep. 2018, 8, 1825. [Google Scholar] [CrossRef] [PubMed]
- Gee, M.S.; Son, S.H.; Jeon, S.H.; Do, J.; Kim, N.; Ju, Y.J.; Lee, S.J.; Chung, E.K.; Inn, K.S.; Kim, N.J.; et al. A selective p38α/β MAPK inhibitor alleviates neuropathology and cognitive impairment, and modulates microglia function in 5XFAD mouse. Alzheimers Res. Ther. 2020, 12, 45. [Google Scholar] [CrossRef]
- Yook, J.S.; Rakwal, R.; Shibato, J.; Takahashi, K.; Koizumi, H.; Shima, T.; Ikemoto, M.J.; Oharomari, L.K.; McEwen, B.S.; Soya, H. Leptin in hippocampus mediates benefits of mild exercise by an antioxidant on neurogenesis and memory. Proc. Natl. Acad. Sci. USA 2019, 116, 10988–10993. [Google Scholar] [CrossRef] [PubMed]
- García-Mesa, Y.; Pareja-Galeano, H.; Bonet-Costa, V.; Revilla, S.; Gómez-Cabrera, M.C.; Gambini, J.; Giménez-Llort, L.; Cristòfol, R.; Viña, J.; Sanfeliu, C. Physical exercise neuroprotects ovariectomized 3xTg-AD mice through BDNF mechanisms. Psychoneuroendocrinology 2014, 45, 154–166. [Google Scholar] [CrossRef]
- Gureev, A.P.; Popov, V.N.; Starkov, A.A. Crosstalk between the mTOR and Nrf2/ARE signaling pathways as a target in the improvement of long-term potentiation. Exp. Neurol. 2020, 328, 113285. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, X.; Gong, D.; Xia, P.; Wang, F.; Xu, S. Bungeanum Improves Cognitive Dysfunction and Neurological Deficits in D-Galactose-Induced Aging Mice via Activating PI3K/Akt/Nrf2 Signaling Pathway. Front. Pharmacol. 2020, 11, 71. [Google Scholar] [CrossRef]
- Ruderman, N.; Prentki, M. AMP kinase and malonyl-CoA: Targets for therapy of the metabolic syndrome. Nat. Rev. Drug Discov. 2004, 3, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- O’Neill, H.M.; Maarbjerg, S.J.; Crane, J.D.; Jeppesen, J.; Jørgensen, S.B.; Schertzer, J.D.; Shyroka, O.; Kiens, B.; van Denderen, B.J.; Tarnopolsky, M.A.; et al. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc. Natl. Acad. Sci. USA 2011, 108, 16092–16097. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Patel, N.V.; Gordon, M.N.; Connor, K.E.; Good, R.A.; Engelman, R.W.; Mason, J.; Morgan, D.G.; Morgan, T.E.; Finch, C.E. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol. Aging 2005, 26, 995–1000. [Google Scholar] [CrossRef]
- Patel, M. Targeting Oxidative Stress in Central Nervous System Disorders. Trends Pharmacol. Sci. 2016, 37, 768–778. [Google Scholar] [CrossRef]
- Scherder, E.; Scherder, R.; Verburgh, L.; Königs, M.; Blom, M.; Kramer, A.F.; Eggermont, L. Executive functions of sedentary elderly may benefit from walking: A systematic review and meta-analysis. Am. J. Geriatr. Psychiatry 2014, 22, 782–791. [Google Scholar] [CrossRef]
- Falck, R.S.; Davis, J.C.; Liu-Ambrose, T. What is the association between sedentary behaviour and cognitive function? A systematic review. Br. J. Sports Med. 2017, 51, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, K.A.; Cotman, C.W. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol. Dis. 2013, 57, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Cummings, J.; Schneider, L.S. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006395. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.R.; Dos Santos, N.B.; Scavone, C.; Munhoz, C.D. Nrf2/ARE Pathway Modulation by Dietary Energy Regulation in Neurological Disorders. Front. Pharmacol. 2019, 10, 33. [Google Scholar] [CrossRef]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.O.; Lee, S.K.; Kim, N.; You, G.Y.; Moon, J.W.; Sha, J.; Kim, S.J.; Park, S.H.; Kim, H.S. Celastrol suppresses breast cancer MCF-7 cell viability via the AMP-activated protein kinase (AMPK)-induced p53-polo like kinase 2 (PLK-2) pathway. Cell Signal. 2013, 25, 805–813. [Google Scholar] [CrossRef]
- Park, Y.J.; Ko, J.W.; Jang, Y.; Kwon, Y.H. Activation of AMP-activated protein kinase alleviates homocysteine-mediated neurotoxicity in SH-SY5Y cells. Neurochem. Res. 2013, 38, 1561–1571. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yu, L.F.; Zhang, L.N.; Qiu, B.Y.; Su, M.B.; Wu, F.; Chen, D.K.; Pang, T.; Gu, M.; Zhang, W.; et al. Novel small-molecule AMPK activator orally exerts beneficial effects on diabetic db/db mice. Toxicol. Appl. Pharmacol. 2013, 273, 325–334. [Google Scholar] [CrossRef]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef]
- Jeppesen, J.; Maarbjerg, S.J.; Jordy, A.B.; Fritzen, A.M.; Pehmøller, C.; Sylow, L.; Serup, A.K.; Jessen, N.; Thorsen, K.; Prats, C.; et al. LKB1 regulates lipid oxidation during exercise independently of AMPK. Diabetes 2013, 62, 1490–1499. [Google Scholar] [CrossRef]
- Yang, T.; Fu, M.; Pestell, R.; Sauve, A.A. SIRT1 and endocrine signaling. Trends Endocrinol. Metab. 2006, 17, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Finley, L.W.; Haigis, M.C. The coordination of nuclear and mitochondrial communication during aging and calorie restriction. Ageing Res. Rev. 2009, 8, 173–188. [Google Scholar] [CrossRef] [PubMed]


| Target | Host | Source | Catalog No. | RRID | Application |
|---|---|---|---|---|---|
| Nrf2 | Rabbit | Cell signaling, | 12721 | AB_2715528 | WB, 1:1000 |
| NQO1 | Rabbit | Cell signaling, | 62262 | AB_2799623 | WB, 1:1000 |
| HO-1 | Rabbit | Cell signaling, | 5853 | AB_10835857 | WB, 1:1000 |
| SOD-1 | Mouse | Santa Cruz Biotechnology, | 271014 | AB_10611197 | WB, 1:1000 |
| Phosphor AMPK alpha | Rabbit | Cell signaling, | 2535 | AB_331250 | WB, 1:500 |
| AMPK alpha | Rabbit | Cell signaling, | 2532 | AB_330331 | WB, 1:500 |
| PGC-1α | Mouse | Santa Cruz Biotechnology, | sc-518025 | AB_2755043 | WB, 1:1000 |
| β-actin (HRP) | Mouse | Santa Cruz Biotechnology, | sc-47778 HRP | AB_2714189 | WB/1:5000 |
| Mouse IgG (HRP) | Goat | Santa Cruz Biotechnology | sc-2005 | AB_631736 | WB/1:5000 |
| Rabbit IgG (HRP) | Goat | Santa Cruz Biotechnology | sc-2054 | AB_631748 | WB/1:5000 |
| Gene Name | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| LKB1 | 5-AGCTGCGCAGGATCCCCAAT-3′ | 5-TGGCACACAGGGAAGCGCTT-3′ |
| SIRT1 | 5′-ACGCTGTGGCAGATTGTTATTA-3′ | 5′-TTGAAGAATGGTCTTGGGTCTT-3′ |
| GAPDH | 5′- TGAATACGGCTACAGCAACA-3′ | 5′-AGGCCCCTCCTGTTATTATG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, J.-S.; Kang, H.-Y.; Lee, J.K. Effect of Treadmill Exercise and Trans-Cinnamaldehyde against d-Galactose- and Aluminum Chloride-Induced Cognitive Dysfunction in Mice. Brain Sci. 2020, 10, 793. https://doi.org/10.3390/brainsci10110793
Ryu J-S, Kang H-Y, Lee JK. Effect of Treadmill Exercise and Trans-Cinnamaldehyde against d-Galactose- and Aluminum Chloride-Induced Cognitive Dysfunction in Mice. Brain Sciences. 2020; 10(11):793. https://doi.org/10.3390/brainsci10110793
Chicago/Turabian StyleRyu, Jong-Sik, Ho-Youl Kang, and Jong Kil Lee. 2020. "Effect of Treadmill Exercise and Trans-Cinnamaldehyde against d-Galactose- and Aluminum Chloride-Induced Cognitive Dysfunction in Mice" Brain Sciences 10, no. 11: 793. https://doi.org/10.3390/brainsci10110793
APA StyleRyu, J.-S., Kang, H.-Y., & Lee, J. K. (2020). Effect of Treadmill Exercise and Trans-Cinnamaldehyde against d-Galactose- and Aluminum Chloride-Induced Cognitive Dysfunction in Mice. Brain Sciences, 10(11), 793. https://doi.org/10.3390/brainsci10110793