Abstract
Mild cognitive impairment (MCI) generally refers to impairment in cognition above that which accompanies the normal age-related cognitive decline and has attracted attention in recent years. Trans-cinnamaldehyde (TCA), which is isolated from cinnamon, has anti-inflammatory and antioxidant properties. Treadmill exercise also has diverse positive effects. The purpose of this study was to investigate the combination effects of TCA and treadmill exercise on learning and memory in a cognitive impairment mouse induced by a combination of d-galactose (d-gal) and aluminum chloride (AlCl3). We found that exercise and TCA attenuated cognitive impairment in mice with induced MCI. This effect was further increased by costimulation of exercise and TCA. To clarify the mechanisms of the positive effects of TCA and exercise, we analyzed the nuclear factor erythroid 2-related factor (Nrf2) and related signaling pathways. We found that TCA and exercise upregulated Nrf2, NAD(P)H dehydrogenase quinone 1 (NQO-1), heme oxygenase 1 (HO-1), and superoxide dismutase 1 (SOD-1); this suggests that TCA and exercise attenuate cognitive dysfunction by reducing oxidative stress. We also found that Nrf2-related signaling pathways, i.e., the AMP-activated protein kinase (AMPK)/Nrf2 and SIRT1/PGC-1a/Nrf2-ARE pathways, exerted antioxidant effects. Together, these results suggest that costimulation with TCA and exercise may be a therapeutic candidate for mild cognitive impairment.
1. Introduction
The brain is the chief main control center of the body, and it is very important to delay the aging of the brain for a healthy life [1]. Cognitive function decreases during the aging process of the brain, causing various problems such as impairment in information processing, attention, working, and long-term memory [2,3,4]. With the aging of societies, cognitive impairment is becoming an increasingly common disease, accompanied by social and economic problems [2]. Mild cognitive impairment (MCI) usually occurs before Alzheimer’s disease (AD), and it distinguished by the severity of cognitive decline that leads to functional impairment. For individuals with MCI, the risk of developing AD is much higher and in general, it is estimated that MCI transits from 5% to 10% per year into AD [5]. Therefore, it is important to explore effective therapeutics for memory dysfunction.
Although the exact pathological mechanism underlying MCI or AD remains unclear, oxidative stress has been proposed as a critical factor in cognitive impairment [6]. Reactive oxygen species (ROS) have been reported to play a related role in the pathogenesis of several neurodegenerative diseases [7]. Lipid peroxidation, nuclear damage, and protein oxidation can occur due to overproduction of ROS, or decreased antioxidants in the brain [8]. Moreover, ROS are potent inducers of apoptosis which in turn contributes to the loss of neurons during memory impairment, thus finally affecting the normal functions of the brain [9].
Previous studies showed that the combined chronic administration of d-galactose (d-gal) and aluminum chloride (AlCl3) damages brain function, including learning and memory, and induces cognitive impairments [10,11,12]. d-gal treatment increases neurodegeneration by increasing the activity of acetylcholinesterase (AChE), ROS levels, and cognitive deficits and by inhibiting antioxidant enzymes [13]. Aluminum is an easily accumulated neurotoxic substance that blocks blood supply to the brain, causing cognitive impairment [14]. Therefore, co-treatment with AlCl3 and d-gal is used to mimic MCI and AD symptoms in rodents.
The benefits of exercise have been demonstrated in many human and experimental studies [15,16,17,18]. Regular exercise can be sufficient to repress the death of neuronal cells, not only animal models but also cognitive impairment patients and this indicates that physical activity or active life can prevent brain damage [19,20]. Physical activity improved cognitive functions by promoting neurogenesis, neurotransmission, and synaptic plasticity in previous animal studies [21,22,23].
Trans-cinnamaldehyde (TCA) is an aromatic aldehyde that is present in the bark extract of cinnamon. Previous studies have reported that cinnamon extracts have not only antioxidant, anti-sugar, and anti-inflammatory effects [24,25,26,27], but also neuroprotective activities in various diseases [28,29]. Considering the previous reports related to the positive effects of exercise and TCA, this study aimed to determine whether costimulation with exercise and TCA can ameliorate the cognitive impairments induced by d-gal and AlCl3 in mice.
2. Materials and Methods
2.1. Animals
Sixty male C57bl/6 mice (8 weeks of age, weighing 24–26 g at the beginning of the experiment) were purchased from Daehan Biolink Co., Ltd. All mice were housed in plastic containers under standard laboratory environments (temperature (23 °C ± 1 °C), humidity (60 °C ± 10 °C), 12-h light/dark cycle, and were given free access to food and water). All animal studies were performed per the “Principles of Laboratory Animal Care” (National Institutes of Health publication number 80–23, revised in 1996) and were approved by the Animal Care and Use Guidelines Committee of Kyung Hee University (approval number: KHUASP(SE)-19-041).
Mice were randomly divided into five experimental groups as follows:
Wild-type control group: control group treated with saline (WT, n = 12).
d-gal + AlCl3 group: mild cognitive impairment group induced by d-gal + AlCl3 (MCI, n = 12).
d-gal + AlCl3 + TCA group: MCI group fed TCA (TCA, n = 12).
d-gal + AlCl3 + treadmill exercise group: MCI group that performed treadmill exercise (EX, n = 12).
d-gal + AlCl3 + TCA + treadmill exercise: MCI group that performed treadmill exercise and was fed TCA (TCA + EX, n = 12).
2.2. Establishment of the Mild Cognitive Impairment Model
d-gal (G0750) and AlCl3 (294713) were purchased from Sigma Aldrich (St. Louis, MO, USA). The model of MCI was established by injecting d-gal (120 mg/kg/day) and AlCl3 (20 mg/kg/day) dissolved in saline (0.9% NaCl) intraperitoneally for 10 weeks, as described in previous studies [30,31,32]. The same volume of saline was injected in the control group during this period.
2.3. Trans-Cinnamaldehyde (TCA) Treatment
TCA (C80687) was purchased from Sigma Aldrich. TCA dissolved in saline (30 mg/kg/day) was injected intraperitoneally for 5 weeks. The same volume of saline was injected to the control group during the experimental period. The dose of TCA was as described previously [29].
2.4. Treadmill Exercise Protocol
The protocol of the treadmill exercise was adapted from previous studies [20,33,34]. An animal electric treadmill machine (JD-A-09, JEUNGDO Bio & Plant Co., Ltd., Seoul, Korea) was used in this study. Mice in the exercise groups were subjected to a familiarization period of treadmill exercise (10 min/day; the treadmill speed was increased from 5 m/min to 10 m/min daily step by step) of 1 week. After the adaptation period, mice performed the treadmill exercise. The details of the treadmill exercise protocol were as follows: 5 m/min for 5 min (warming-up), 8 m/min for 5 min, 12 m/min for 30 min, and 5 m/min for 5 min (cooling-down). Mice exercised five times a week between 5 p.m. and 8 p.m. for 9 weeks. During the treadmill excise, light electric stimulation was given to the end of the belt to induce the mice to run forward.
2.5. Y-Maze
The Y-maze methods was adapted from previous studies with a slight modification [35,36]. The Y-maze was 60 cm long and made of plastic, consisting of three passages in a triangular shape. The mice started in the center of the Y-maze and were given 8 min [37]. It was wiped with 70% ethanol and left for a sufficient time to avoid affecting the experiment during the trials. The number of correct alternations (as a percentage of all alternations) was taken as an index of the spatial working memory performance. The correct alternation was considered when the three passages were passed consecutively (i.e., ABC, BCA, or CAB but not ABA). The results were manually scored using the recorded videos.
2.6. Morris Water Maze (MWM)
The Morris water maze (MWM) was performed as previously described [38]. In brief, the mice swam in white colored water until arriving the hidden platform for maximum 60 s in one trial. One mouse had three trials in one day and trained for seven days. The latency times of each trial were checked. At day 8, the hidden platform was removed, and a probe task was performed. All trials and probe task were recorded, and the tracing data were analyzed using Toxtrac software (https://toxtrac.sourceforge.io).
2.7. Immunoblotting
Immunoblotting was performed as previously described [38]. In brief, the immunoblotting samples were made using the RIPA buffered lysates of mouse hippocampus. The samples (20 µg/10 μL) were separated by SDS-PAGE, followed by transfer to PVDF membranes (Merck, #IPVH00010, Darmstadt, Germany). The membranes were blocked for one-hour and incubated with primary antibodies at 4 °C overnight, followed by one-hour incubation with matched secondary antibodies. After antibody incubation, the membranes were developed with an enhanced chemiluminescence (ECL, Bio-Rad, 1705061) detection system and imaged using Fusion Solo S (Vilber, Collegien, France). The image analysis was conducted using Image J software (NIH, Bethesda, MD, USA). The antibodies were diluted in a 5% skim milk in TBS-T solution and all antibodies used in this study are listed in Table 1.

Table 1.
Information of immunostaining antibodies used in this study.
2.8. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed as previously described [38]. In brief, total RNA of mouse hippocampus were extracted and converted to cDNA (3 μg/20 µL). The cDNA samples were amplified using SYBR Green (Enzynomics Daejeon, Republic of Korea) and the CFX Connect Real-Time PCR System (Bio-Rad Laboratories, Hercules, CA, USA). The primers used in this study listed in Table 2.

Table 2.
Information of quantitative real-time polymerase chain reaction (qRT-PCR) primers used in this study.
2.9. Statistical Analysis
All data were analyzed using SPSS version 25 (IBM corporation, Armonk, NY, USA). Statistical significance was defined as a p value less than 0.05. The date was shown as the mean ± standard error of the mean (SEM). Parametric tests such as ANOVA were used when the data satisfied the null hypothesis of the Levene’s test. Tukey’s post hoc test was performed if the p value was < 0.05 in one-way analysis of variance (ANOVA). For the non-parametric test, the Kruskal–Wallis test followed by Dunn’s post hoc multiple comparisons were used. GraphPad Prism 5.0 software (GraphPad software Inc., San Diego, CA, USA) was used to draw all graphs.
3. Results
3.1. TCA and Treadmill Exercise Improved Cognitive Function in Mice with a d-gal- and AlCl3-Induced Cognitive Deficiencies
To clarify whether TCA and treadmill exercise improve cognitive performance, we used the d-gal- and AlCl3-induced mouse model of cognitive deficiency (Figure 1A). During the experimental period, the body weight did not differ among the groups (Figure 1B).
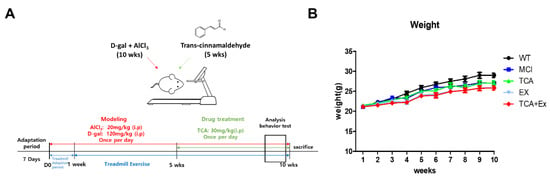
Figure 1.
Experimental design and weight variation. (A) Schedule of the experiment. Eight-week-old C57Bl/6 mice were injected intraperitoneally with d-gal (120 mg/kg/day) and AlCl3 (20 mg/kg/day) for 10 weeks. Trans-cinnamaldehyde (TCA) (30 mg/kg/day) was injected intraperitoneally for 5 weeks. Treadmill exercise lasted 9 weeks and was initiated after 1 week of adaptation. Mice were euthanized and brain samples were collected for further analysis after the Morris water maze (MWM). (B) Ten weeks of d-gal and AlCl3 treatment induced cognitive impairment, and 5 weeks of TCA treatment and 9 weeks of treadmill exercise yielded no significant differences in weight among the groups. WT; wild-type control group, MCI; mild Cognitive impairment group, TCA; MCI group fed TCA, EX; MCI group that performed treadmill exercise. TCA+EX; MCI group that performed treadmill exercise and was fed TCA.
Spatial memory and working memory performance of mice were measured through Y-maze and MWM task. First, we conducted the Y-maze test to see how TCA and exercise treatments affect working memory performance in mice. In the case of the Y-maze, the MCI group showed significantly lower alternations than did the WT group, but this impairment was recovered in the TCA, EX, and TCA + EX groups (F = 8.765) (Figure 2A). The MWM task was used to assess spatial learning and memory by determining the time to find and reach the hidden platform. In the MWM task, the MCI group exhibited markedly impaired learning and memory compared with the WT group. Similar to the Y-maze results, the EX and TCA + EX groups exhibited recovery of memory compared with the MCI group (Figure 2B). Although there was no significant difference in the escape latency times between the MCI and TCA groups, the latter group exhibited an improvement tendency regarding memory (F = 4.771) (Figure 2B). The platform was removed after 7 consecutive days, and the mice were given 60 s to find the missing platform during the probe trial. The time spent in the target quadrant was significantly shorter in the MCI group compared with the WT, EX, and TCA + EX groups (Figure 2C). The probe task showed that there was no significant difference among the groups regarding the swimming speed and the total distance traveled (Figure 2D,E). Taken together, our results suggest that TCA or EX ameliorate memory dysfunction in mice with d-gal- and AlCl3-induced MCI, and that costimulation with exercise and TCA further increases memory function.
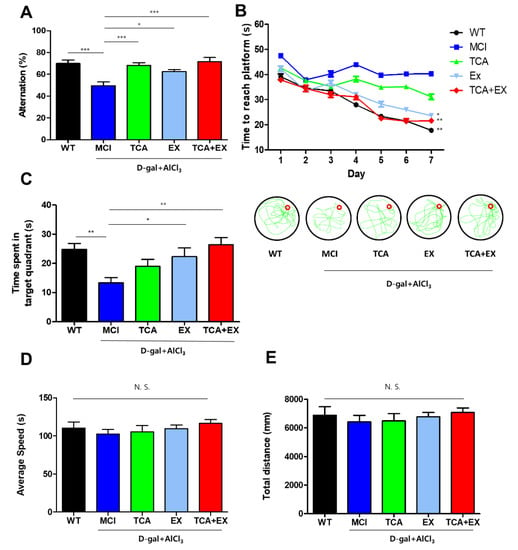
Figure 2.
Exercise and TCA improved cognitive function in mice with d-gal- and AlCl3-induced cognitive deficiencies. (A) Spontaneous alternation scores in the Y-maze task. The occurrences of correct alternations are expressed as a percentage the total alternations (mean + standard error of the mean (SEM); n = 12 per group). (B) Spatial learning and memory of mice were analyzed by the time to reach platform for 7 days on the MWM. To analyze cognitive function, we measured the time required to reach the platform. (C) After 7 days, a probe task was performed. The amount of time that the mouse spent in the target quadrant was measured during 60 s probe test. To accurate analysis of memory, the average swim speed (D) and total distance traveled (E) were identified in the probe test. All results are expressed as the mean ± SEM; n = 12 per group). N.S.; no significant. * p < 0.05 compared with the MCI group; ** p < 0.01 compared with the MCI group; *** p < 0.001 compared with the MCI group. WT; wild-type control group, MCI; mild cognitive impairment group, TCA; MCI group fed TCA, EX; MCI group that performed treadmill exercise. TCA+EX; MCI group that performed treadmill exercise and was fed TCA.
3.2. Effects of TCA and Exercise on the Nrf2 Signaling Pathway in the Brain of d-gal- and AlCl3-Treated Mice
Previous studies have shown that TCA and EX are effective antioxidants [25,39,40]. Antioxidant proteins are regulated by the expression of Nrf2, which protects against the oxidative damage triggered by inflammation and injury. Moreover, previous studies reported that cognitive function is associated with the Nrf2 signaling pathway [41,42]. Therefore, we examined whether the improved cognitive performance observed in MCI mice after the administration of TCA and treadmill exercise was related to the Nrf2 signaling pathway. As shown in Figure 3A, the MCI group exhibited decreased Nrf2 levels compared with the WT group. Levels of Nrf2 were increased in the TCA and EX groups, although this difference did not reach statistical significance (Figure 3A and Figure S1). The TCA+EX group exhibited a dramatically increased Nrf2 level compared with the MCI group (F = 3.377) (Figure 3A and Figure S1A), indicating that costimulation with TCA and treadmill exercise might restore cognition through the Nrf2 signaling pathway. To clarify the antioxidant effect induced by Nrf2 signals after treadmill exercise and TCA, the hippocampal levels of the NQO1, HO-1, and SOD-1 proteins, which are representative antioxidant enzymes, were evaluated using immunoblotting. The antioxidant proteins were downregulated in the MCI group compared with the WT group, but were significantly elevated in the TCA and EX groups. Similar to the Nrf2 expression pattern, costimulation further upregulated NQO1 (F = 3.880), HO-1 (F = 3.727) and SOD-1 (F = 6.501) (Figure 3B,D and Figure S1B,D). These results suggest that the memory improvement afforded by TCA and EX might be related to an antioxidant effect regulated by the Nrf2 signaling pathway.
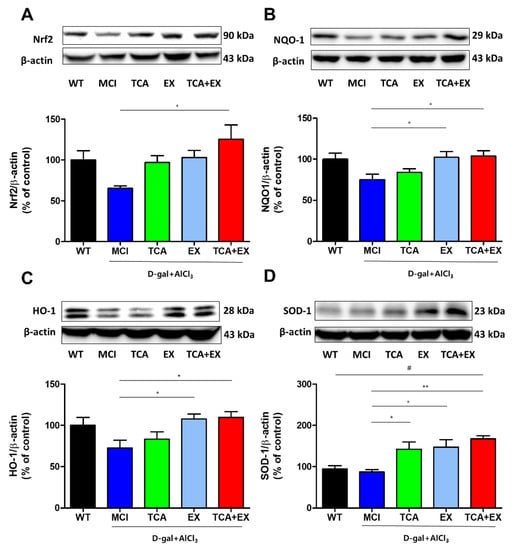
Figure 3.
Effects of TCA and treadmill exercise on the Nrf2 signaling pathway in the brains of d-gal- and AlCl3-treated mice. (A–D) Representative immunoblot images and quantification of proteins related to the Nrf2 signaling pathway. Immunoblotting was carried out using antibodies against Nrf2 (A), NQO1 (B), HO-1 (C), and SOD-1 (D) on total protein lysates from the brain. All results are expressed as the mean ± SEM; n = 5–6 per group). * p < 0.05 compared with the MCI group; ** p < 0.01 compared with the MCI group; # p < 0.01 compared with the WT group. WT; wild-type control group, MCI; mild cognitive impairment group, TCA; MCI group fed TCA, EX; MCI group that performed treadmill exercise. TCA+EX; MCI group that performed treadmill exercise and was fed TCA.
3.3. TCA and Treadmill Exercise Triggered LKB1/AMPK and SIRT1/PGC1-α Expression in d-gal- and AlCl3-Treated Mice
AMPK is heterotrimeric serine/threonine enzyme which consists of a catalytic subunit and two regulatory subunits, that function monitoring sensor of the cellular energy status. In addition, AMPK diminishes oxidative stress and other potentially adverse cellular events [43]. To examine whether the antioxidant effect of TCA and EX was induced by AMPK activation, we used immunoblotting analyses to measure the changes in cerebral AMPK activity. We found that the activity of AMPK was decreased in the MCI group. In contrast, the activity of AMPK was increased in the EX group and, furthermore, there was a marked increase in the costimulation group (F = 4.144) (Figure 4A and Figure S2A). An upstream kinase, serine threonine liver kinase B1 (LKB1), was also increased in the TCA, EX, and costimulation groups compared with the MCI group (F = 4.819) (Figure 4B). Next, we examined the silent information regulator T1 (SIRT1), which is a protein that coexists with AMPK [44]. Similar to AMPK activation, SIRT1 was also increased in the TCA, EX, and costimulation groups (F = 5.417) (Figure 4C). The mitochondrial peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1α) is the critical regulator of the activation of Nrf2 [45,46]. Both AMPK and SIRT1 regulate each other and they share common target molecules, for example PGC-1 α [47]. Therefore, we analyzed PGC-1α expression. As expected, PGC-1α was also upregulated in the TCA, EX, and TCA + EX groups compared with the MCI group (F = 5.065) (Figure 4D and Figure S2D). Collectively, our data indicate that TCA and EX regulate the SIRT1/LKB1AMPK signaling pathway.
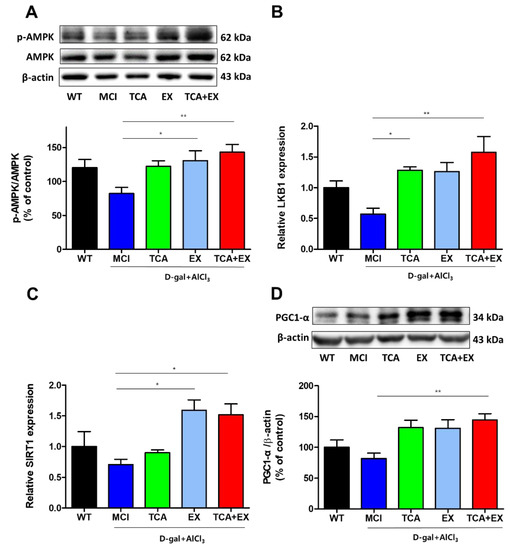
Figure 4.
Effects of TCA and treadmill exercise on LKB1/AMPK and SIRT1/PGC-1α expression in d-gal- and AlCl3-treated mice. (A) Representative immunoblot images and quantification of the AMPK (A) and PGC-1α proteins (D). mRNA expression levels of LKB1 (B) and SIRT1 (C). All results are expressed as the mean ± SEM; n = 5–6 per group). * p < 0.05 compared with the MCI group; ** p < 0.01 compared with the MCI group. WT; wild-type control group, MCI; mild cognitive impairment group, TCA; MCI group fed TCA, EX; MCI group that performed treadmill exercise. TCA+EX; MCI group that performed treadmill exercise and was fed TCA.
4. Discussion
The aim of this study was to examine the effect of TCA and treadmill exercise on behavioral dysfunction and neurological deficiencies of a mouse model with induced cognitive impairment. We found that TCA treatment and exercise activated the SIRT1/LKB1/AMPK signaling mechanism-mediated Nrf2 pathway, to exert antioxidant effects. Therefore, the combination of TCA and exercise might be an effective therapy for aging-associated diseases, including MCI. Notably, this was the first description of the co-stimulatory effect of TCA and exercise on the learning and memory impairment of the d-gal and AlCl3-induced mouse model.
The vast majority of cases of cognitive impairment are sporadic; however, the causes of these cases remain largely unknown. Therefore, the choice of animal model depends on the experimental hypothesis. Oxidative stress promotes neurotoxicity via inflammatory events, such as the production of ROS; therefore, oxidative stress is widely recognized to be very important in CNS physiology and pathophysiology [48]. Overproduction of ROS can cause protein oxidation, that acts as an important trigger of cognitive impairments and also lipid peroxidation, nuclear and mitochondrial DNA damage [8]. Because of these negative effects, oxidative stress has been consistently noted as a therapeutic target to treat neurodegenerative diseases [49]. In Our study, we chose a cognitive impairment mouse model induced by oxidative stress. d-gal treatment increases neurodegeneration by elevating ROS levels via the inhibition of antioxidant enzymes. Moreover, treatment with AlCl3 induces neurodegenerative symptoms similar to those of AD in rodents. Therefore, combination of d-gal- and AlCl3-treated mice were a suitable choice in this study to examine the effects of TCA and exercise on oxidative stress-induced cognitive impairment.
TCA, a major component of cinnamon, is known to be effective in anti-inflammatory, antidiabetic, and antioxidant effects [24,25,26,27]. Exercise also improves health in various ways, including via anti-inflammatory and antioxidant actions and even by improving cognitive functions [21,50,51]. In particular, aerobic exercise has improved cognitive processes not only in animal studies but also in studies of older human subject with cognitive disabilities, even the elderly without cognitive impairment. [52,53]. Because of these effects, we hypothesized that TCA and exercise might protect against d-gal- and AlCl3-induced cognitive impairment in mice. We showed that TCA and exercise yielded an improvement in cognitive dysfunction, and that costimulation using TCA combined with exercise further increased these effects.
In this study, we investigated whether combined treatment of nutrition (through TCA) and exercise (through treadmill exercise) might improve the learning and memory of mice induced with cognitive impairment. For this purpose, Y-maze and MWM task were used. Our results showed that TCA and treadmill exercise yielded an improvement in cognitive dysfunction, and that costimulation using TCA combined with exercise further increased these effects.
Nrf2 and its target antioxidants provide a defense system against oxidative stress. Nrf2 is pivotal in the regulation of the cellular redox role by controlling the expression of over 200 downstream genes encoding Phase II response enzymes during oxidative challenge [54]. When Nrf2 activated it translocates into the nucleus. And Nrf2 binds to antioxidants response elements (AREs) to activate the expression of target genes, including NQO1, HO-1, and SOD-1 [55]. To determine the manner in which TCA and exercise improve cognitive function in the mouse model with d-gal and AlCl3-induced MCI, we analyzed the Nrf2 signaling pathway. We found that TCA and exercise significantly upregulated the Nrf2/ARE pathway, resulting in the elevation of NQO1, HO-1, and SOD-1. These results suggest that TCA and exercise improve memory function via antioxidant effects, and that costimulation with TCA and exercise further increased these effects.
Several signaling cascades, such as the mitogen-activated protein kinases and AMPK, regulate the nuclear activation of Nrf2. AMPK is known to be effective in various diseases, such as neurological disorders, cancer, diabetes, and cardiovascular disease [56,57,58]. Previous studies reported that AMPK also mediated enhancement of Nrf2 signaling [59]. We found that TCA and exercise increased AMPK activation. We also found that TCA and exercise upregulated LKB1, which is an upstream molecule of AMPK and a member (together with another 12 proteins) of the Ser/Thr kinase family, which is closely related to AMPK [60]. LKB1 is an important enzyme, the deacetylation or phosphorylation of which is crucial to the activation of AMPK. SIRT1 is expressed widely in mammalian cells and has been reported in many tissues, including the brain [61]. Several reports have suggested that SIRT1 and AMPK have common activators, actions, and target molecules [44,47,62]. Similar to previous works showing the partnership between SIRT1 and AMPK, we found that SIRT1 was also increased in the exercise and costimulation groups. PGC-1α, which is a key enzyme associated with increased expression of Nrf2, was a common target molecule regulated by AMPK and SIRT1. TCA and treadmill exercise had a significant effect on the expression of PGC-1α. All things taken together, our studies suggest that TCA and exercise activate the SIRT/LKB1/AMPK signaling mechanism and result in an Nrf2-mediated antioxidant effect.
5. Conclusions
In summary, cognitive improvement afforded by TCA and exercise in mice with MCI induced by d-gal and AlCl3 was demonstrated in this study. TCA and exercise yielded improvement of learning and memory through Nrf2-mediated antioxidant effects. Moreover, the positive effects of TCA and exercise were related with the SIRT/LKB1/AMPK signaling pathway. Although many aspects of the exact partnership between SIRT1 and AMPK require further study, our results indicate that TCA and exercise stimulate AMPK and SIRT1, suggesting a significant correlation between these two molecules. Based on our results, we propose that the combined treatment of nutrition (through TCA) and exercise (through treadmill exercise) can help diseases associated with cognitive deficiency.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3425/10/11/793/s1, Figure S1: Original images for blot of Nrf2, NQO1, HO-1 and SOD-1, Figure S2: Original images for blot of p-AMPK and PGC1-α.
Author Contributions
Conceptualization, J.K.L. and H.-Y.K.; methodology, analysis and investigation, J.-S.R.; writing—original draft preparation, J.-S.R.; writing—review and editing, J.K.L. supervision, J.K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1D1A1B07050547).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singer, J.; Trollor, J.N.; Baune, B.T.; Sachdev, P.S.; Smith, E. Arterial stiffness, the brain and cognition: A systematic review. Ageing Res. Rev. 2014, 15, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Moller, J.T.; Cluitmans, P.; Rasmussen, L.S.; Houx, P.; Rasmussen, H.; Canet, J.; Rabbitt, P.; Jolles, J.; Larsen, K.; Hanning, C.D.; et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998, 351, 857–861. [Google Scholar] [CrossRef]
- Hovens, I.B.; Schoemaker, R.G.; van der Zee, E.A.; Heineman, E.; Izaks, G.J.; van Leeuwen, B.L. Thinking through postoperative cognitive dysfunction: How to bridge the gap between clinical and pre-clinical perspectives. Brain Behav. Immun. 2012, 26, 1169–1179. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative stress and β-amyloid protein in Alzheimer’s disease. Neuromolecular Med. 2011, 13, 223–250. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Lovell, M.A.; Markesbery, W.R. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res. 2007, 35, 7497–7504. [Google Scholar] [CrossRef]
- Bartley, M.G.; Marquardt, K.; Kirchhof, D.; Wilkins, H.M.; Patterson, D.; Linseman, D.A. Overexpression of amyloid-β protein precursor induces mitochondrial oxidative stress and activates the intrinsic apoptotic cascade. J. Alzheimers Dis. 2012, 28, 855–868. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Chen, Z.B.; Jiang, H.; Li, L.L.; Li, E.G.; Xu, Y. Alteration of Aβ metabolism-related molecules in predementia induced by AlCl3 and D-galactose. Age 2009, 31, 277–284. [Google Scholar] [CrossRef]
- Peng, X.M.; Gao, L.; Huo, S.X.; Liu, X.M.; Yan, M. The Mechanism of Memory Enhancement of Acteoside (Verbascoside) in the Senescent Mouse Model Induced by a Combination of d-gal and AlCl3. Phytother. Res. 2015, 29, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, X.G.; Zhang, X.Y.; Hou, J.D.; Lin, L.F.; Gao, Q.; Luo, H.M. Combined administration of D-galactose and aluminium induces Alzheimer-like lesions in brain. Neurosci. Bull. 2011, 27, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, R.; You, X.; Luo, F.; He, H.; Chang, X.; Zhu, L.; Ding, X.; Yan, T. Salidroside suppresses inflammation in a D-galactose-induced rat model of Alzheimer’s disease via SIRT1/NF-κB pathway. Metab. Brain Dis. 2016, 31, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Jayant, S.; Sharma, B.; Sharma, B. Protective effect of transient receptor potential vanilloid subtype 1 (TRPV1) modulator, against behavioral, biochemical and structural damage in experimental models of Alzheimer’s disease. Brain Res. 2016, 1642, 397–408. [Google Scholar] [CrossRef]
- Chan, L.; Chin, L.M.K.; Kennedy, M.; Woolstenhulme, J.G.; Nathan, S.D.; Weinstein, A.A.; Connors, G.; Weir, N.A.; Drinkard, B.; Lamberti, J.; et al. Benefits of intensive treadmill exercise training on cardiorespiratory function and quality of life in patients with pulmonary hypertension. Chest 2013, 143, 333–343. [Google Scholar] [CrossRef]
- Daniele, T.M.; de Bruin, V.M.; e Forte, A.C.; de Oliveira, D.S.; Pompeu, C.M.; de Bruin, P.F. The relationship between physical activity, restless legs syndrome, and health-related quality of life in type 2 diabetes. Endocrine 2013, 44, 125–131. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Volek, J.S.; Clark, K.L.; Gordon, S.E.; Incledon, T.; Puhl, S.M.; Triplett-McBride, N.T.; McBride, J.M.; Putukian, M.; Sebastianelli, W.J. Physiological adaptations to a weight-loss dietary regimen and exercise programs in women. J. Appl. Physiol. 1997, 83, 270–279. [Google Scholar] [CrossRef]
- Vadstrup, E.S.; Frølich, A.; Perrild, H.; Borg, E.; Røder, M. Lifestyle intervention for type 2 diabetes patients: Trial protocol of The Copenhagen Type 2 Diabetes Rehabilitation Project. BMC Public Health 2009, 9, 166. [Google Scholar] [CrossRef]
- Fratiglioni, L.; Paillard-Borg, S.; Winblad, B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004, 3, 343–353. [Google Scholar] [CrossRef]
- Um, H.S.; Kang, E.B.; Koo, J.H.; Kim, H.T.; Jin, L.; Kim, E.J.; Yang, C.H.; An, G.Y.; Cho, I.H.; Cho, J.Y. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease. Neurosci. Res. 2011, 69, 161–173. [Google Scholar] [CrossRef]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Trigiani, L.J.; Lacalle-Aurioles, M.; Bourourou, M.; Li, L.; Greenhalgh, A.D.; Zarruk, J.G.; David, S.; Fehlings, M.G.; Hamel, E. Benefits of physical exercise on cognition and glial white matter pathology in a mouse model of vascular cognitive impairment and dementia. Glia 2020, 68, 1925–1940. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.X.; Liu, G.C.; Chen, H.L.; Lu, M.N.; Chen, B.; Hu, T.; Zhang, L.; Mao, R.; Li, S.; Mei, R.; et al. Exercise-Induced Cognitive Improvement Is Associated with Sodium Channel-Mediated Excitability in APP/PS1 Mice. Neural Plast. 2020, 2020, 9132720. [Google Scholar] [CrossRef] [PubMed]
- Gowder, S.J.; Devaraj, H. Effect of the food flavour cinnamaldehyde on the antioxidant status of rat kidney. Basic. Clin. Pharmacol. Toxicol. 2006, 99, 379–382. [Google Scholar] [CrossRef]
- Tanaka, Y.; Uchi, H.; Furue, M. Antioxidant cinnamaldehyde attenuates UVB-induced photoaging. J. Dermatol. Sci. 2019, 96, 151–158. [Google Scholar] [CrossRef]
- Kim, S.H.; Hyun, S.H.; Choung, S.Y. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J. Ethnopharmacol. 2006, 104, 119–123. [Google Scholar] [CrossRef]
- Schink, A.; Naumoska, K.; Kitanovski, Z.; Kampf, C.J.; Fröhlich-Nowoisky, J.; Thines, E.; Pöschl, U.; Schuppan, D.; Lucas, K. Anti-inflammatory effects of cinnamon extract and identification of active compounds influencing the TLR2 and TLR4 signaling pathways. Food Funct. 2018, 9, 5950–5964. [Google Scholar] [CrossRef]
- Mustafa, H.N. Neuro-amelioration of cinnamaldehyde in aluminum-induced Alzheimer’s disease rat model. J. Histotechnol. 2020, 43, 11–20. [Google Scholar] [CrossRef]
- Do, J.; Kim, N.; Jeon, S.H.; Gee, M.S.; Ju, Y.J.; Kim, J.H.; Oh, M.S.; Lee, J.K. Trans-Cinnamaldehyde Alleviates Amyloid-Beta Pathogenesis via the SIRT1-PGC1α-PPARγ Pathway in 5XFAD Transgenic Mice. Int. J. Mol. Sci. 2020, 21, 4492. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Lu, W.; Zhang, S.; Guan, X.; Li, Z.; Wang, D. Anti-Oxidative Stress Activity Is Essential for Amanita caesarea Mediated Neuroprotection on Glutamate-Induced Apoptotic HT22 Cells and an Alzheimer’s Disease Mouse Model. Int. J. Mol. Sci. 2017, 18, 1623. [Google Scholar] [CrossRef]
- Gao, L.; Peng, X.M.; Huo, S.X.; Liu, X.M.; Yan, M. Memory Enhancement of Acteoside (Verbascoside) in a Senescent Mice Model Induced by a Combination of d-gal and AlCl3. Phytother. Res. 2015, 29, 1131–1136. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, D.; Zheng, Y.; Li, H.; Hao, C.; Ouyang, W. Protective effects of kinetin against aluminum chloride and D-galactose induced cognitive impairment and oxidative damage in mouse. Brain Res. Bull. 2017, 134, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, Q.; Huang, T.; Zhao, N.; Liang, F.; Xu, B.; Chen, X.; Li, T.; Bi, J. Treadmill Exercise Decreases Aβ Deposition and Counteracts Cognitive Decline in APP/PS1 Mice, Possibly via Hippocampal Microglia Modifications. Front. Aging Neurosci. 2019, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Kang, E.B.; Oh, Y.S.; Yang, D.S.; Cho, J.Y. Treadmill exercise decreases amyloid-β burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer’s disease. Exp. Neurol. 2017, 288, 142–152. [Google Scholar] [CrossRef]
- Plucińska, K.; Crouch, B.; Koss, D.; Robinson, L.; Siebrecht, M.; Riedel, G.; Platt, B. Knock-in of human BACE1 cleaves murine APP and reiterates Alzheimer-like phenotypes. J. Neurosci. 2014, 34, 10710–10728. [Google Scholar] [CrossRef]
- Crouch, B.; Yeap, J.M.; Pais, B.; Riedel, G.; Platt, B. Of mice and motion: Behavioural-EEG phenotyping of Alzheimer’s disease mouse models. J. Neurosci. Methods 2019, 319, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Crouch, B.; Sommerlade, L.; Veselcic, P.; Riedel, G.; Schelter, B.; Platt, B. Detection of time-, frequency- and direction-resolved communication within brain networks. Sci. Rep. 2018, 8, 1825. [Google Scholar] [CrossRef] [PubMed]
- Gee, M.S.; Son, S.H.; Jeon, S.H.; Do, J.; Kim, N.; Ju, Y.J.; Lee, S.J.; Chung, E.K.; Inn, K.S.; Kim, N.J.; et al. A selective p38α/β MAPK inhibitor alleviates neuropathology and cognitive impairment, and modulates microglia function in 5XFAD mouse. Alzheimers Res. Ther. 2020, 12, 45. [Google Scholar] [CrossRef]
- Yook, J.S.; Rakwal, R.; Shibato, J.; Takahashi, K.; Koizumi, H.; Shima, T.; Ikemoto, M.J.; Oharomari, L.K.; McEwen, B.S.; Soya, H. Leptin in hippocampus mediates benefits of mild exercise by an antioxidant on neurogenesis and memory. Proc. Natl. Acad. Sci. USA 2019, 116, 10988–10993. [Google Scholar] [CrossRef] [PubMed]
- García-Mesa, Y.; Pareja-Galeano, H.; Bonet-Costa, V.; Revilla, S.; Gómez-Cabrera, M.C.; Gambini, J.; Giménez-Llort, L.; Cristòfol, R.; Viña, J.; Sanfeliu, C. Physical exercise neuroprotects ovariectomized 3xTg-AD mice through BDNF mechanisms. Psychoneuroendocrinology 2014, 45, 154–166. [Google Scholar] [CrossRef]
- Gureev, A.P.; Popov, V.N.; Starkov, A.A. Crosstalk between the mTOR and Nrf2/ARE signaling pathways as a target in the improvement of long-term potentiation. Exp. Neurol. 2020, 328, 113285. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, X.; Gong, D.; Xia, P.; Wang, F.; Xu, S. Bungeanum Improves Cognitive Dysfunction and Neurological Deficits in D-Galactose-Induced Aging Mice via Activating PI3K/Akt/Nrf2 Signaling Pathway. Front. Pharmacol. 2020, 11, 71. [Google Scholar] [CrossRef]
- Ruderman, N.; Prentki, M. AMP kinase and malonyl-CoA: Targets for therapy of the metabolic syndrome. Nat. Rev. Drug Discov. 2004, 3, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- O’Neill, H.M.; Maarbjerg, S.J.; Crane, J.D.; Jeppesen, J.; Jørgensen, S.B.; Schertzer, J.D.; Shyroka, O.; Kiens, B.; van Denderen, B.J.; Tarnopolsky, M.A.; et al. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc. Natl. Acad. Sci. USA 2011, 108, 16092–16097. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Patel, N.V.; Gordon, M.N.; Connor, K.E.; Good, R.A.; Engelman, R.W.; Mason, J.; Morgan, D.G.; Morgan, T.E.; Finch, C.E. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol. Aging 2005, 26, 995–1000. [Google Scholar] [CrossRef]
- Patel, M. Targeting Oxidative Stress in Central Nervous System Disorders. Trends Pharmacol. Sci. 2016, 37, 768–778. [Google Scholar] [CrossRef]
- Scherder, E.; Scherder, R.; Verburgh, L.; Königs, M.; Blom, M.; Kramer, A.F.; Eggermont, L. Executive functions of sedentary elderly may benefit from walking: A systematic review and meta-analysis. Am. J. Geriatr. Psychiatry 2014, 22, 782–791. [Google Scholar] [CrossRef]
- Falck, R.S.; Davis, J.C.; Liu-Ambrose, T. What is the association between sedentary behaviour and cognitive function? A systematic review. Br. J. Sports Med. 2017, 51, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, K.A.; Cotman, C.W. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol. Dis. 2013, 57, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Cummings, J.; Schneider, L.S. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006395. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.R.; Dos Santos, N.B.; Scavone, C.; Munhoz, C.D. Nrf2/ARE Pathway Modulation by Dietary Energy Regulation in Neurological Disorders. Front. Pharmacol. 2019, 10, 33. [Google Scholar] [CrossRef]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.O.; Lee, S.K.; Kim, N.; You, G.Y.; Moon, J.W.; Sha, J.; Kim, S.J.; Park, S.H.; Kim, H.S. Celastrol suppresses breast cancer MCF-7 cell viability via the AMP-activated protein kinase (AMPK)-induced p53-polo like kinase 2 (PLK-2) pathway. Cell Signal. 2013, 25, 805–813. [Google Scholar] [CrossRef]
- Park, Y.J.; Ko, J.W.; Jang, Y.; Kwon, Y.H. Activation of AMP-activated protein kinase alleviates homocysteine-mediated neurotoxicity in SH-SY5Y cells. Neurochem. Res. 2013, 38, 1561–1571. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yu, L.F.; Zhang, L.N.; Qiu, B.Y.; Su, M.B.; Wu, F.; Chen, D.K.; Pang, T.; Gu, M.; Zhang, W.; et al. Novel small-molecule AMPK activator orally exerts beneficial effects on diabetic db/db mice. Toxicol. Appl. Pharmacol. 2013, 273, 325–334. [Google Scholar] [CrossRef]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef]
- Jeppesen, J.; Maarbjerg, S.J.; Jordy, A.B.; Fritzen, A.M.; Pehmøller, C.; Sylow, L.; Serup, A.K.; Jessen, N.; Thorsen, K.; Prats, C.; et al. LKB1 regulates lipid oxidation during exercise independently of AMPK. Diabetes 2013, 62, 1490–1499. [Google Scholar] [CrossRef]
- Yang, T.; Fu, M.; Pestell, R.; Sauve, A.A. SIRT1 and endocrine signaling. Trends Endocrinol. Metab. 2006, 17, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Finley, L.W.; Haigis, M.C. The coordination of nuclear and mitochondrial communication during aging and calorie restriction. Ageing Res. Rev. 2009, 8, 173–188. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).