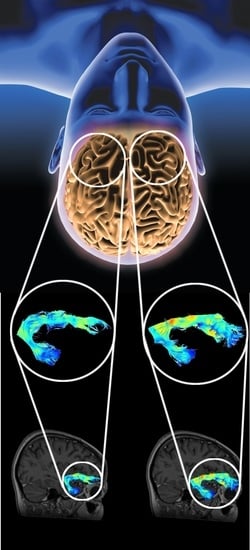
Tractography-Based Analysis of Morphological and Anatomical Characteristics of the Uncinate Fasciculus in Human Brains
Abstract
1. Introduction
2. Materials and Methods
2.1. MRI and DTI Acquisition
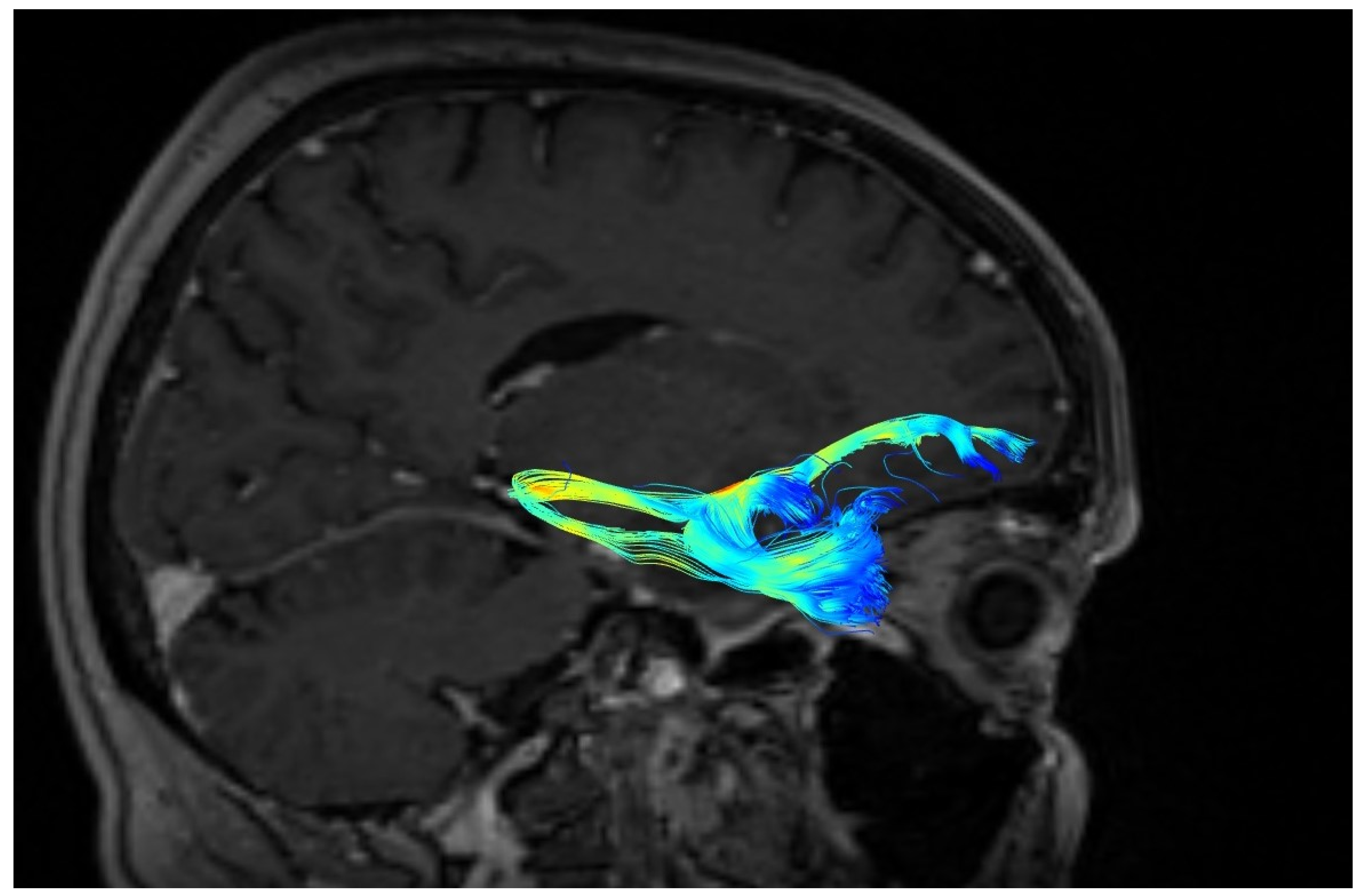
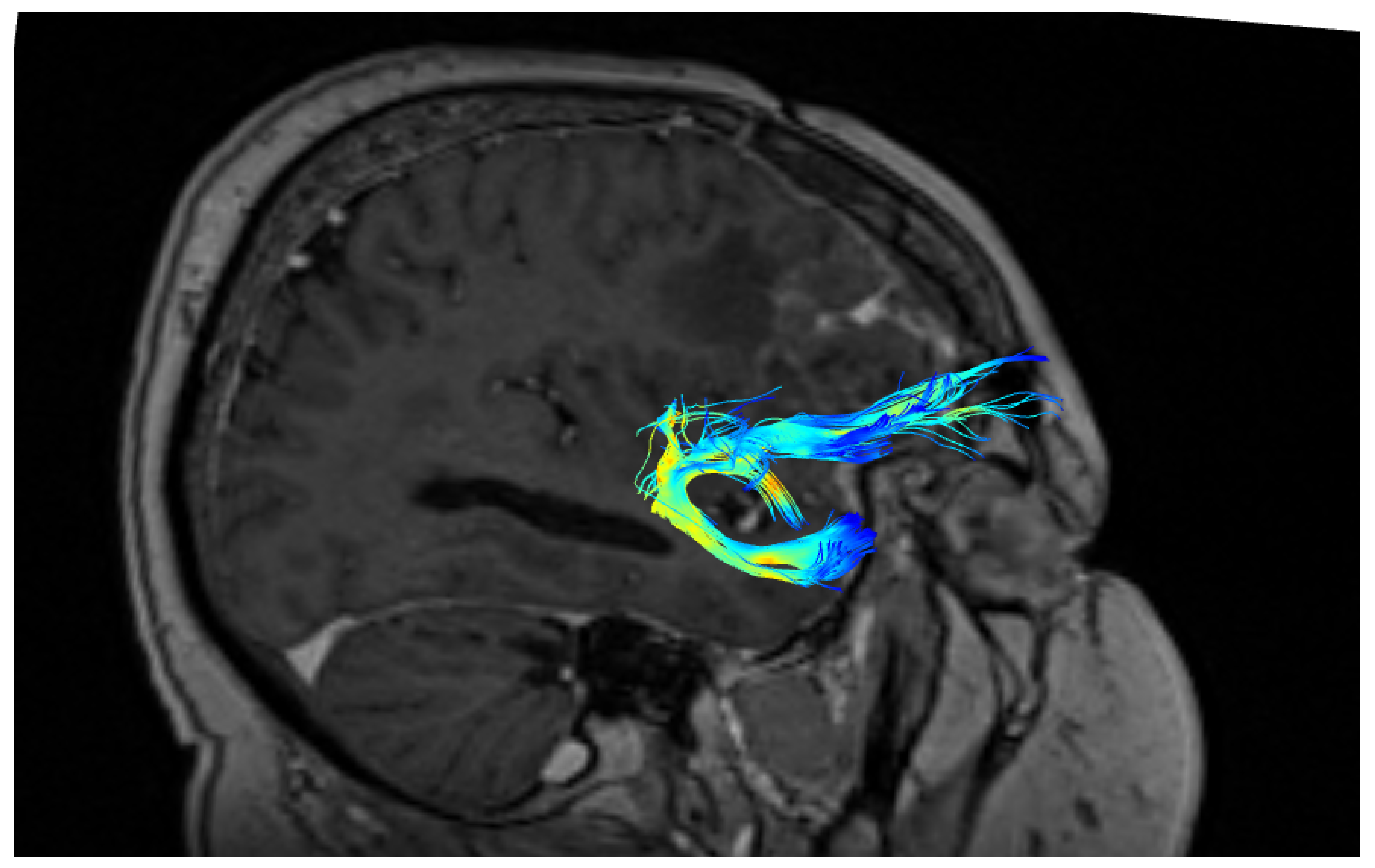
2.2. Fiber Tracking
2.3. Statistical Analyses
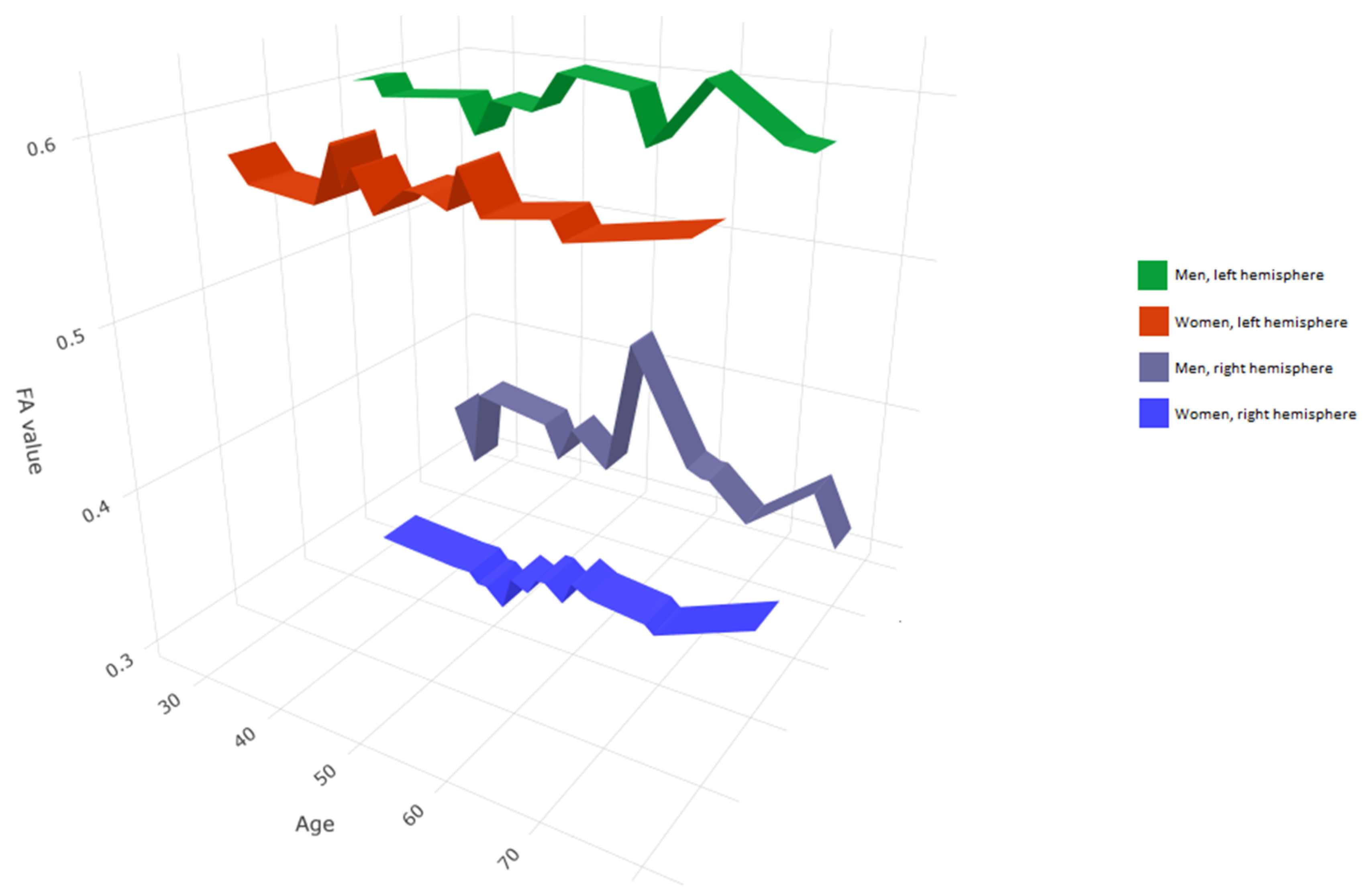
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olson, I.R.; Von Der Heide, R.J.; Alm, K.H.; Vyas, G. Development of the uncinate fasciculus: Implications for theory and developmental disorders. Dev. Cogn. Neurosci. 2015, 14, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Donner, F. Untersuchungen über den Bau des grossen Gehirn im Menschen. DMW Dtsch. Med. Wochenschr. 1878, 4, 471–473. [Google Scholar]
- Von Der Heide, R.J.; Skipper, L.M.; Klobusicky, E.; Olson, I.R. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain 2013, 136, 1692–1707. [Google Scholar] [CrossRef] [PubMed]
- Hau, J.; Sarubbo, S.; Houde, J.C.; Corsini, F.; Girard, G.; Deledalle, C.; Crivello, F.; Zago, L.; Mellet, E.; Jobard, G.; et al. Revisiting the human uncinate fasciculus, its subcomponents and asymmetries with stem-based tractography and microdissection validation. Brain Struct. Funct. 2017, 222, 1645–1662. [Google Scholar] [CrossRef] [PubMed]
- Wycoco, V.; Shroff, M.; Sudhakar, S.; Lee, W. White Matter Anatomy. What the Radiologist Needs to Know. Neuroimaging Clin. N. Am. 2013, 23, 197–216. [Google Scholar] [CrossRef]
- Fujiwara, S.; Sasaki, M.; Kanbara, Y.; Inoue, T.; Hirooka, R.; Ogawa, A. Feasibility of 1.6-mm isotropic voxel diffusion tensor tractography in depicting limbic fibers. Neuroradiology 2008, 50, 131–136. [Google Scholar] [CrossRef]
- Heimer, L.; Van Hoesen, G.; Trimble, M. The Anatomy of Neuropsychiatry: The New Anatomy of the Basal Forebrain and and Its Implication for the Neuropsychiatric Illness, 1st ed.; Academic Press: Cambridge, MA, USA, 2008; ISBN 9780123742391. [Google Scholar]
- Krestel, H.; Annoni, J.M.; Jagella, C. White matter in aphasia: A historical review of the Dejerines’ studies. Brain Lang. 2013, 127, 526–532. [Google Scholar] [CrossRef][Green Version]
- Bhatia, K.; Henderson, L.; Yim, M.; Hsu, E.; Dhaliwal, R. Diffusion Tensor Imaging Investigation of Uncinate Fasciculus Anatomy in Healthy Controls: Description of a Subgenual Stem. Neuropsychobiology 2018, 75, 132–140. [Google Scholar] [CrossRef]
- Seitz, J.; Zuo, J.X.; Lyall, A.E.; Makris, N.; Kikinis, Z.; Bouix, S.; Pasternak, O.; Fredman, E.; Duskin, J.; Goldstein, J.M.; et al. Tractography analysis of 5 white matter bundles and their clinical and cognitive correlates in early-course schizophrenia. Schizophr. Bull. 2016, 42, 762–771. [Google Scholar] [CrossRef]
- Li, Z.; Peck, K.K.; Brennan, N.P.; Jenabi, M.; Hsu, M.; Zhang, Z.; Holodny, A.I.; Young, R.J. Diffusion tensor tractography of the arcuate fasciculus in patients with brain tumors: Comparison between deterministic and probabilistic models. J. Biomed. Sci. Eng. 2013, 6, 192–200. [Google Scholar] [CrossRef]
- Ebeling, U.; Cramon, D.V. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir. 1992, 115, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Yağmurlu, K.; Oguz, K.K.; Shaffrey, M.E.; Mut, M. Orbitofrontal extensions of the insular glioma based on subdivision of the uncinate fasciculus. J. Clin. Neurosci. 2020, 78, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Kavcic, V.; Zhu, T.; Ekholm, S.; Zhong, J. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. Am. J. Neuroradiol. 2006, 27, 1776–1781. [Google Scholar] [PubMed]
- Jones, D.K. The Effect of Gradient Sampling Schemes on Measures Derived from Diffusion Tensor MRI: A Monte Carlo Study. Magn. Reson. Med. 2004, 51, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Lebel, C.; Benner, T.; Beaulieu, C. Six is enough? Comparison of diffusion parameters measured using six or more diffusion-encoding gradient directions with deterministic tractography. Magn. Reson. Med. 2012, 68, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Yoldemir, B.; Acar, B.; Firat, Z.; Kiliçkesmez, Ö. SMT: A reliability based interactive DTI tractography algorithm. IEEE Trans. Med. Imaging 2012, 31, 1929–1940. [Google Scholar] [CrossRef]
- Larroza, A.; Moratal, D.; D’ocón Alcañiz, V.; Arana, E. Tractography of the uncinate fasciculus and the posterior cingulate fasciculus in patients with mild cognitive impairment and Alzheimer disease. Neurologia (Engl. Ed.) 2014, 29, 11–20. [Google Scholar] [CrossRef]
- Sato, T.; Maruyama, N.; Hoshida, T.; Minato, K. Correlation between uncinate fasciculus and memory tasks in healthy individual using diffusion tensor tractography. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 424–427. [Google Scholar] [CrossRef]
- Witwer, B.P.; Moftakhar, R.; Hasan, K.M.; Deshmukh, P.; Haughton, V.; Field, A.; Arfanakis, K.; Noyes, J.; Moritz, C.H.; Meyerand, M.E.; et al. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J. Neurosurg. 2002, 97, 568–575. [Google Scholar] [CrossRef]
- Oishi, K.; Faria, A.V.; Hsu, J.; Tippett, D.; Mori, S.; Hillis, A.E. Critical Role of the Right Uncinate Fasciculus in Emotional Empathy. Ann. Neurol. 2008, 23, 1–7. [Google Scholar] [CrossRef]
- Steffens, D.C.; Taylor, W.D.; Denny, K.L.; Bergman, S.R.; Wang, L. Structural integrity of the uncinate fasciculus and resting state functional connectivity of the ventral prefrontal cortex in late life depression. PLoS ONE 2011, 6, e22697. [Google Scholar] [CrossRef]
- Yagmurlu, K.; Vlasak, A.L.; Rhoton, A.L., Jr. Three-dimensional topographic fiber tract anatomy of the cerebrum. Oper. Neurosurg. 2015, 11, 274–305. [Google Scholar] [CrossRef] [PubMed]
- Gaffan, D.; Wilson, C.R.E. Medial temporal and prefrontal function: Recent behavioural disconnection studies in the macaque monkey. Cortex 2008, 44, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Kitis, O.; Ozalay, O.; Zengin, E.B.; Haznedaroglu, D.; Eker, M.C.; Yalvac, D.; Oguz, K.; Coburn, K.; Gonul, A.S. Reduced left uncinate fasciculus fractional anisotropy in deficit schizophrenia but not in non-deficit schizophrenia. Psychiatry Clin. Neurosci. 2012, 66, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.M.; Burks, J.D.; Briggs, R.G.; Smitherman, A.D.; Glenn, C.A.; Conner, A.K.; Wu, D.H.; Sughrue, M.E. The crossed frontal aslant tract: A possible pathway involved in the recovery of supplementary motor area syndrome. Brain Behav. 2018, 8, e00926. [Google Scholar] [CrossRef]
- Mahon, K.; Burdick, K.E.; Szeszko, P.R. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci. Biobehav. Rev. 2010, 34, 533–554. [Google Scholar] [CrossRef]
- Dalamagkas, K.; Tsintou, M.; Rathi, Y.; O’Donnell, L.J.; Pasternak, O.; Gong, X.; Zhu, A.; Savadjiev, P.; Papadimitriou, G.M.; Kubicki, M.; et al. Individual variations of the human corticospinal tract and its hand-related motor fibers using diffusion MRI tractography. Brain Imaging Behav. 2019, 1–40. [Google Scholar] [CrossRef]
- Highley, J.R. Asymmetry of the Uncinate Fasciculus: A Post-mortem Study of Normal Subjects and Patients with Schizophrenia. Cereb. Cortex 2002, 12, 1218–1224. [Google Scholar] [CrossRef]
- Motzkin, J.C.; Newman, J.P.; Kiehl, K.A.; Koenigs, M. Reduced prefrontal connectivity in psychopathy. J. Neurosci. 2011, 31, 17348–17357. [Google Scholar] [CrossRef]
- Sundram, F.; Deeley, Q.; Sarkar, S.; Daly, E.; Latham, R.; Craig, M.; Raczek, M.; Fahy, T.; Picchioni, M.; Barker, G.J.; et al. White matter microstructural abnormalities in the frontal lobe of adults with antisocial personality disorder. Cortex 2012, 48, 216–229. [Google Scholar] [CrossRef]
- Catani, M.; Dell’Acqua, F.; Bizzi, A.; Forkel, S.J.; Williams, S.C.; Simmons, A.; Murphy, D.G.; de Schotten, M.T. Beyond cortical localization in clinico-anatomical correlation. Cortex 2012, 48, 1262–1287. [Google Scholar] [CrossRef]
- Pugliese, L.; Catani, M.; Ameis, S.; Dell’Acqua, F.; de Schotten, M.T.; Murphy, C.; Robertson, D.; Deeley, Q.; Daly, E.; Murphy, D.G.M. The anatomy of extended limbic pathways in Asperger syndrome: A preliminary diffusion tensor imaging tractography study. Neuroimage 2009, 47, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Z.; Chang, C.; Qian, L.; Li, C.; Xiao, T.; Xiao, X.; Chu, K.; Fang, H.; Ke, X. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry 2019, 19, 399. [Google Scholar] [CrossRef]
- Malykhin, N.; Concha, L.; Seres, P.; Beaulieu, C.; Coupland, N.J. Diffusion tensor imaging tractography and reliability analysis for limbic and paralimbic white matter tracts. Psychiatry Res. Neuroimaging 2008, 164, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kou, N.; Boudrias, M.H.; Playford, E.D.; Ward, N.S. Assessing a standardised approach to measuring corticospinal integrity after stroke with DTI. NeuroImage Clin. 2013, 2, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Fjell, A.M.; Westlye, L.T.; Greve, D.N.; Fischl, B.; Benner, T.; Van Der Kouwe, A.J.W.; Salat, D.; Bjørnerud, A.; Due-Tønnessen, P.; Walhovd, K.B. The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. Neuroimage 2008, 42, 1654–1668. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, D.J.; Hallquist, M.N.; Asato, M.; Luna, B. Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. Neuroimage 2014, 92, 356–368. [Google Scholar] [CrossRef]
- Ingalhalikar, M.; Smith, A.; Parker, D.; Satterthwaite, T.D.; Elliott, M.A.; Ruparel, K.; Hakonarson, H.; Gur, R.E.; Gur, R.C.; Verma, R. Sex differences in the structural connectome of the human brain. Proc. Natl. Acad. Sci. USA 2014, 111, 823–828. [Google Scholar] [CrossRef]
- Kitamura, S.; Morikawa, M.; Kiuchi, K.; Taoka, T.; Fukusumi, M.; Kichikawa, K.; Kishimoto, T. Asymmetry, sex differences and age-related changes in the white matter in the healthy elderly: A tract-based study. BMC Res. Notes 2011, 4, 378. [Google Scholar] [CrossRef]
- Kubicki, M.; Westin, C.; Maier, S.E.; Frumin, M.; Nestor, P.G.; Ph, D.; Salisbury, D.F.; Ph, D.; Kikinis, R.; Jolesz, F.A.; et al. Uncinate Fasciculus Findings in Schizophrenia: A Magnetic Resonance Diffusion Tensor Imaging Study. Am. J. Psychiatry 2002, 5, 813–820. [Google Scholar] [CrossRef]
- Hasan, K.M.; Iftikhar, A.; Kamali, A.; Kramer, L.A.; Ashtari, M.; Cirino, P.T.; Papanicolaou, A.C.; Fletcher, J.M.; Ewing-cobbs, L. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res. 2009, 1276, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.A.; Valsasina, P.; Ceccarelli, A.; Absinta, M.; Ghezzi, A.; Riccitelli, G.; Pagani, E.; Falini, A.; Comi, G.; Scotti, G.; et al. Structural and functional MRI correlates of stroop control in benign MS. Hum. Brain Mapp. 2009, 30, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Danielian, L.E.; Iwata, N.K.; Thomasson, D.M.; Floeter, M.K. Reliability of Fiber Tracking Measurements in Diffusion Tensor Imaging for Longitudinal Study. NIH Public Access 2011, 49, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Eluvathingal, T.J.; Hasan, K.M.; Kramer, L.; Fletcher, J.M.; Ewing-Cobbs, L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb. Cortex 2007, 17, 2760–2768. [Google Scholar] [CrossRef]
- Rodrigo, S.; Oppenheim, C.; Chassoux, F.; Golestani, N. Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. Eur. Radiol. 2007, 17, 1663–1668. [Google Scholar] [CrossRef]
- Park, H.J.; Westin, C.F.; Kubicki, M.; Maier, S.E.; Niznikiewicz, M.; Baer, A.; Frumin, M.; Kikinis, R.; Jolesz, F.A.; McCarley, R.W.; et al. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: A diffusion tensor MRI study. Neuroimage 2004, 23, 213–223. [Google Scholar] [CrossRef]
- Yasmin, H.; Aoki, S.; Abe, O. Tract-specific analysis of white matter pathways in healthy subjects: A pilot study using diffusion tensor MRI. Neuroradiology 2009, 831–840. [Google Scholar] [CrossRef]
- Alm, K.H.; Rolheiser, T.; Mohamed, F.B.; Olson, I.R. Fronto-temporal white matter connectivity predicts reversal learning errors. Front. Hum. Neurosci. 2015, 9, 343. [Google Scholar] [CrossRef]
- Kurki, T.J.I.; Laalo, J.P.; Oksaranta, O.M. Diffusion tensor tractography of the uncinate fasciculus: Pitfalls in quantitative analysis due to traumatic volume changes. J. Magn. Reson. Imaging 2013, 38, 46–53. [Google Scholar] [CrossRef]
- Hsu, J.; Leemans, A.; Bai, C.; Lee, C.; Tsai, Y.; Chiu, H.; Chen, W. Gender differences and age-related white matter changes of the human brain: A diffusion tensor imaging study. Neuroimage 2008, 39, 566–577. [Google Scholar] [CrossRef]
- Borkowski, K.; Krzyżak, A.T. Analysis and correction of errors in DTI-based tractography due to diffusion gradient inhomogeneity. J. Magn. Reson. 2018, 296, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Bammer, R.; Markl, M.; Barnett, A.; Acar, B.; Alley, M.T.; Pelc, N.J.; Glover, G.H.; Moseley, M.E. Analysis and generalized correction of the effect of spatial gradient field distortions in diffusion-weighted imaging. Magn. Reson. Med. 2003, 50, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Krzyzak, A.T.; Olejniczak, Z. Improving the accuracy of PGSE DTI experiments using the spatial distribution of b matrix. Magn. Reson. Imaging 2015, 33, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Kłodowski, K.; Krzyzak, A.T. Innovative anisotropic phantoms for calibration of diffusion tensor imaging sequences. Magn. Reson. Imaging 2016, 34, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, K.; Krzyżak, A.T. Assessment of the systematic errors caused by diffusion gradient inhomogeneity in DTI-computer simulations. NMR Biomed. 2019, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.K.; Knösche, T.R.; Turner, R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage 2013, 73, 239–254. [Google Scholar] [CrossRef]
- Jeurissen, B.; Descoteaux, M.; Mori, S.; Leemans, A. Diffusion MRI fiber tractography of the brain. NMR Biomed. 2019, 32, 1–22. [Google Scholar] [CrossRef]
- Maier-Hein, K.H.; Neher, P.F.; Houde, J.C.; Côté, M.A.; Garyfallidis, E.; Zhong, J.; Chamberland, M.; Yeh, F.C.; Lin, Y.C.; Ji, Q.; et al. The challenge of mapping the human connectome based on diffusion tractography. Nat. Commun. 2017, 8, 1349. [Google Scholar] [CrossRef]
- Jones, D.K. Challenges and limitations of quantifying brain connectivity in vivo with diffusion MRI. Imaging Med. 2010, 2, 341–355. [Google Scholar] [CrossRef]
- Bajada, C.J.; Schreiber, J.; Caspers, S. Fiber length profiling: A novel approach to structural brain organization. Neuroimage 2019, 186, 164–173. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kierońska, S.; Sokal, P.; Dura, M.; Jabłońska, M.; Rudaś, M.; Jabłońska, R. Tractography-Based Analysis of Morphological and Anatomical Characteristics of the Uncinate Fasciculus in Human Brains. Brain Sci. 2020, 10, 709. https://doi.org/10.3390/brainsci10100709
Kierońska S, Sokal P, Dura M, Jabłońska M, Rudaś M, Jabłońska R. Tractography-Based Analysis of Morphological and Anatomical Characteristics of the Uncinate Fasciculus in Human Brains. Brain Sciences. 2020; 10(10):709. https://doi.org/10.3390/brainsci10100709
Chicago/Turabian StyleKierońska, Sara, Paweł Sokal, Marta Dura, Magdalena Jabłońska, Marcin Rudaś, and Renata Jabłońska. 2020. "Tractography-Based Analysis of Morphological and Anatomical Characteristics of the Uncinate Fasciculus in Human Brains" Brain Sciences 10, no. 10: 709. https://doi.org/10.3390/brainsci10100709
APA StyleKierońska, S., Sokal, P., Dura, M., Jabłońska, M., Rudaś, M., & Jabłońska, R. (2020). Tractography-Based Analysis of Morphological and Anatomical Characteristics of the Uncinate Fasciculus in Human Brains. Brain Sciences, 10(10), 709. https://doi.org/10.3390/brainsci10100709