Expression and Distribution Pattern of Pnn in Ischemic Cerebral Cortex and Cultured Neural Cells Exposed to Oxygen-Glucose Deprivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals and Middle Cerebral Artery Occlusion Operation
2.3. Culturing of Prenatal Primary Neurons and Astrocytes
2.4. Western Blot
2.5. Histopathological and Immunofluorescent Staining
2.6. Statistical Analysis
3. Results
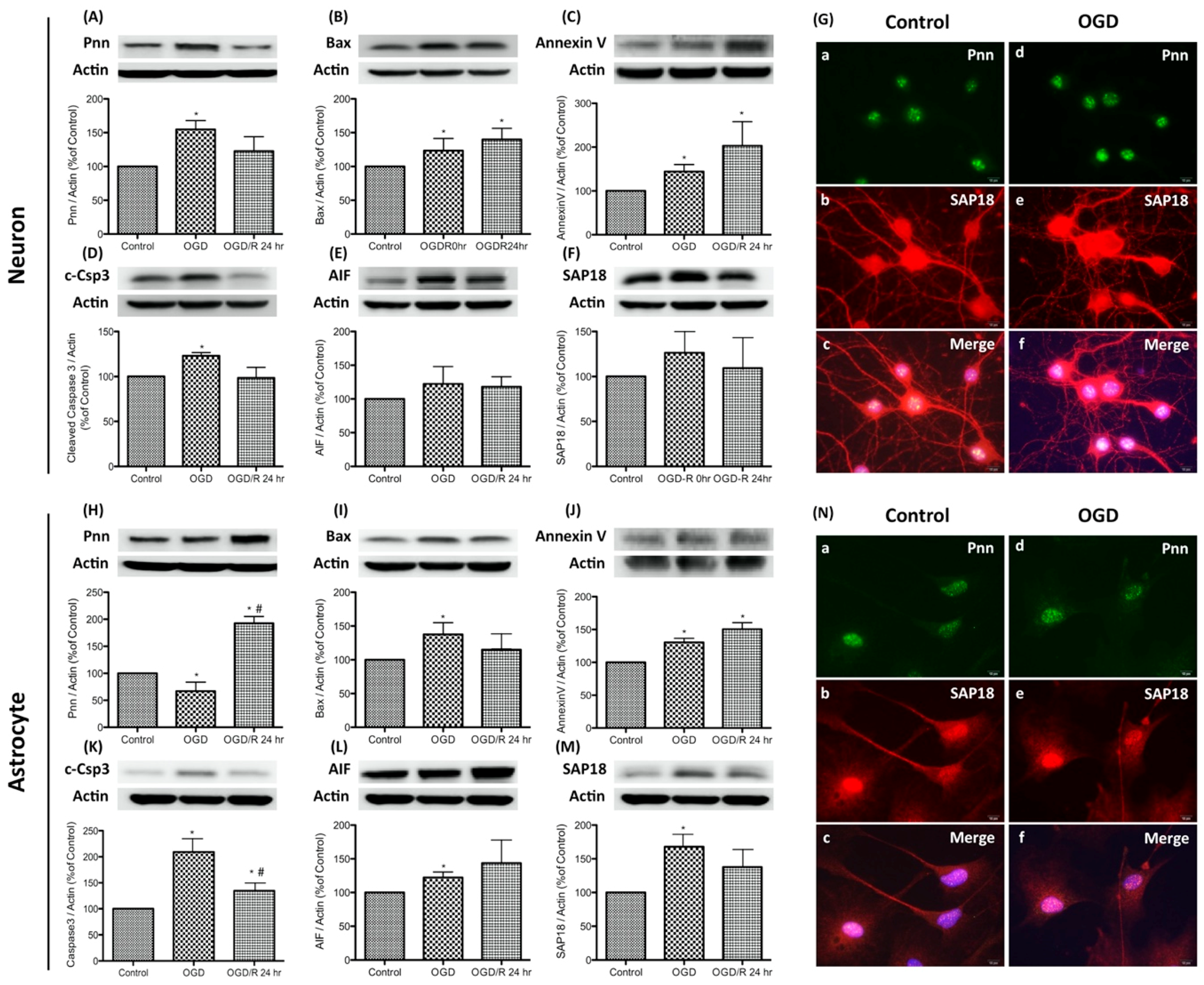
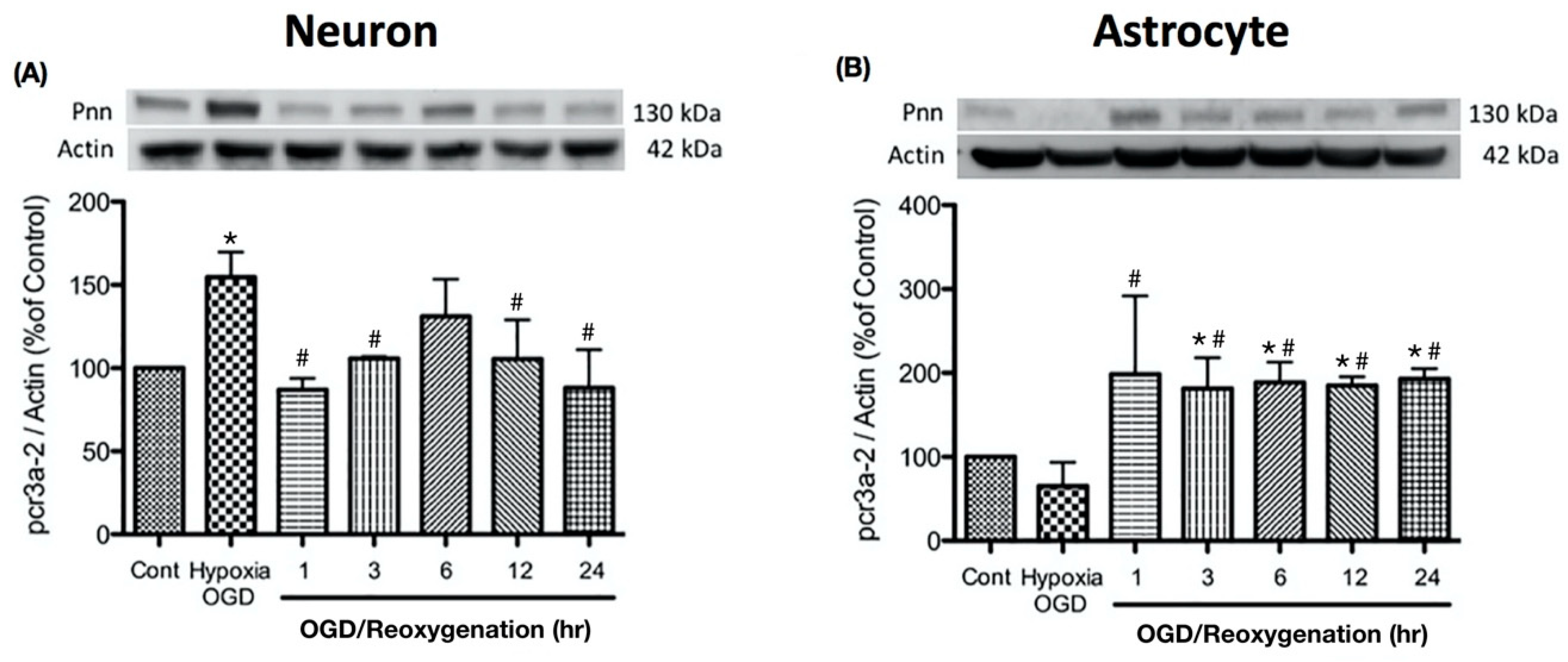
3.1. Oxygen-Glucose Deprivation and Reoxygenation Leads to Distinct Pattern of Pnn Expression in Neurons and Astrocytes
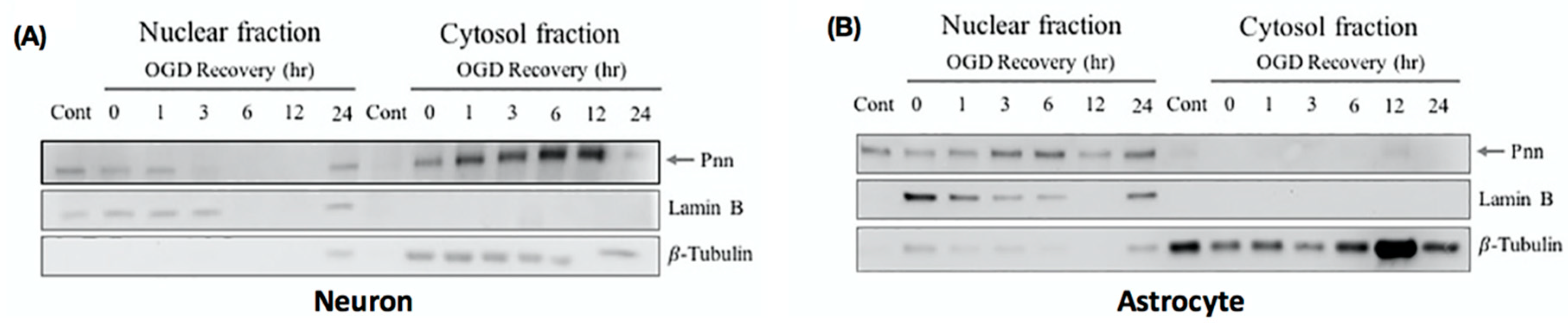
3.2. Oxygen-Glucose Deprivation and Reoxygenation Induces the Nuclear–Cytoplasmic Translocation of Pnn in Neurons
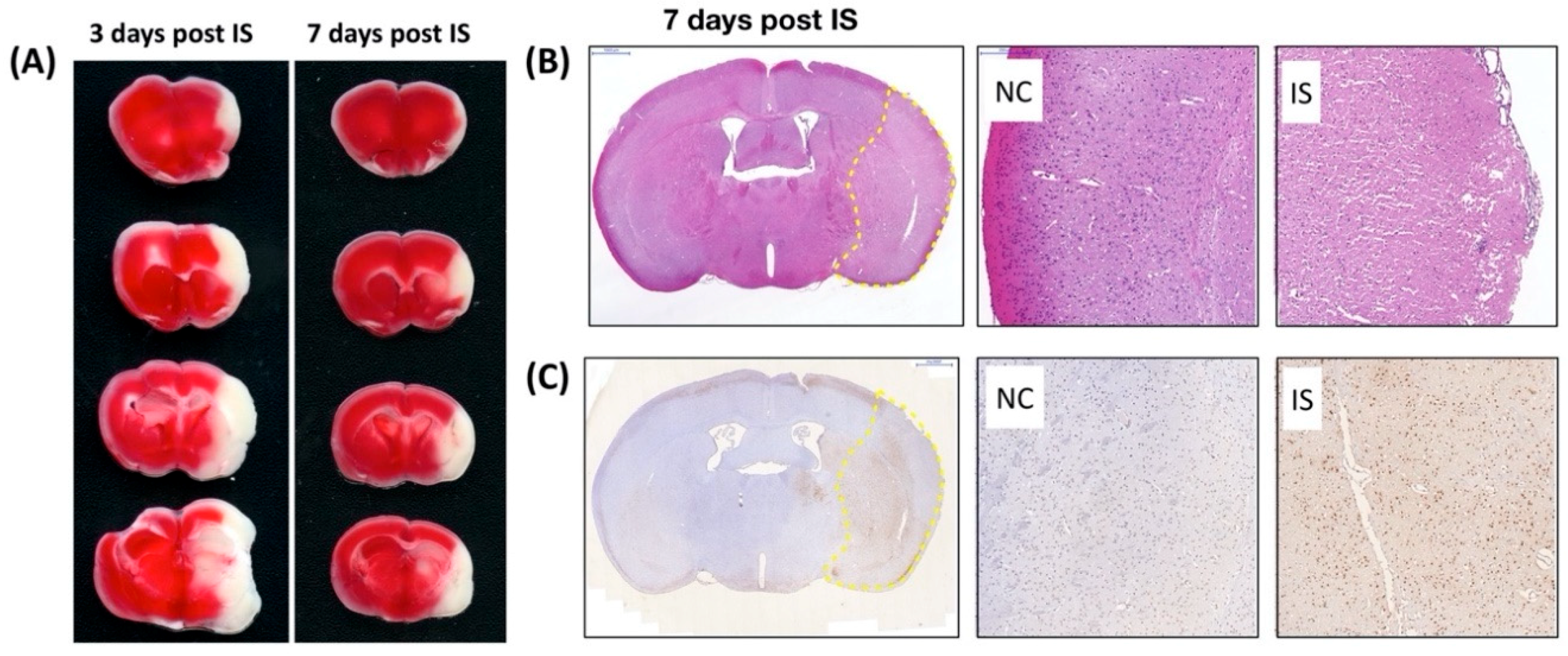
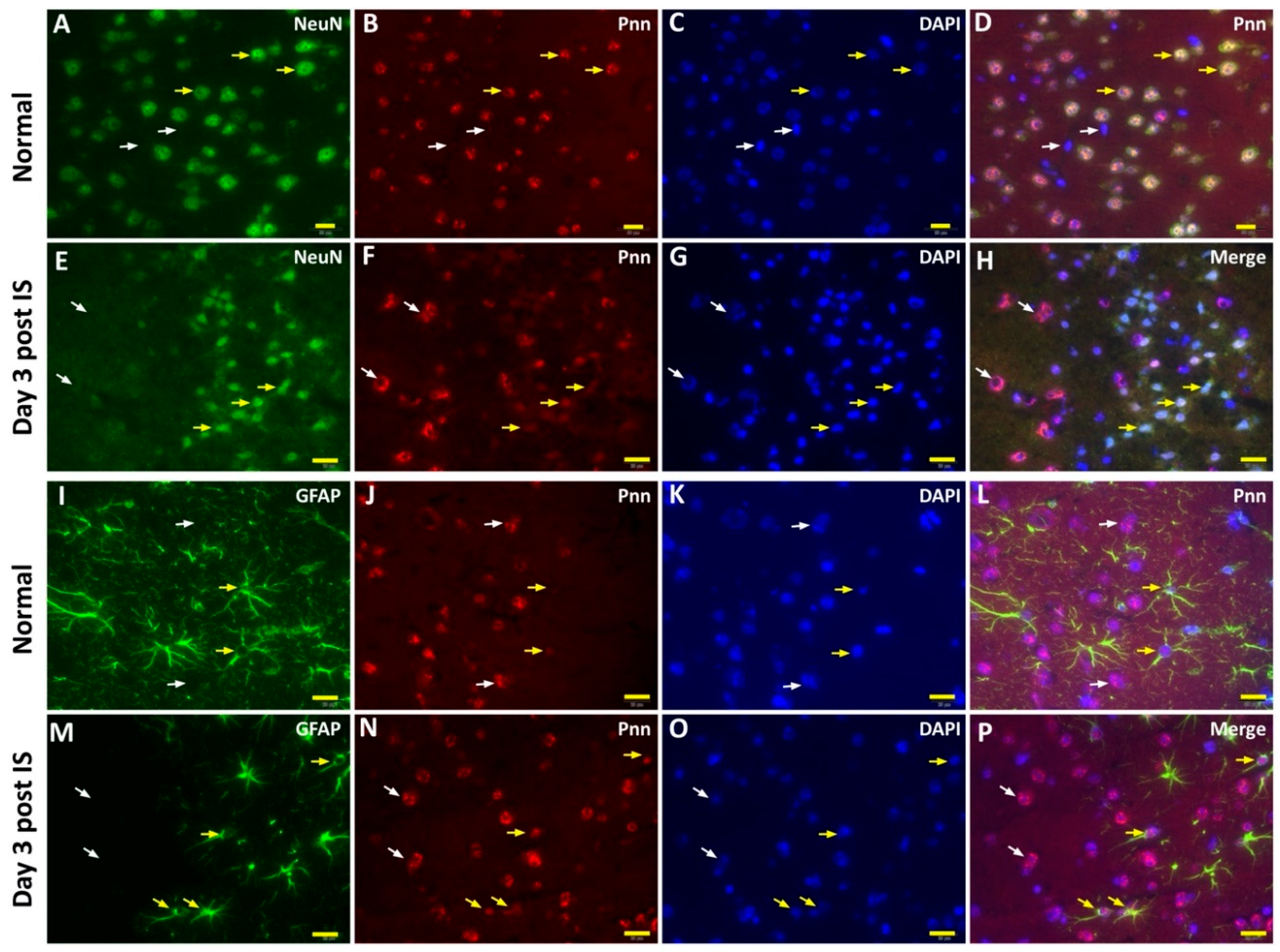
3.3. The Cell Type-Specific Distribution of Pnn in the Mouse Cerebrum with Ischemic Stroke
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leu, S.; Lin, Y.M.; Wu, C.H.; Ouyang, P. Loss of Pnn expression results in mouse early embryonic lethality and cellular apoptosis through SRSF1-mediated alternative expression of Bcl-xS and ICAD. J. Cell Sci. 2012, 125 Pt 13, 3164–3172. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.Y.; Chen, Y.J.; Ouyang, P. Pnn and SR family proteins are differentially expressed in mouse central nervous system. Histochem. Cell Biol. 2011, 135, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Kim, Y.H.; Dunn, N.W.; Sugrue, S.P. Disruption of mouse corneal epithelial differentiation by conditional inactivation of pnn. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1927–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, J.H.; Lee, Y.J.; Munguba, G.C.; Park, S.; Taxter, T.J.; Elsagga, M.Y.; Jackson, M.R.; Oh, S.P.; Sugrue, S.P. Role of Pinin in neural crest, dorsal dermis, and axial skeleton development and its involvement in the regulation of Tcf/Lef activity in mice. Dev. Dyn. 2007, 236, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lou, P.J.; Leu, S.; Ouyang, P. Modulation of alternative pre-mRNA splicing in vivo by pinin. Biochem. Biophys. Res. Commun. 2002, 294, 448–455. [Google Scholar] [CrossRef]
- Wei, Z.; Ma, W.; Qi, X.; Zhu, X.; Wang, Y.; Xu, Z.; Luo, J.; Wang, D.; Guo, W.; Li, X.; et al. Pinin facilitated proliferation and metastasis of colorectal cancer through activating EGFR/ERK signaling pathway. Oncotarget 2016, 7, 29429–29439. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, D.; Dong, C.; Tian, Y.; Gao, Z.; Wang, L. Pinin associates with prognosis of hepatocellular carcinoma through promoting cell proliferation and suppressing glucose deprivation-induced apoptosis. Oncotarget 2016, 7, 39694–39704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kwok, J.S.; Choi, P.W.; Liu, M.; Yang, J.; Singh, M.; Ng, S.K.; Welch, W.R.; Muto, M.G.; Tsui, S.K.; et al. Pinin interacts with C-terminal binding proteins for RNA alternative splicing and epithelial cell identity of human ovarian cancer cells. Oncotarget 2016, 7, 11397–11411. [Google Scholar] [CrossRef] [Green Version]
- Murachelli, A.G.; Ebert, J.; Basquin, C.; Le Hir, H.; Conti, E. The structure of the ASAP core complex reveals the existence of a Pinin-containing PSAP complex. Nat. Struct. Mol. Biol. 2012, 19, 378–386. [Google Scholar] [CrossRef]
- Chalmers, J. Global impact of stroke. Heart Dis. 2000, 2, S13–S17. [Google Scholar]
- Kalache, A.; Aboderin, I. Stroke: The global burden. Health Policy Plan. 1995, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.; Orr, S.; Briand, M.; Piazza, C.; Veydt, A.; McCoy, S. Acute ischemic stroke update. Pharmacotherapy 2010, 30, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Broughton, B.R.; Reutens, D.C.; Sobey, C.G. Apoptotic mechanisms after cerebral ischemia. Stroke 2009, 40, e331–e339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Infante, S.K.; Oberhauser, A.F.; Perez-Polo, J.R. Bax phosphorylation association with nucleus and oligomerization after neonatal hypoxia-ischemia. J. Neurosci. Res. 2013, 91, 1152–1164. [Google Scholar] [CrossRef] [PubMed]
- Chelluboina, B.; Klopfenstein, J.D.; Gujrati, M.; Rao, J.S.; Veeravalli, K.K. Temporal regulation of apoptotic and anti-apoptotic molecules after middle cerebral artery occlusion followed by reperfusion. Mol. Neurobiol. 2014, 49, 50–65. [Google Scholar] [CrossRef] [Green Version]
- Mukda, S.; Tsai, C.Y.; Leu, S.; Yang, J.L.; Chan, S.H.H. Pinin protects astrocytes from cell death after acute ischemic stroke via maintenance of mitochondrial anti-apoptotic and bioenergetics functions. J. Biomed. Sci. 2019, 26, 43. [Google Scholar] [CrossRef]
- Yang, J.L.; Lin, Y.T.; Chuang, P.C.; Bohr, V.A.; Mattson, M.P. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromolecular Med. 2014, 16, 161–174. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Cheng, Y.C.; Shih, H.Y.; Ouyang, P. Dissection of the role of Pinin in the development of zebrafish posterior pharyngeal cartilages. Histochem. Cell Biol. 2012, 138, 127–140. [Google Scholar] [CrossRef]
- Tang, T.; Yang, L.; Cao, Y.; Wang, M.; Zhang, S.; Gong, Z.; Xiong, F.; He, Y.; Zhou, Y.; Liao, Q.; et al. LncRNA AATBC regulates Pinin to promote metastasis in nasopharyngeal carcinoma. Mol. Oncol. 2020, 14, 2251–2270. [Google Scholar] [CrossRef]
- Li, F.; Yuan, P.; Rao, M.; Jin, C.H.; Tang, W.; Rong, Y.F.; Hu, Y.P.; Zhang, F.; Wei, T.; Yin, Q.; et al. piRNA-independent function of PIWIL1 as a co-activator for anaphase promoting complex/cyclosome to drive pancreatic cancer metastasis. Nat. Cell Biol. 2020, 22, 425–438. [Google Scholar] [CrossRef]
- Kang, G.J.; Park, M.K.; Byun, H.J.; Kim, H.J.; Kim, E.J.; Yu, L.; Kim, B.; Shim, J.G.; Lee, H.; Lee, C.H. SARNP, a participant in mRNA splicing and export, negatively regulates E-cadherin expression via interaction with pinin. J. Cell Physiol. 2020, 235, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kwok, J.S.; Choi, P.W.; Liu, M.; Yang, J.; Singh, M.; Ng, S.K.; Welch, W.R.; Muto, M.G.; Tsui, S.K.; et al. Correction: Pinin interacts with C-terminal binding proteins for RNA alternative splicing and epithelial cell identity of human ovarian cancer cells. Oncotarget 2017, 8, 12533. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Correia, G.P.; Li, J.L.; Lopez, M.C.; Baker, H.V.; Sugrue, S.P. Transcriptomic analysis of PNN- and ESRP1-regulated alternative pre-mRNA splicing in human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Akin, D.; Newman, J.R.; McIntyre, L.M.; Sugrue, S.P. RNA-seq analysis of impact of PNN on gene expression and alternative splicing in corneal epithelial cells. Mol. Vis. 2016, 22, 40–60. [Google Scholar] [PubMed]
- Lindenboim, L.; Zohar, H.; Worman, H.J.; Stein, R. The nuclear envelope: Target and mediator of the apoptotic process. Cell Death Discov. 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Munguba, G.C.; Alpatov, R.; Jackson, M.R.; Sugrue, S.P. Hypoxia Alters Nuclear Distribution of Nuclear Speckle Protein, Pnn, in Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4915. [Google Scholar]
- Schwerk, C.; Prasad, J.; Degenhardt, K.; Erdjument-Bromage, H.; White, E.; Tempst, P.; Kidd, V.J.; Manley, J.L.; Lahti, J.M.; Reinberg, D. ASAP, a novel protein complex involved in RNA processing and apoptosis. Mol. Cell Biol 2003, 23, 2981–2990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, E.; Beltran, S.; Espinas, M.L. Drosophila melanogaster SAP18 protein is required for environmental stress responses. FEBS Lett. 2011, 585, 275–280. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, S.-Y.; Mukda, S.; Leu, S. Expression and Distribution Pattern of Pnn in Ischemic Cerebral Cortex and Cultured Neural Cells Exposed to Oxygen-Glucose Deprivation. Brain Sci. 2020, 10, 708. https://doi.org/10.3390/brainsci10100708
Hsu S-Y, Mukda S, Leu S. Expression and Distribution Pattern of Pnn in Ischemic Cerebral Cortex and Cultured Neural Cells Exposed to Oxygen-Glucose Deprivation. Brain Sciences. 2020; 10(10):708. https://doi.org/10.3390/brainsci10100708
Chicago/Turabian StyleHsu, Shu-Yuan, Sujira Mukda, and Steve Leu. 2020. "Expression and Distribution Pattern of Pnn in Ischemic Cerebral Cortex and Cultured Neural Cells Exposed to Oxygen-Glucose Deprivation" Brain Sciences 10, no. 10: 708. https://doi.org/10.3390/brainsci10100708




