Association between Chronic Pain and Alterations in the Mesolimbic Dopaminergic System
Abstract
1. Introduction
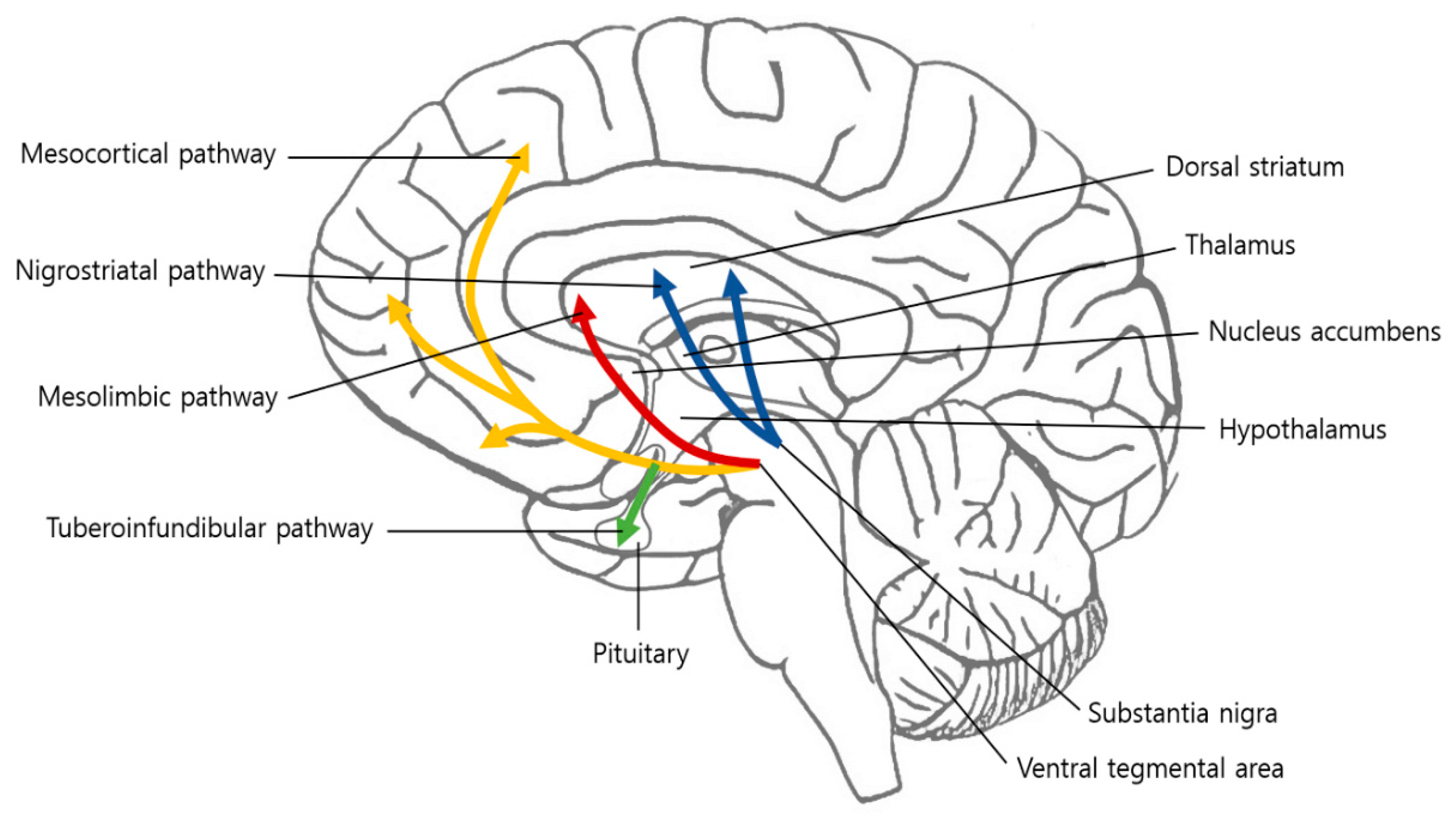
2. Dopamine
3. Mesolimbic System
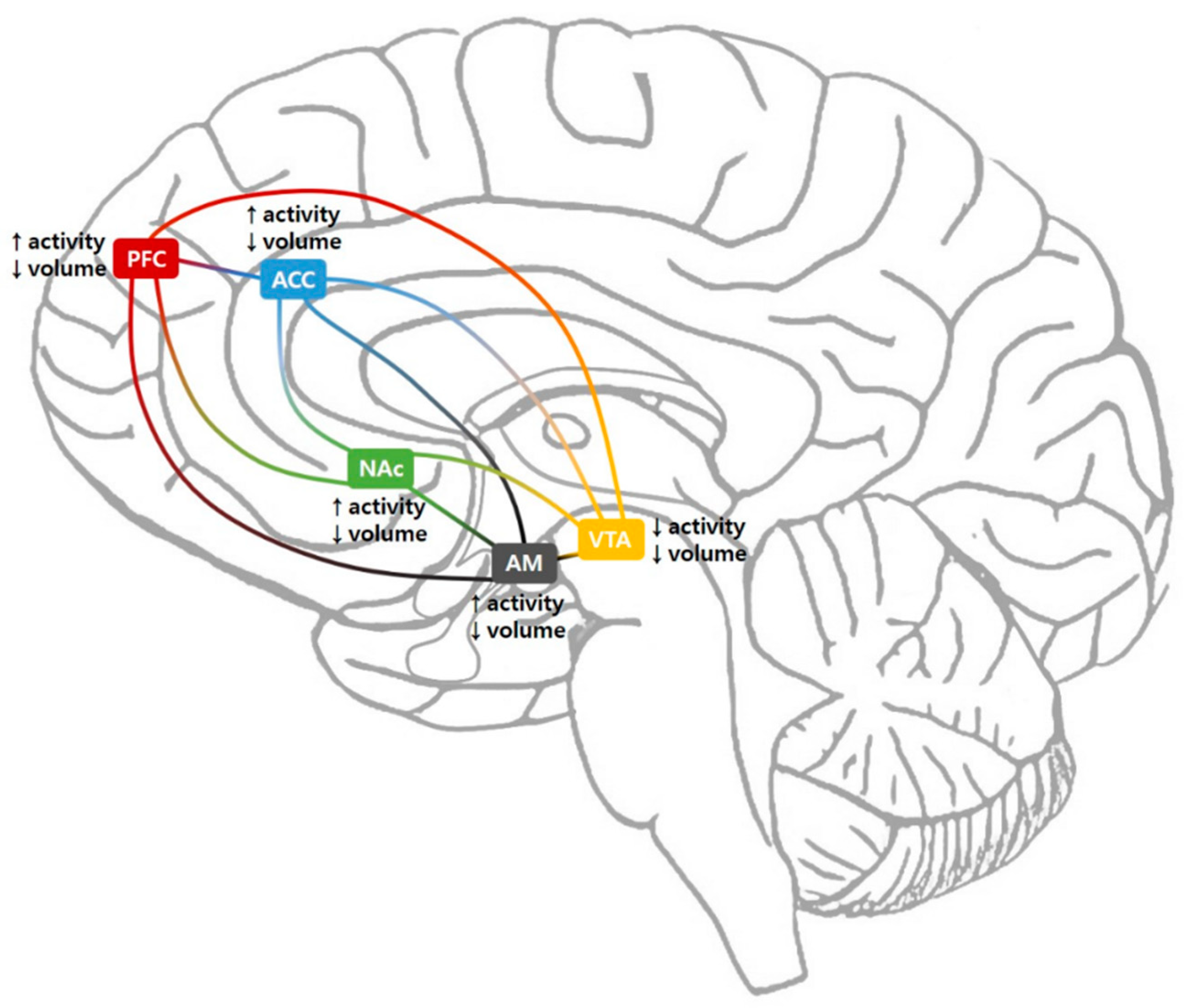
3.1. Ventral Tegmental Area
3.2. Nucleus Accumbens
3.3. Prefrontal Cortex
3.4. Anterior Cingulate Cortex
3.5. Amygdala
4. Administration of Dopaminergic Drugs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| VTA | ventral tegmental area |
| NAc | nucleus accumbens |
| PFC | prefrontal cortex |
| ACC | anterior cingulate cortex |
| MDD | major depressive disorder |
| MRI | magnetic resonance imaging |
| PET | positron emission tomography |
| fMRI | functional magnetic resonance imaging |
| TMS | transcranial magnetic stimulation |
| rTMS | repetitive transcranial magnetic stimulation |
| tDCS | transcranial direct current stimulation |
| VAS | visual analogue scale |
References
- Raja, S.N.; Carrf, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.; Mogil, J.; Ringkamp, M.; Sluka, K.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020. [Google Scholar] [CrossRef] [PubMed]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Korff, M.V.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chang, M.C. Chronic Pain: Structural and Functional Changes in Brain Structures and Associated Negative Affective States. Int. J. Mol. Sci. 2019, 20, 3130. [Google Scholar] [CrossRef]
- Bair, M.J.; Robinson, R.L.; Katon, W.; Kroenke, K. Depression and pain comorbidity: A literature review. Arch. Intern. Med. 2013, 163, 2433–2445. [Google Scholar] [CrossRef]
- Dahan, A.; van Velzen, M.; Niesters, M. Comorbidities and the complexities of chronic pain. Anesthesiology 2014, 121, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.S.; Jones, W.J.; Shen, J.; Robinson, R.L.; Weinberger, M.; Kroenke, K. Prevalence and impact of depression and pain in neurology outpatients. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1587–1589. [Google Scholar] [CrossRef]
- Tetsunaga, T.; Tesunaga, T.; Nishida, K.; Kanzaki, H.; Misawa, H.; Takigawa, T.; Shiozaki, Y.; Ozaki, T. Drug dependence in patients with chronic pain: A retrospective study. Medicine 2018, 97, e12748. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Involvement of the nucleus accumbens and dopamine system in chronic pain. Neurology 2016, 87, 1720–1726. [Google Scholar] [CrossRef]
- Finan, P.H.; Smith, M.T. The comorbidity of insomnia, chronic pain, and depression: Dopamine as a putative mechanism. Sleep Med. Rev. 2013, 17, 173–183. [Google Scholar] [CrossRef]
- Serafini, R.A.; Pryce, K.D.; Zachariou, V. The Mesolimbic Dopamine System in Chronic Pain and Associated Affective Comorbidities. Biol. Psychiatry 2020, 87, 64–73. [Google Scholar]
- Haddad, M.; Pud, D.; Treister, R.; Suzan, E.; Eisenberg, E. The effects of a dopamine agonist (apomorphine) on experimental and spontaneous pain in patients with chronic radicular pain: A randomized, double-blind, placebo-controlled, cross-over study. PLoS ONE 2018, 13, e0195287. [Google Scholar] [CrossRef] [PubMed]
- Holman, A.J.; Myers, R.R. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum. 2005, 52, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Stuginski-Barbosa, J.; Rodrigues, G.G.; Bigal, M.E.; Speciali, J.G. Burning mouth syndrome responsive to pramipexol. J. Headache Pain 2008, 9, 43–45. [Google Scholar] [CrossRef][Green Version]
- Ikemoto, S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci. Biobehav. Rev. 2010, 35, 129–150. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, D.Y.; Chun, M.H.; Kim, Y.G. The effect of repetitive transcranial magnetic stimulation on fibromyalgia: A randomized sham-controlled trial with 1-mo follow-up. Am. J. Phys. Med. Rehabil. 2012, 91, 1077–1085. [Google Scholar] [CrossRef]
- Yu, S.; Li, W.; Shen, W.; Edwards, R.R.; Gollub, R.L.; Wilson, G.; Park, J.; Ortiz, A.; Cao, J.; Gerber, J.; et al. Impaired mesocorticolimbic connectivity underlies increased pain sensitivity in chronic low back pain. NeuroImage 2020, 218, 116969. [Google Scholar] [CrossRef]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
- Dreyer, J.K.; Herrik, K.F.; Berg, R.W.; Hounsgaard, J.D. Influence of phasic and tonic dopamine release on receptor activation. J. Neurosci. 2010, 30, 14273–14283. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, S.; Shukla, S. Physiological and Functional Basis of Dopamine Receptors and Their Role in Neurogenesis: Possible Implication for Parkinson’s disease. J. Exp. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.B. Role of central dopamine in pain and analgesia. Expert Rev. Neurother. 2008, 8, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Torres, G.E.; Gainetdinov, R.R.; Caron, M.G. Plasma membrane monoamine transporters: Structure, regulation and function. Nat. Rev. Neurosci. 2003, 4, 13–25. [Google Scholar] [CrossRef] [PubMed]
- DeGroot, S.R.; Zhao-Shea, R.; Chung, L.; Klenowski, P.M.; Sun, F.; Molas, S.; Gardner, P.D.; Li, Y.; Tapper, A.R. Midbrain Dopamine Controls Anxiety-like Behavior by Engaging Unique Interpeduncular Nucleus Microcircuitry. Biol. Psychiatry 2020. [Google Scholar] [CrossRef]
- Augustin, S.M.; Loewinger, G.C.; O’Neal, T.J.; Kravitz, A.V.; Lovinger, D.M. Dopamine D2 receptor signaling on iMSNs is required for initiation and vigor of learned actions. Neuropsychopharmacology 2020, 1–11. [Google Scholar] [CrossRef]
- Chen, Y.C.; Baronio, D.; Semenova, S.; Abdurakhmanova, S.; Panula, P. Cerebral Dopamine Neurotrophic Factor Regulates Multiple Neuronal Subtypes and Behavior. J. Neurosci. 2020, 40, 6146–6164. [Google Scholar] [CrossRef]
- Abdallah, K.; Monconduit, L.; Artola, A.; Luccarini, P.; Dallel, R. GABAAergic inhibition or dopamine denervation of the A11 hypothalamic nucleus induces trigeminal analgesia. Pain 2015, 156, 644–655. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Lu, X.; Tao, F. Role of Descending Dopaminergic Pathways in Pain Modulation. Curr. Neuropharmacol. 2019, 17, 1176–1182. [Google Scholar] [CrossRef]
- Li, Z.; Yan, C.; Xie, W.Z.; Li, K.; Zeng, Y.W.; Jin, Z.; Cheung, E.F.; Chan, R.C. Anticipatory pleasure predicts effective connectivity in the mesolimbic system. Front. Behav. Neurosci. 2015, 9, 217. [Google Scholar] [CrossRef][Green Version]
- Dubol, M.; Trichard, C.; Leroy, C.; Sandu, A.L.; Rahim, M.; Granger, B.; Tzavara, E.T.; Karila, L.; Martinot, J.L.; Artiges, E. Dopamine Transporter and Reward Anticipation in a Dimensional Perspective: A Multimodal Brain Imaging Study. Neuropsychopharmacology 2018, 43, 820–827. [Google Scholar] [CrossRef]
- Farmer, M.A.; Baliki, M.N.; Apkarian, A.V. A dynamic network perspective of chronic pain. Neurosci. Lett. 2012, 520, 197–203. [Google Scholar] [CrossRef]
- Navratilova, E.; Porreca, F. Reward and motivation in pain and pain relief. Nat. Neurosci. 2014, 17, 1304–1312. [Google Scholar] [CrossRef]
- Huang, S.; Borgland, S.L.; Zamponi, G.W. Peripheral nerve injury-induced alterations in VTA neuron firing properties. Mol. Brain 2019, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Castonguay, A.; Taylor, A.J.; Murphy, N.P.; Ghogha, A.; Cook, C.; Xue, L.; Olmstead, M.C.; De Koninck, Y.; Evans, C.J.; et al. Microglia disrupt mesolimbic reward circuitry in chronic pain. J. Neurosci. 2015, 35, 8442–8450. [Google Scholar] [CrossRef]
- Anderegg, A.; Poulin, J.F.; Awatramani, R. Molecular heterogeneity of midbrain dopaminergic neurons--Moving toward single cell resolution. FEBS Lett. 2015, 589, 3714–3726. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.; Hauser, T.U.; Papoutsi, M.; Magerkurth, J.; Dolan, R.J.; Rutledge, R.B. Endogenous fluctuations in the dopaminergic midbrain drive behavioral choice variability. Proc. Natl. Acad. Sci. USA 2019, 116, 18732–18737. [Google Scholar] [CrossRef]
- Schott, B.H.; Minuzzi, L.; Krebs, R.M.; Elmenhorst, D.; Lang, M.; Winz, O.H.; Seidenbecher, C.I.; Coenen, H.H.; Heinze, H.J.; Zilles, K.; et al. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J. Neurosci. 2008, 28, 14311–14319. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E. Serotonin-dopamine interaction as a focus of novel antidepressant drugs. Curr. Drug Targets 2006, 7, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Nestler, E.J. Neural Substrates of Depression and Resilience. Neurotherapeutics 2017, 14, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.J.; Chen, D.Q.; Zhong, J.; Lin, A.; Behan, B.; Walker, M.; Hodaie, M. Affective Circuitry Alterations in Patients with Trigeminal Neuralgia. Front. Neuroanat. 2017, 11, 73. [Google Scholar] [CrossRef]
- Loggia, M.L.; Berna, C.; Kim, J.; Cahalan, C.M.; Gollub, R.L.; Wasan, A.D.; Harris, R.E.; Edwards, R.R.; Napadow, V. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014, 66, 203–212. [Google Scholar] [CrossRef]
- Akram, H.; Miller, S.; Lagrata, S.; Hyam, J.; Jahanshahi, M.; Hariz, M.; Matharu, M.; Zrinzo, L. Ventral tegmental area deep brain stimulation for refractory chronic cluster headache. Neurology 2016, 86, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Salgado, S.; Kaplitt, M.G. The Nucleus Accumbens: A Comprehensive Review. Stereotact. Funct. Neurosurg. 2015, 93, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Funayama, T.; Tateno, A.; Fukayama, H.; Okubo, Y.; Suzuki, H. Bupropion increases activation in nucleus accumbens during anticipation of monetary reward. Psychopharmacology 2019, 236, 3655–3665. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.D.; Zubieta, J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 2005, 9, 463–484. [Google Scholar] [CrossRef]
- Bushnell, M.C.; Ceko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef]
- Kucyi, A.; Davis, K.D. The dynamic pain connectome. Trends Neurosci. 2015, 38, 86–95. [Google Scholar] [CrossRef]
- Selley, D.E.; Lazenka, M.F.; Sim-Selley, L.J.; Secor McVoy, J.R.; Potter, D.N.; Chartoff, E.H.; Carlezon, W.A., Jr.; Negus, S.S. Attenuated dopamine receptor signaling in nucleus accumbens core in a rat model of chemically-induced neuropathy. Neuropharmacology 2020, 166, 107935. [Google Scholar] [CrossRef]
- Seifert, C.L.; Magon, S.; Sprenger, T.; Lang, U.E.; Huber, C.G.; Denier, N.; Vogel, M.; Schmidt, A.; Radue, E.W.; Borgwardt, S.; et al. Reduced volume of the nucleus accumbens in heroin addiction. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 265, 637–645. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Yuan, R.; Patel, D.; Chandrasekaran, S.; Weng, H.H.; Yang, J.T.; Lin, C.P.; Biswal, B.B. Altered structure and functional connection in patients with classical trigeminal neuralgia. Hum. Brain Mapp. 2017, 39, 609–621. [Google Scholar] [CrossRef]
- Grubert, C.; Hurlemann, R.; Bewernick, B.H.; Kayser, S.; Hadrysiewicz, B.; Axmacher, N.; Sturm, V.; Schlaepfer, T.E. Neuropsychological safety of nucleus accumbens deep brain stimulation for major depression: Effects of 12-month stimulation. World J. Biol. Psychiatry 2011, 12, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.M.; Polosecki, P.; Cecchi, G.A.; DeAraujo, I.E.; Barron, D.S.; Constable, T.R.; Whang, P.G.; Thomas, D.A.; Mowafi, H.; Small, D.M.; et al. Loss of nucleus accumbens low-frequency fluctuations is a signature of chronic pain. Proc. Natl. Acad. Sci. USA 2020, 117, 10015–10023. [Google Scholar] [CrossRef] [PubMed]
- Froeliger, B.; McConnell, P.A.; Stankeviciute, N.; McClure, E.A.; Kalivas, P.W.; Gray, K.M. The effects of N-acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: A double-blind, placebo-controlled fMRI pilot study. Drug Alcohol Depend. 2015, 156, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, C.S.R. Ventral striatal dysfunction in cocaine dependence—Difference mapping for subregional resting state functional connectivity. Transl. Psychiatry 2018, 8, 119. [Google Scholar] [CrossRef]
- Lara, A.H.; Wallis, J.D. The Role of Prefrontal Cortex in Working Memory: A Mini Review. Front. Syst. Neurosci. 2015, 9, 173. [Google Scholar] [CrossRef]
- Arnsten, A.F. Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat. Neurosci. 2015, 18, 1376–1385. [Google Scholar] [CrossRef]
- Seminowicz, D.A.; Wideman, T.H.; Naso, L.; Hatami-Khoroushahi, S.; Fallatah, S.; Ware, M.A.; Jarzem, P.; Buchnell, M.C.; Shir, Y.; Ouellet, J.A.; et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 2011, 31, 7540–7550. [Google Scholar] [CrossRef]
- Flodin, P.; Martinsen, S.; Altawil, R.; Waldheim, E.; Lampa, J.; Kosek, E.; Fransson, P. Intrinsic brain connectivity in chronic pain: A resting-state fMRI study in patients with rheumatoid arthritis. Front. Hum. Neurosci. 2016, 10, 107. [Google Scholar] [CrossRef]
- Tanwar, S.; Mattoo, B.; Kumar, U.; Bhatia, R. Repetitive transcranial magnetic stimulation of the prefrontal cortex for fibromyalgia syndrome: A randomised controlled trial with 6-months follow up. Adv. Rheumatol. 2020, 60, 34. [Google Scholar] [CrossRef]
- Stevens, F.L.; Hurley, R.A.; Taber, K.H. Anterior cingulate cortex: Unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef]
- Truini, A.; Tinelli, E.; Gerardi, M.C.; Calistri, V.; Iannuccelli, C.; La Cesa, S.; Tarsitani, L.; Mainero, C.; Sarzi-Puttini, P.; Cruccu, G.; et al. Abnormal resting state functional connectivity of the periaqueductal grey in patients with fibromyalgia. Clin. Exp. Rheumatol. 2016, 34, S129–S133. [Google Scholar] [PubMed]
- Barthas, F.; Sellmeijer, J.; Hugel, S.; Waltisperger, E.; Barrot, M.; Yalcin, I. The anterior cingulate cortex is a critical hub for pain-induced depression. Biol. Psychiatry 2015, 77, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Urien, L.; Xiao, Z.; Dale, J.; Bauer, E.P.; Chen, Z.; Wang, J. Rate and Temporal Coding Mechanisms in the Anterior Cingulate Cortex for Pain Anticipation. Scientific Rep. 2018, 8, 8298. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.N.; Peng, Y.B.; Boyette-Davis, J.A.; Uhelski, M.L. The anterior cingulate cortex and pain processing. Front. Integr. Neurosci. 2014, 8, 35. [Google Scholar] [CrossRef]
- Russell, M.D.; Barrick, T.R.; Howe, F.A.; Sofat, N. Reduced anterior cingulate grey matter volume in painful hand osteoarthritis. Rheumatol. Int. 2018, 38, 1429–1435. [Google Scholar] [CrossRef]
- Deng, Z.; Pan, Y.; Li, D.; Zhang, C.; Jin, H.; Wang, T.; Zhan, S.; Sun, B. Effect of bilateral anterior cingulotomy on chronic neuropathic pain with severe depression. World Neurosurg. 2019, 121, 196–200. [Google Scholar] [CrossRef]
- Schmidt-Wilcke, T.; Ichesco, E.; Hampson, J.P.; Kairys, A.; Peltier, S.; Harte, S.; Clauw, D.J.; Harris, R.E. Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. Neuroimage Clin. 2014, 6, 252–261. [Google Scholar] [CrossRef]
- Kiernan, J.A. Anatomy of the temporal lobe. Epilepsy Res. Treat. 2012, 2012, 176157. [Google Scholar] [CrossRef]
- Phelps, E.A.; LeDoux, J.E. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 2005, 48, 175–187. [Google Scholar] [CrossRef]
- Gonçalves, L.; Silva, R.; Pinto-Ribeiro, F.; Pêgo, J.M.; Bessa, J.M.; Pertovaara, A.; Sousa, N.; Almeida, A. Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Exp. Neurol. 2008, 213, 48–56. [Google Scholar] [CrossRef]
- Burgmer, M.; Gaubitz, M.; Konrad, C.; Wrenger, M.; Hilgart, S.; Heuft, G.; Pfleiderer, B. Decreased gray matter volumes in the cingulofrontal cortex and the amygdala in patients with fibromyalgia. Psychosom. Med. 2009, 71, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Bogers, W.; Raichle, M.E. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur. Neuropsychopharmacol. 2002, 12, 527–544. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kilpatrick, L.A.; Labus, J.; Gupta, A.; Jiang, Z.; Ashe-McNalley, C.; Stains, J.; Heendeniya, N.; Ebrat, B.; Smith, S.; et al. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J. Neurosci. 2013, 33, 11994–12002. [Google Scholar] [CrossRef] [PubMed]
- Veinante, P.; Yalcin, I.; Barrot, M. The amygdala between sensation and affect: A role in pain. J. Mol. Psychiatry 2013, 1, 9. [Google Scholar] [CrossRef]
- Vachon-Presseau, E.; Tetreault, P.; Petre, B.; Huang, L.; Berger, S.E.; Torbey, S.; Baria, A.T.; Mansour, A.R.; Hashmi, J.A.; Griffith, J.W.; et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 2016, 139, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Younger, J.W.; Chu, L.F.; D’Arcy, N.T.; Trott, K.E.; Jastrzab, L.E.; MacKey, S.C. Prescription opioid analgesics rapidly change the human brain. Pain 2011, 152, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Liu, C.; Ke, J.; Xu, Q.; Ye, Y.; Jia, L.; Wang, F.; Zhang, L.J.; Lu, G.M. Abnormal amygdala resting-state functional connectivity in irritable bowel syndrome. AJNR Am. J. Neuroradiol. 2016, 37, 1139–1145. [Google Scholar] [CrossRef]
- Mallory, G.W.; Abulseoud, O.; Hwang, S.C.; Gorman, D.A.; Stead, S.M.; Klassen, B.T.; Sandroni, P.; Watson, J.C.; Lee, K.H. The nucleus accumbens as a potential target for central poststroke pain. Mayo. Clin. Proc. 2012, 87, 1025–1031. [Google Scholar] [CrossRef]
- Choi, G.S.; Chang, M.C. Effects of high-frequency repetitive transcranial magnetic stimulation on reducing hemiplegic shoulder pain in patients with chronic stoke: A randomized controlled trial. Int. J. Neurosci. 2018, 128, 110–116. [Google Scholar] [CrossRef]
- Choi, G.S.; Kwak, S.G.; Lee, H.D.; Chang, M.C. Effect of high-frequency repetitive transcranial magnetic stimulation on chronic central pain after mild traumatic brain injury: A pilot study. J. Rehabil. Med. 2018, 50, 246–252. [Google Scholar] [CrossRef]
- Yang, S.; Chang, M.C. Effect of Repetitive Transcranial Magnetic Stimulation on Pain Management: A Systematic Narrative Review. Front. Neurol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Brefel-Courbon, C.; Payoux, P.; Thalamas, C.; Ory, F.; Quelven, I.; Chollet, F.; Montastruc, J.L.; Rascol, O. Effect of levodopa on pain threshold in Parkinson’s disease: A clinical and positron emission tomography study. Mov. Disord. 2005, 20, 1557–1563. [Google Scholar] [CrossRef]
- Gerdelat-Mas, A.; Simonetta-Moreau, M.; Thalamas, C.; Ory-Magne, F.; Slaoui, T.; Rascol, O.; Brefel-Courbon, C. Levodopa raises objective pain threshold in Parkinson’s disease: A RIII reflex study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Slaoui, T.; Mas-Gerdelat, A.; Ory-Magne, F.; Rascol, O.; Brefel-Courbon, C. Levodopa modifies pain thresholds in Parkinson’s disease patients. Rev. Neurol. 2007, 163, 66–71. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Carli, G.; Casoni, F.; Galbiati, A. Restless Legs Syndrome and Parkinson Disease: A Causal Relationship Between the Two Disorders? Front. Neurol. 2018, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Ruottinen, H.M.; Partinen, M.; Hublin, C.; Bergman, J.; Haaparanta, M.; Solin, O.; Rinne, J.O. An FDOPA PET study in patients with periodic limb movement disorder and restless legs syndrome. Neurology 2000, 54, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Högl, B.; Comella, C. Therapeutic advances in restless legs syndrome (RLS). Mov. Disord. 2015, 30, 1574–1579. [Google Scholar] [CrossRef]
- Jahan, F.; Nanji, K.; Qidwai, W.; Qasim, R. Fibromyalgia syndrome: An overview of pathophysiology, diagnosis and management. Oman. Med. J. 2012, 27, 192–195. [Google Scholar] [CrossRef]
- Bennett, R.M. Emerging concepts in the neurobiology of chronic pain: Evidence of abnormal sensory processing in fibromyalgia. Mayo. Clin. Proc. 1999, 74, 385–398. [Google Scholar] [CrossRef]
- Mountz, J.M.; Bradley, L.A.; Modell, J.G.; Alexander, R.W.; Triana-Alexander, M.; Aaron, L.A.; Stewart, K.E.; Alarcón, G.S.; Mountz, J.D. Fibromyalgia in women. Abnormalities of regional cerebral blood flow in the thalamus are associated with low pain threshold levels. Arthritis Rheum. 1995, 38, 926–938. [Google Scholar] [CrossRef]
- Wood, P.B.; Patterson, J.C.; Sunderland, J.J.; Tainter, K.H.; Glabus, M.F.; Lilien, D.L. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: A pilot study. J. Pain 2007, 8, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, S.A.; Oak, J.N.; Jackson, M.F.; Perez, Y.; Orser, B.A.; van Tol, H.H.; MacDonald, J.F. A D2 class dopamine receptor transactivates a receptor kinase to inhibit NMDA receptor transmission. Neuron 2002, 35, 1111–1122. [Google Scholar] [CrossRef]
- Yunus, M.B. Use of a dopamine agonist in fibromyalgia: Where is the evidence? J. Clin. Rheumatol. 2003, 9, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P. Pharmacology of dopamine agonists in the treatment of Parkinson’s disease. Neurology 2002, 58 (Suppl. 1), S1–S8. [Google Scholar] [CrossRef]
- Hagelberg, N.; Forssell, H.; Rinne, J.O.; Scheinin, H.; Taiminen, T.; Aalto, S.; Luutonen, S.; Någren, K.; Jääskeläinen, S. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain 2003, 101, 149–154. [Google Scholar] [CrossRef]
- Jääskeläinen, S.K.; Rinne, J.O.; Forssell, H.; Tenovuo, O.; Kaasinen, V.; Sonninen, P.; Bergman, J. Role of the dopaminergic system in chronic pain—A fluorodopa-PET study. Pain 2001, 90, 257–260. [Google Scholar] [CrossRef]
- Choo, T.J.; Chang, M.C. Effectiveness of orthoses for treatment in patients with spinal pain. Yeungnam Univ. J. Med. 2020, 37, 84–89. [Google Scholar] [CrossRef]
- Chang, M.C.; Lee, D.G. Clinical effectiveness of caudal epidural pulsed radiofrequency stimulation in managing refractory chronic leg pain in patients with postlumbar surgery syndrome. J. Back Musculoskelet. Rehabil. 2020, 33, 523–528. [Google Scholar] [CrossRef]
- Chang, M.C.; Lee, D.G. Outcome of transforaminal epidural steroid injection according to the severity of lumbar foraminal spinal stenosis. Pain Physician 2018, 21, 67–72. [Google Scholar] [CrossRef]
- Do, K.H.; Kim, T.H.; Chang, M.C. Effects of interlaminar epidural steroid injection in patients with moderate to severe lumber central spinal stenosis: A prospective study. Ann. Palliat. Med. 2020, 9, 163–168. [Google Scholar] [CrossRef]
- Zeng, Z.F.; Liang, Y.R.; Chen, Y.; Jing, X.N.; Peng, S.D.; Tao, E.X. Chronic back pain cured by low-dose levodopa: It it a variant of restless legs syndrome? J. Pain Res. 2018, 11, 277–279. [Google Scholar] [CrossRef]
- Faron-Górecka, A.; Kuśmider, M.; Inan, S.Y.; Siwanowicz, J.; Piwowarczyk, T.; Dziedzicka-Wasylewska, M. Long-term exposure of rats to tramadol alters brain dopamine and alpha 1-adrenoceptor function that may be related to antidepressant potency. Eur. J. Pharmacol. 2004, 501, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Field, T.; Hernandez-Reif, M.; Diego, M.; Schanberg, S.; Kuhn, C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int. J. Neurosci. 2005, 115, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Chopra, K.; Arora, V. An intricate relationship between pain and depression: Clinical correlates, coactivation factors and therapeutic targets. Expert Opin. Ther. Targets 2014, 18, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Correa, M. The mysterious motivational functions of mesolimbic dopamine. Neuron 2012, 76, 470–485. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Boudier-Revéret, M.; Choo, Y.J.; Chang, M.C. Association between Chronic Pain and Alterations in the Mesolimbic Dopaminergic System. Brain Sci. 2020, 10, 701. https://doi.org/10.3390/brainsci10100701
Yang S, Boudier-Revéret M, Choo YJ, Chang MC. Association between Chronic Pain and Alterations in the Mesolimbic Dopaminergic System. Brain Sciences. 2020; 10(10):701. https://doi.org/10.3390/brainsci10100701
Chicago/Turabian StyleYang, Seoyon, Mathieu Boudier-Revéret, Yoo Jin Choo, and Min Cheol Chang. 2020. "Association between Chronic Pain and Alterations in the Mesolimbic Dopaminergic System" Brain Sciences 10, no. 10: 701. https://doi.org/10.3390/brainsci10100701
APA StyleYang, S., Boudier-Revéret, M., Choo, Y. J., & Chang, M. C. (2020). Association between Chronic Pain and Alterations in the Mesolimbic Dopaminergic System. Brain Sciences, 10(10), 701. https://doi.org/10.3390/brainsci10100701






