The Effect of Tactile Training on Sustained Attention in Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Participants
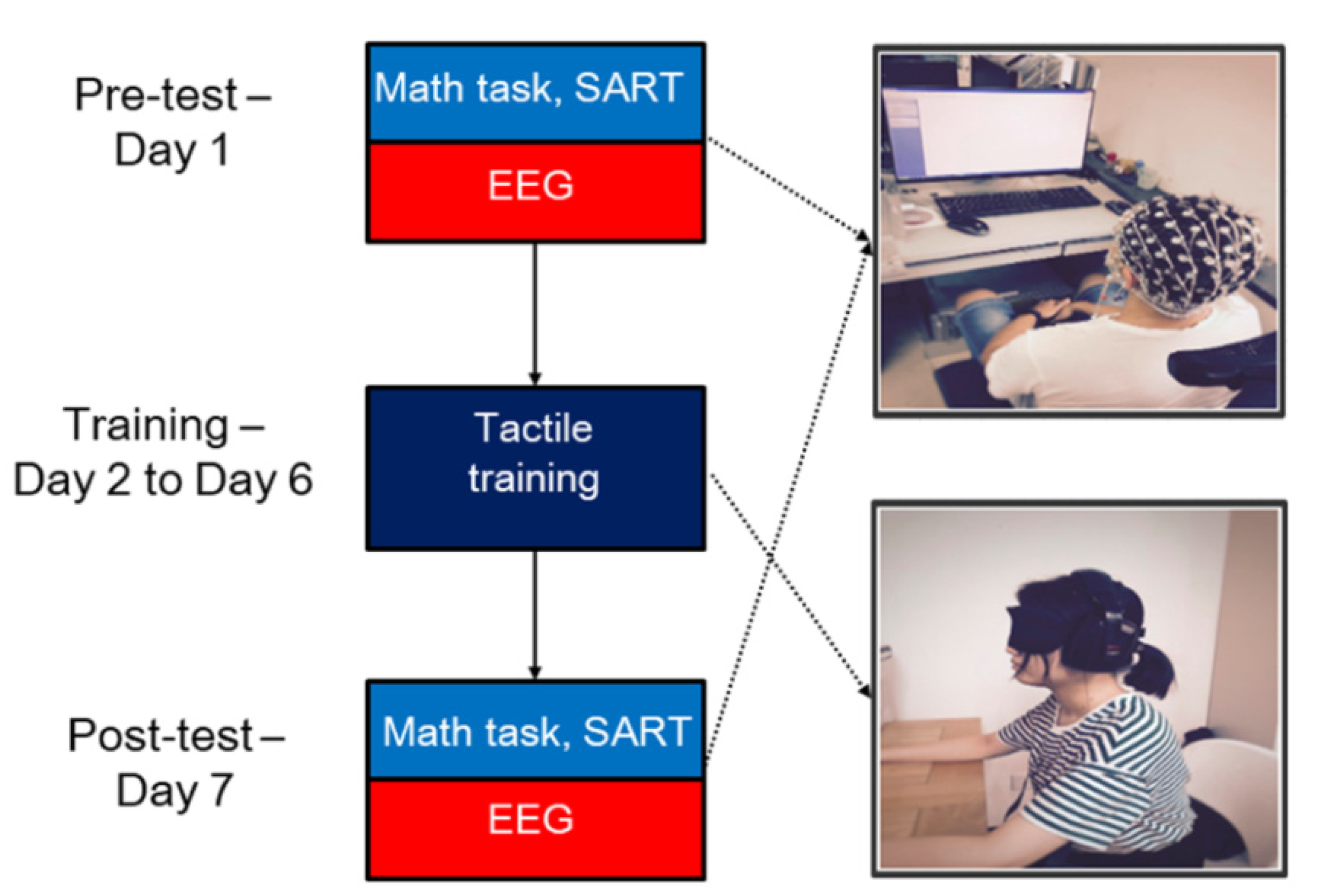
2.3. Experimental Design
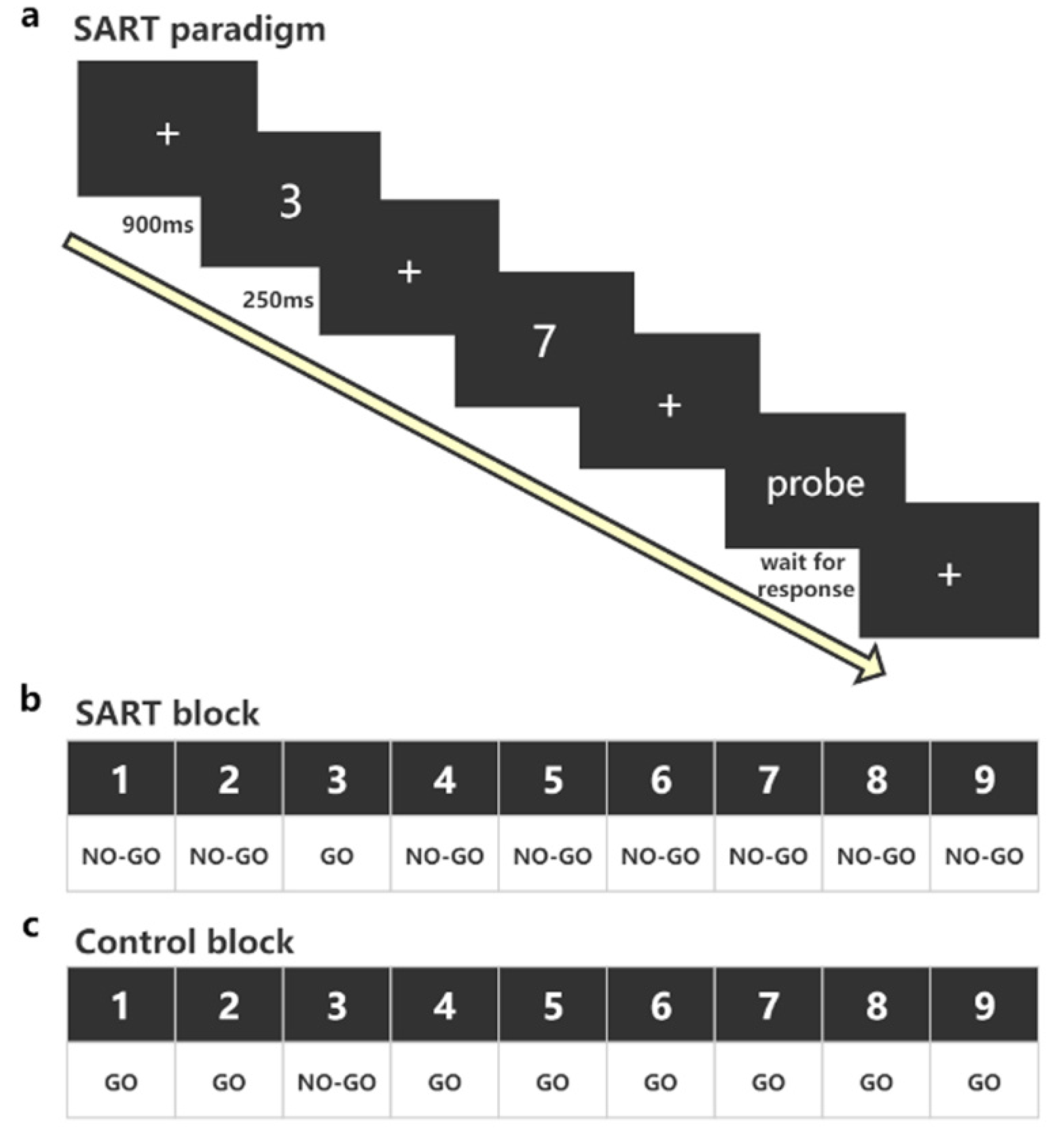
2.4. The SART
2.5. The Math Task
2.6. The Tactile Training
2.7. EEG Acquisition and Preprocessing
2.8. EEG Source Imaging
2.9. Statistical Analyses
3. Results
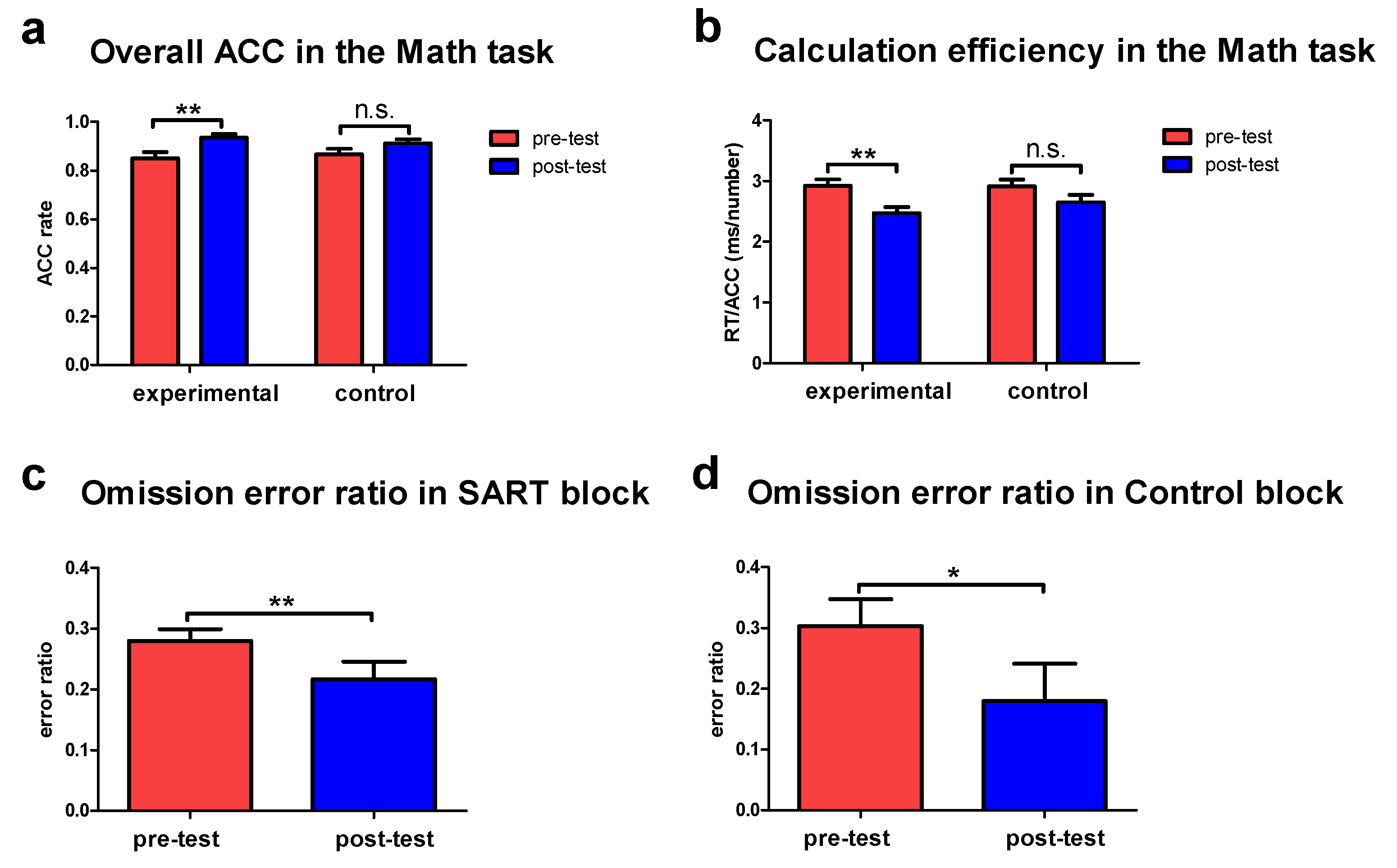
3.1. Improved Behavioral Performance following the Tactile Training
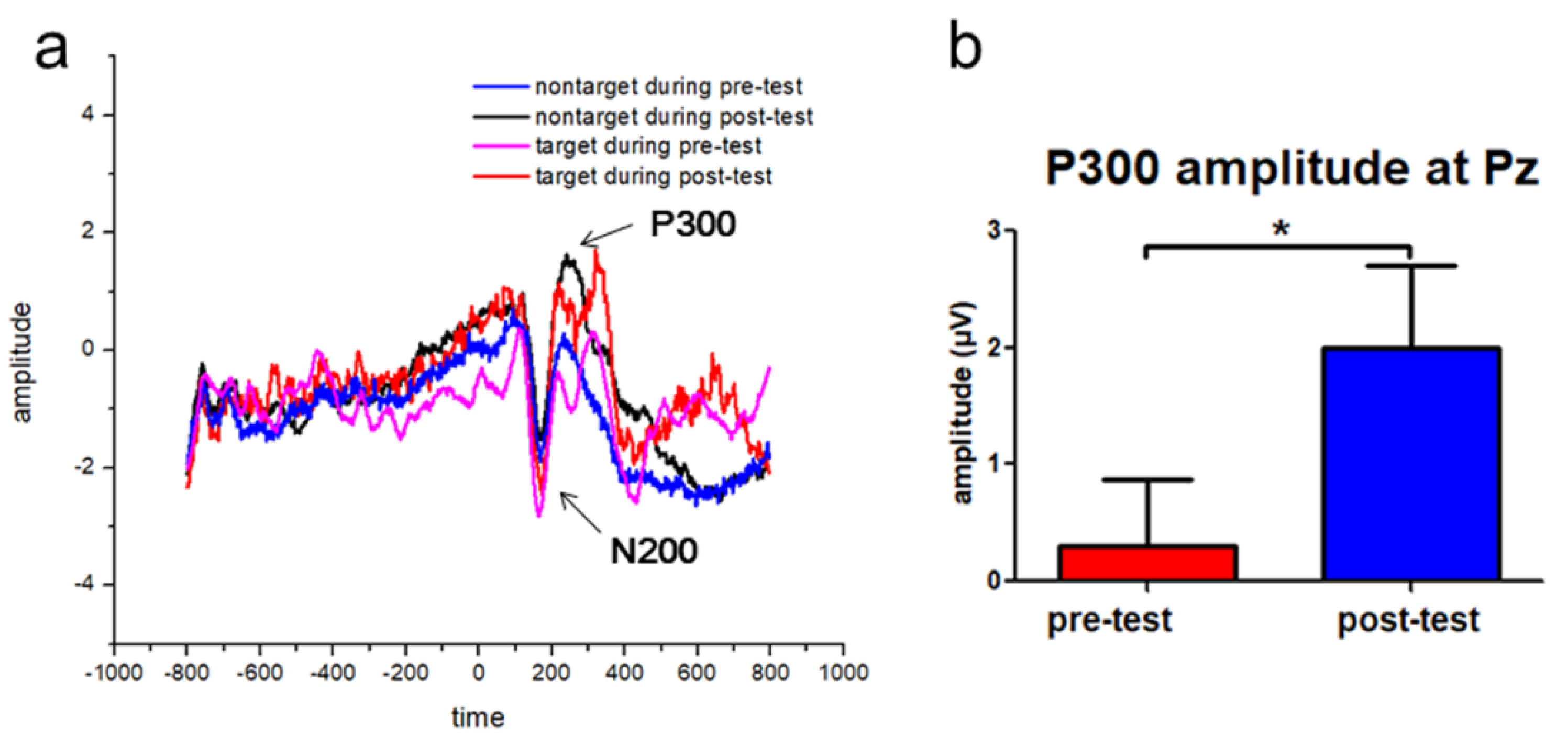
3.2. Increased P300 Event-Related Potentials (ERP) Amplitudes following the Tactile Training
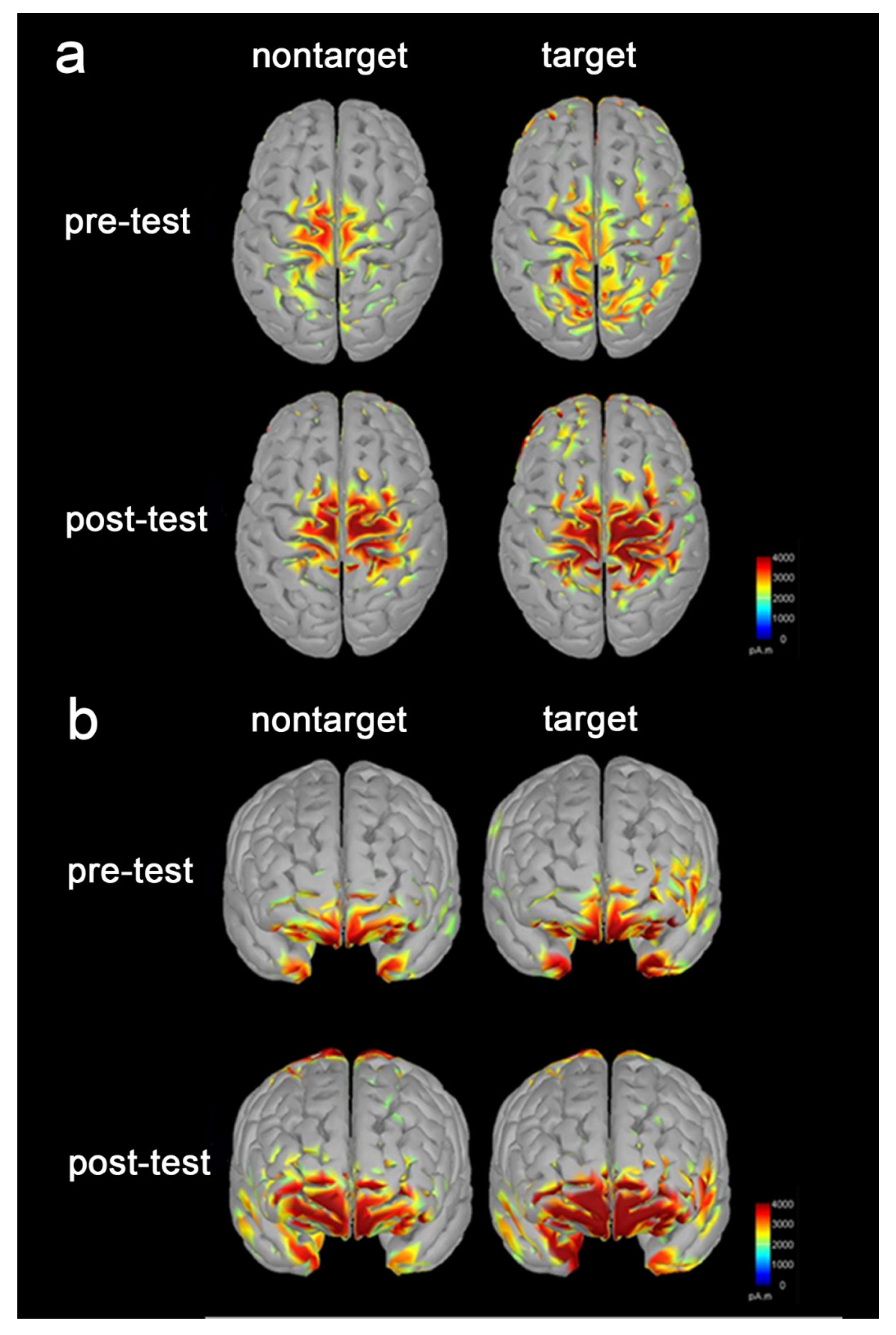
3.3. Increased Brain Activation following the Tactile Training
4. Discussion
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moisala, M.; Salmela, V.; Hietajärvi, L.; Salo, E.; Carlson, S.; Salonen, O.; Lonka, K.; Hakkarainen, K.; Salmela-Aro, K.; Alho, K. Media multitasking is associated with distractibility and increased prefrontal activity in adolescents and young adults. NeuroImage 2016, 134, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ophir, E.; Nass, C.; Wagner, A.D. Cognitive control in media multitaskers. Proc. Natl. Acad. Sci. USA 2009, 106, 15583–15587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralph, B.C.; Thomson, D.R.; Cheyne, J.A.; Smilek, D. Media multitasking and failures of attention in everyday life. Psychol. Res. 2014, 78, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.A.; Simon, A.J.; Gallen, C.L.; Skinner, S.; Janowich, J.R.; Volponi, J.J.; Rolle, C.E.; Mishra, J.; Kornfield, J.; Anguera, J.A. Closed-loop digital meditation improves sustained attention in young adults. Nat. Hum. Behav. 2019, 3, 746–757. [Google Scholar] [CrossRef]
- Rideout, V.J.; Foehr, U.G.; Roberts, D.F. Generation M2: Media in the Lives of 8-to 18-Year-Olds; Henry J. Kaiser Family Foundation: San Francisco, CA, USA, 2010. [Google Scholar]
- Chun, M.M.; Golomb, J.D.; Turk-Browne, N.B. A taxonomy of external and internal attention. Annu. Rev. Psychol. 2011, 62, 73–101. [Google Scholar] [CrossRef] [Green Version]
- Todd, R.M.; Cunningham, W.A.; Anderson, A.K.; Thompson, E. Affect-biased attention as emotion regulation. Trends Cogn. Sci. 2012, 16, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Westlye, L.T.; Grydeland, H.; Walhovd, K.B.; Fjell, A.M. Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cereb. Cortex 2010, 21, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Cortese, S.; Ferrin, M.; Brandeis, D.; Buitelaar, J.; Daley, D.; Dittmann, R.W.; Holtmann, M.; Santosh, P.; Stevenson, J.; Stringaris, A. Cognitive training for attention-deficit/hyperactivity disorder: Meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Anguera, J.A.; Boccanfuso, J.; Rintoul, J.L.; Al-Hashimi, O.; Faraji, F.; Janowich, J.; Kong, E.; Larraburo, Y.; Rolle, C.; Johnston, E. Video game training enhances cognitive control in older adults. Nature 2013, 501, 97. [Google Scholar] [CrossRef] [Green Version]
- Weyandt, L.L.; Oster, D.R.; Marraccini, M.E.; Gudmundsdottir, B.G.; Munro, B.A.; Rathkey, E.S.; McCallum, A. Prescription stimulant medication misuse: Where are we and where do we go from here? Exp. Clin. Psychopharmacol. 2016, 24, 400. [Google Scholar] [CrossRef]
- DeSantis, A.D.; Webb, E.M.; Noar, S.M. Illicit use of prescription ADHD medications on a college campus: A multimethodological approach. J. Am. Coll. Health 2008, 57, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.; Flory, K.; Humphreys, K.L.; Lee, S.S. Misuse of stimulant medication among college students: A comprehensive review and meta-analysis. Clin. Child Fam. Psychol. Rev. 2015, 18, 50–76. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-Y.; Posner, M.I. Attention training and attention state training. Trends Cogn. Sci. 2009, 13, 222–227. [Google Scholar] [CrossRef]
- Zeidan, F.; Johnson, S.K.; Gordon, N.S.; Goolkasian, P. Effects of brief and sham mindfulness meditation on mood and cardiovascular variables. J. Altern. Complement. Med. 2010, 16, 867–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deci, E.L.; Koestner, R.; Ryan, R.M. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol. Bull. 1999, 125, 627. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Yang, X.; Yang, G.; Yang, Y. Force control tasks with pure haptic feedback promote short-term focused attention. IEEE Trans. Haptics 2014, 7, 467–476. [Google Scholar] [CrossRef]
- Ostry, D.J.; Gribble, P.L. Sensory plasticity in human motor learning. Trends Neurosci. 2016, 39, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Diamond, A.; Barnett, W.S.; Thomas, J.; Munro, S. Preschool program improves cognitive control. Science 2007, 318, 1387–1388. [Google Scholar] [CrossRef] [Green Version]
- Rueda, M.R.; Checa, P.; Combita, L.M. Enhanced efficiency of the executive attention network after training in preschool children: Immediate changes and effects after two months. Dev. Cogn. Neurosci. 2012, 2 (Suppl. S1), S192–S204. [Google Scholar] [CrossRef]
- Chan, E.; Pradhan, A.K.; Pollatsek, A.; Knodler, M.A.; Fisher, D.L. Are driving simulators effective tools for evaluating novice drivers’ hazard anticipation, speed management, and attention maintenance skills. Transp. Res. Part F Traffic Psychol. Behav. 2010, 13, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Jha, A.P.; Morrison, A.B.; Dainer-Best, J.; Parker, S.; Rostrup, N.; Stanley, E.A. Minds “at attention”: Mindfulness training curbs attentional lapses in military cohorts. PLoS ONE 2015, 10, e0116889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, J.L.; Antrobus, J.S. Daydreaming, imaginal processes, and personality: A normative study. In The Function and Nature of Imagery; Academic Press: Cambridge, MA, USA, 1972; pp. 175–202. [Google Scholar]
- Whyte, J.; Grieb-Neff, P.; Gantz, C.; Polansky, M. Measuring sustained attention after traumatic brain injury: Differences in key findings from the sustained attention to response task (SART). Neuropsychologia 2006, 44, 2007–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keil, A.; Debener, S.; Gratton, G.; Junghöfer, M.; Kappenman, E.S.; Luck, S.J.; Luu, P.; Miller, G.A.; Yee, C.M. Committee report: Publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology 2014, 51, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011, 8. [Google Scholar] [CrossRef]
- He, B.; Astolfi, L.; Valdes-Sosa, P.A.; Marinazzo, D.; Palva, S.; Benar, C.G.; Michel, C.M.; Koenig, T. Electrophysiological Brain Connectivity: Theory and Implementation. IEEE Trans. Biomed. Eng. 2019. [Google Scholar] [CrossRef] [Green Version]
- Adamek, J.; Luo, Y.; Ewen, J. Using Connectivity to Explain Autism. PsyArXiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhang, J.; Wang, C.; Zhao, X.; Chang, Q.; Wang, H.; Wang, C. Discriminating schizophrenia disease progression using a P50 sensory gating task with dense-array EEG, clinical assessments, and cognitive tests. Expert Rev. Neurother. 2019, 19, 459–470. [Google Scholar] [CrossRef]
- Negra, Y.; Chaabene, H.; Sammoud, S.; Bouguezzi, R.; Abbes, M.A.; Hachana, Y.; Granacher, U. Effects of plyometric training on physical fitness in prepuberal soccer athletes. Int. J. Sports Med. 2017, 38, 370–377. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J. Educ. Behav. Stat. 2000, 25, 60–83. [Google Scholar] [CrossRef] [Green Version]
- Hart, E.; Dumas, E.; Reijntjes, R.; Van Der Hiele, K.; Van Den Bogaard, S.; Middelkoop, H.; Roos, R.; Van Dijk, J. Deficient sustained attention to response task and P300 characteristics in early Huntington’s disease. J. Neurol. 2012, 259, 1191–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanger, K.L.; Dorjee, D. Mindfulness training for adolescents: A neurodevelopmental perspective on investigating modifications in attention and emotion regulation using event-related brain potentials. Cogn. Affect. Behav. Neurosci. 2015, 15, 696–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, A.; Cusack, R.; Hawkins, K.; Heutink, J.; Rorden, C.; Robertson, I.H.; Manly, T. The P300 as a marker of waning attention and error propensity. Comput. Intell. Neurosci. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Molenberghs, P.; Gillebert, C.R.; Schoofs, H.; Dupont, P.; Peeters, R.; Vandenberghe, R. Lesion neuroanatomy of the Sustained Attention to Response task. Neuropsychologia 2009, 47, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Cheyne, J.A.; Solman, G.J.; Carriere, J.S.; Smilek, D. Anatomy of an error: A bidirectional state model of task engagement/disengagement and attention-related errors. Cognition 2009, 111, 98–113. [Google Scholar] [CrossRef]
- Johnson, K.A.; Robertson, I.H.; Kelly, S.P.; Silk, T.J.; Barry, E.; Dáibhis, A.; Watchorn, A.; Keavey, M.; Fitzgerald, M.; Gallagher, L. Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia 2007, 45, 2234–2245. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, R.G.; Bellgrove, M.A.; Dockree, P.M.; Robertson, I.H. Reduced electrodermal response to errors predicts poor sustained attention performance in attention deficit hyperactivity disorder. Neuroreport 2004, 15, 2535–2538. [Google Scholar] [CrossRef]
- Manly, T.; Owen, A.M.; McAvinue, L.; Datta, A.; Lewis, G.H.; Scott, S.K.; Rorden, C.; Pickard, J.; Robertson, I.H. Enhancing the sensitivity of a sustained attention task to frontal damage: Convergent clinical and functional imaging evidence. Neurocase 2003, 9, 340–349. [Google Scholar] [CrossRef]
- Smallwood, J.; Schooler, J.W. The restless mind. Psychol. Bull. 2006, 132, 946. [Google Scholar] [CrossRef]
- Christoff, K.; Gordon, A.M.; Smallwood, J.; Smith, R.; Schooler, J.W. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci. USA 2009, 106, 8719–8724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killingsworth, M.A.; Gilbert, D.T. A wandering mind is an unhappy mind. Science 2010, 330, 932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dinteren, R.; Arns, M.; Jongsma, M.L.; Kessels, R.P. Combined frontal and parietal P300 amplitudes indicate compensated cognitive processing across the lifespan. Front. Aging Neurosci. 2014, 6, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huster, R.J.; Enriquez-Geppert, S.; Lavallee, C.F.; Falkenstein, M.; Herrmann, C.S. Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. Int. J. Psychophysiol. 2013, 87, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Dippel, G.; Chmielewski, W.; Mückschel, M.; Beste, C. Response mode-dependent differences in neurofunctional networks during response inhibition: An EEG-beamforming study. Brain Struct. Funct. 2016, 221, 4091–4101. [Google Scholar] [CrossRef]
- Sur, S.; Sinha, V. Event-related potential: An overview. Ind. Psychiatry J. 2009, 18, 70. [Google Scholar] [CrossRef]
- Szuromi, B.; Czobor, P.; Komlósi, S.; Bitter, I. P300 deficits in adults with attention deficit hyperactivity disorder: A meta-analysis. Psychol. Med. 2011, 41, 1529. [Google Scholar] [CrossRef]
- Karaaslan, F.; Gonul, A.S.; Oguz, A.; Erdinc, E.; Esel, E. P300 changes in major depressive disorders with and without psychotic features. J. Affect. Disord. 2003, 73, 283–287. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.H.; Yoon, J.; Jung, K.-Y. Influence of task difficulty on the features of event-related potential during visual oddball task. Neurosci. Lett. 2008, 445, 179–183. [Google Scholar] [CrossRef]
- Choi, J.W.; Jung, K.-Y.; Kim, C.H.; Kim, K.H. Changes in gamma-and theta-band phase synchronization patterns due to the difficulty of auditory oddball task. Neurosci. Lett. 2010, 468, 156–160. [Google Scholar] [CrossRef]
- Kok, A. Effects of degradation of visual stimuli on components of the event-related potential (ERP) in go/nogo reaction tasks. Biol. Psychol. 1986, 23, 21–38. [Google Scholar] [CrossRef]
- Eimer, M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol. Psychol. 1993, 35, 123–138. [Google Scholar] [CrossRef]
- Jackson, S.R.; Jackson, G.M.; Roberts, M. The selection and suppression of action: ERP correlates of executive control in humans. Neuroreport 1999, 10, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kok, A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology 2001, 38, 557–577. [Google Scholar] [CrossRef] [Green Version]
- Mückschel, M.; Dippel, G.; Beste, C. Distinguishing stimulus and response codes in theta oscillations in prefrontal areas during inhibitory control of automated responses. Hum. Brain Mapp. 2017, 38, 5681–5690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampshire, A.; Chamberlain, S.R.; Monti, M.M.; Duncan, J.; Owen, A.M. The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage 2010, 50, 1313–1319. [Google Scholar] [CrossRef] [Green Version]
- Grahn, J.A.; Manly, T. Common neural recruitment across diverse sustained attention tasks. PLoS ONE 2012, 7, e49556. [Google Scholar] [CrossRef]
- Dux, P.E.; Tombu, M.N.; Harrison, S.; Rogers, B.P.; Tong, F.; Marois, R. Training improves multitasking performance by increasing the speed of information processing in human prefrontal cortex. Neuron 2009, 63, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Sakai, K.; Hikosaka, O.; Miyauchi, S.; Takino, R.; Sasaki, Y.; Pütz, B. Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J. Neurosci. 1998, 18, 1827–1840. [Google Scholar] [CrossRef] [Green Version]
- Erickson, K.I.; Colcombe, S.J.; Wadhwa, R.; Bherer, L.; Peterson, M.S.; Scalf, P.E.; Kim, J.S.; Alvarado, M.; Kramer, A.F. Training-induced functional activation changes in dual-task processing: An FMRI study. Cereb. Cortex 2007, 17, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Dosenbach, N.U.; Visscher, K.M.; Palmer, E.D.; Miezin, F.M.; Wenger, K.K.; Kang, H.C.; Burgund, E.D.; Grimes, A.L.; Schlaggar, B.L.; Petersen, S.E. A core system for the implementation of task sets. Neuron 2006, 50, 799–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, S.; Han, J.; Gu, Y.; Wang, X.; Song, W.; Li, D.; Liu, Z.; Yang, J.; Li, X. Reduced prefrontal cortex activation in children with attention-deficit/hyperactivity disorder during go/no-go task: A functional near-infrared spectroscopy study. Front. Neurosci. 2017, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Rubia, K.; Halari, R.; Smith, A.B.; Mohammad, M.; Scott, S.; Brammer, M.J. Shared and disorder-specific prefrontal abnormalities in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J. Child Psychol. Psychiatry 2009, 50, 669–678. [Google Scholar] [CrossRef]
- Wahn, B.; König, P. Is attentional resource allocation across sensory modalities task-dependent? Adv. Cogn. Psychol. 2017, 13, 83–96. [Google Scholar] [CrossRef]
- Dux, P.E.; Ivanoff, J.; Asplund, C.L.; Marois, R. Isolation of a central bottleneck of information processing with time-resolved fMRI. Neuron 2006, 52, 1109–1120. [Google Scholar] [CrossRef] [Green Version]
- Bishop, S.J. Trait anxiety and impoverished prefrontal control of attention. Nat. Neurosci. 2009, 12, 92–98. [Google Scholar] [CrossRef]
- Blasi, G.; Goldberg, T.E.; Elvevåg, B.; Rasetti, R.; Bertolino, A.; Cohen, J.; Alce, G.; Zoltick, B.; Weinberger, D.R.; Mattay, V.S. Differentiating allocation of resources and conflict detection within attentional control processing. Eur. J. Neurosci. 2007, 25, 594–602. [Google Scholar] [CrossRef]
- Du, Y.; Zatorre, R.J. Musical training sharpens and bonds ears and tongue to hear speech better. Proc. Natl. Acad. Sci. USA 2017, 114, 13579–13584. [Google Scholar] [CrossRef] [Green Version]
- Busse, L.; Roberts, K.C.; Crist, R.E.; Weissman, D.H.; Woldorff, M.G. The spread of attention across modalities and space in a multisensory object. Proc. Natl. Acad. Sci. USA 2005, 102, 18751–18756. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.-L.; Wang, D.-X.; Zhang, Y.-R.; Tang, Y.-Y. Enhancing Attention by Synchronizing Respiration and Fingertip Pressure: A Pilot Study Using Functional Near-Infrared Spectroscopy. Front. Neurosci. 2019, 13, 1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Experimental Group (n = 17) | Control Group (n = 17) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p Value | Cohen’s d | |
| Gender (M/F) | 8/9 | — | 8/9 | — | — | — |
| Age (years) | 22.5 | 1. 6 | 22.9 | 1.1 | 0.9 | 0.3 |
| Education (years) | 16.5 | 0.9 | 16.7 | 1.2 | 0.5 | 0.2 |
| Daydreaming frequency subscale (scores) | 33.9 | 7.2 | 33.7 | 5.8 | 0.1 | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Zhang, J. The Effect of Tactile Training on Sustained Attention in Young Adults. Brain Sci. 2020, 10, 695. https://doi.org/10.3390/brainsci10100695
Luo Y, Zhang J. The Effect of Tactile Training on Sustained Attention in Young Adults. Brain Sciences. 2020; 10(10):695. https://doi.org/10.3390/brainsci10100695
Chicago/Turabian StyleLuo, Yu, and Jicong Zhang. 2020. "The Effect of Tactile Training on Sustained Attention in Young Adults" Brain Sciences 10, no. 10: 695. https://doi.org/10.3390/brainsci10100695




