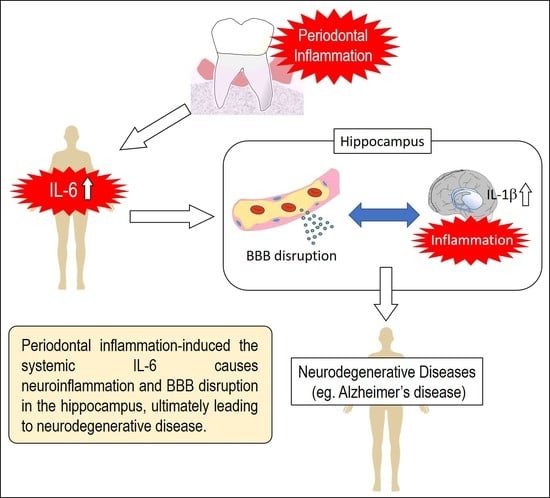
IL-6 Induced by Periodontal Inflammation Causes Neuroinflammation and Disrupts the Blood–Brain Barrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Real-Time PCR
2.3. ELISA
2.4. Immunofluorescence
2.5. Dextran Imaging
2.6. Statistical Analysis
3. Results
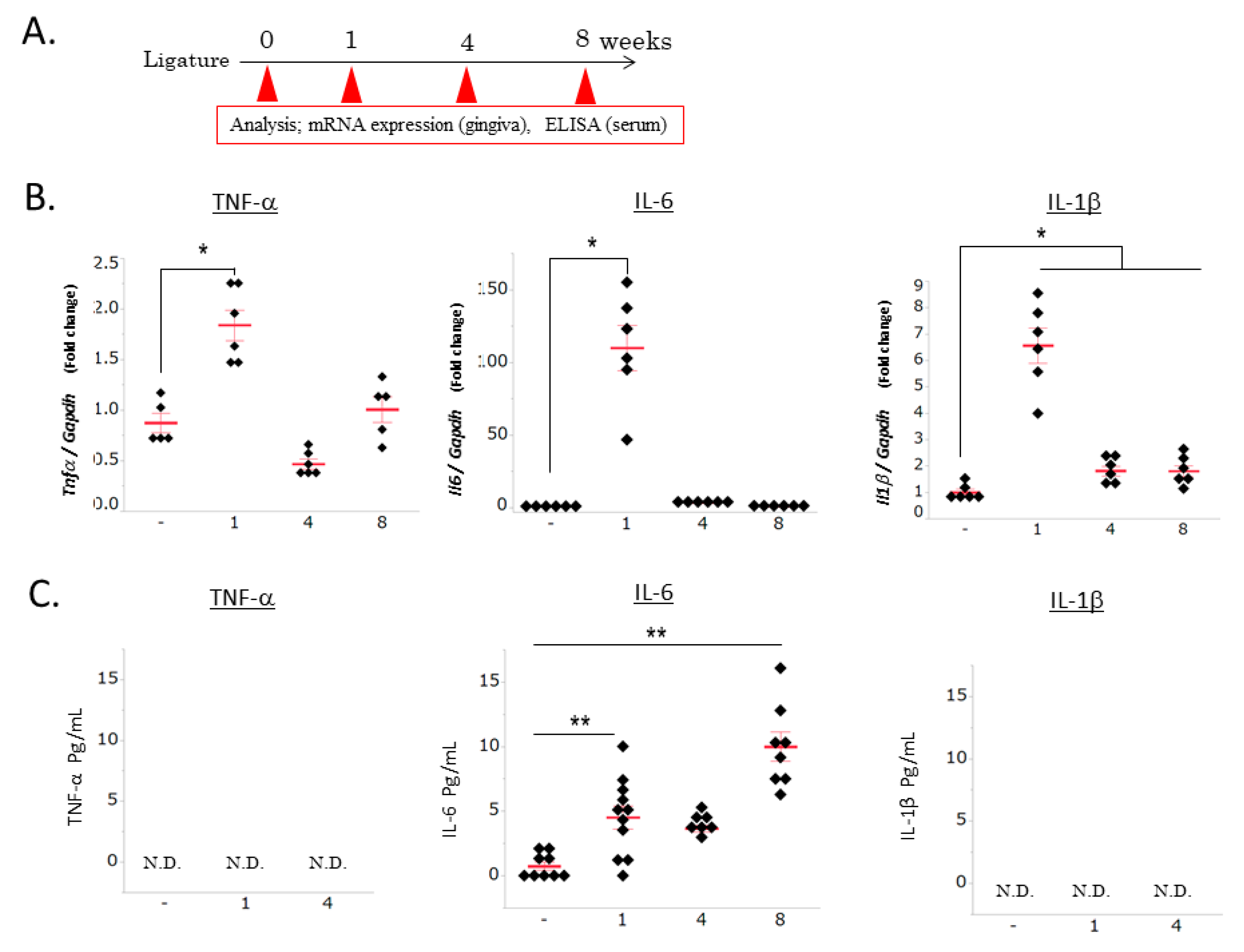
3.1. Ligature-Induced Gingival and Systemic Inflammatory Responses
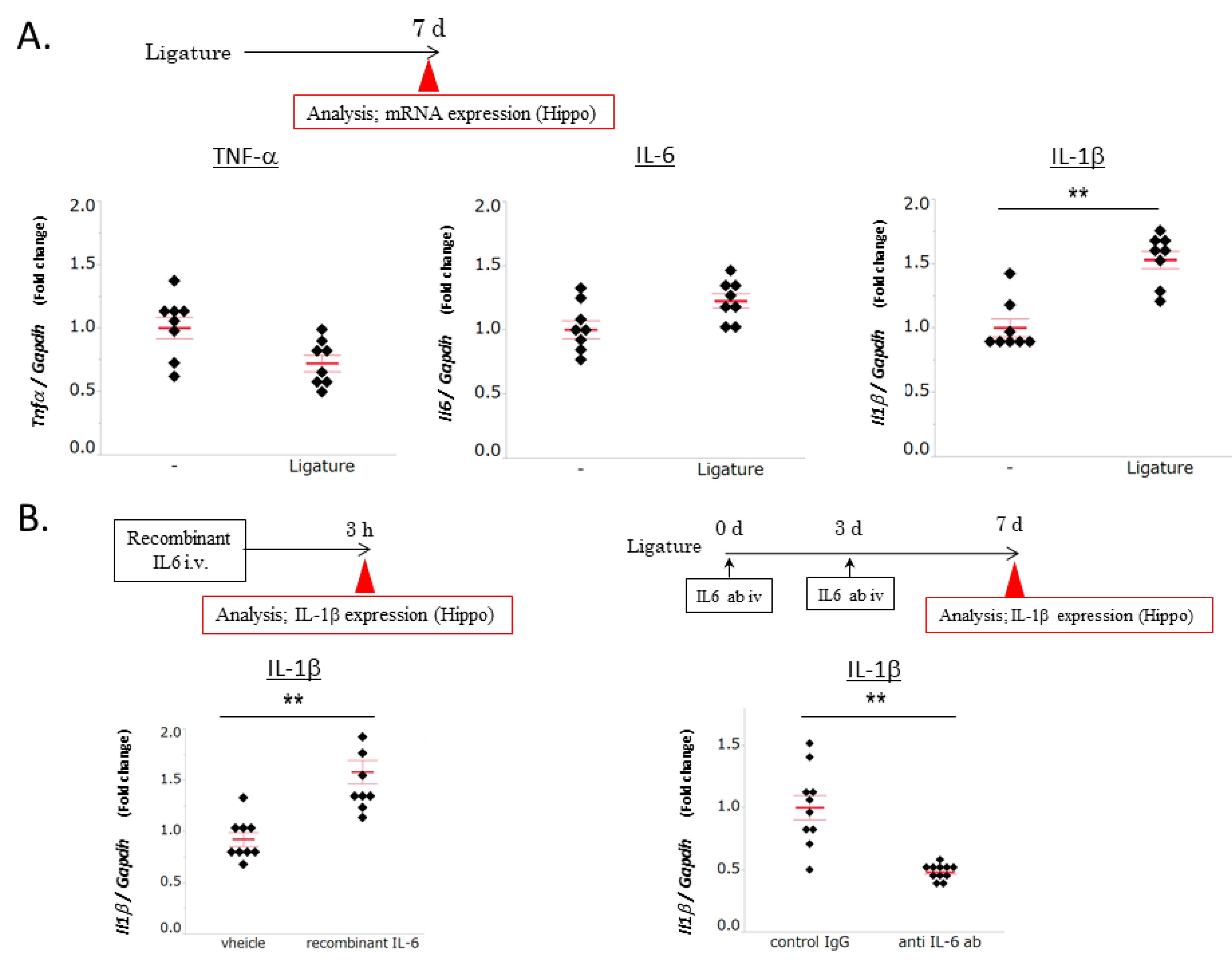
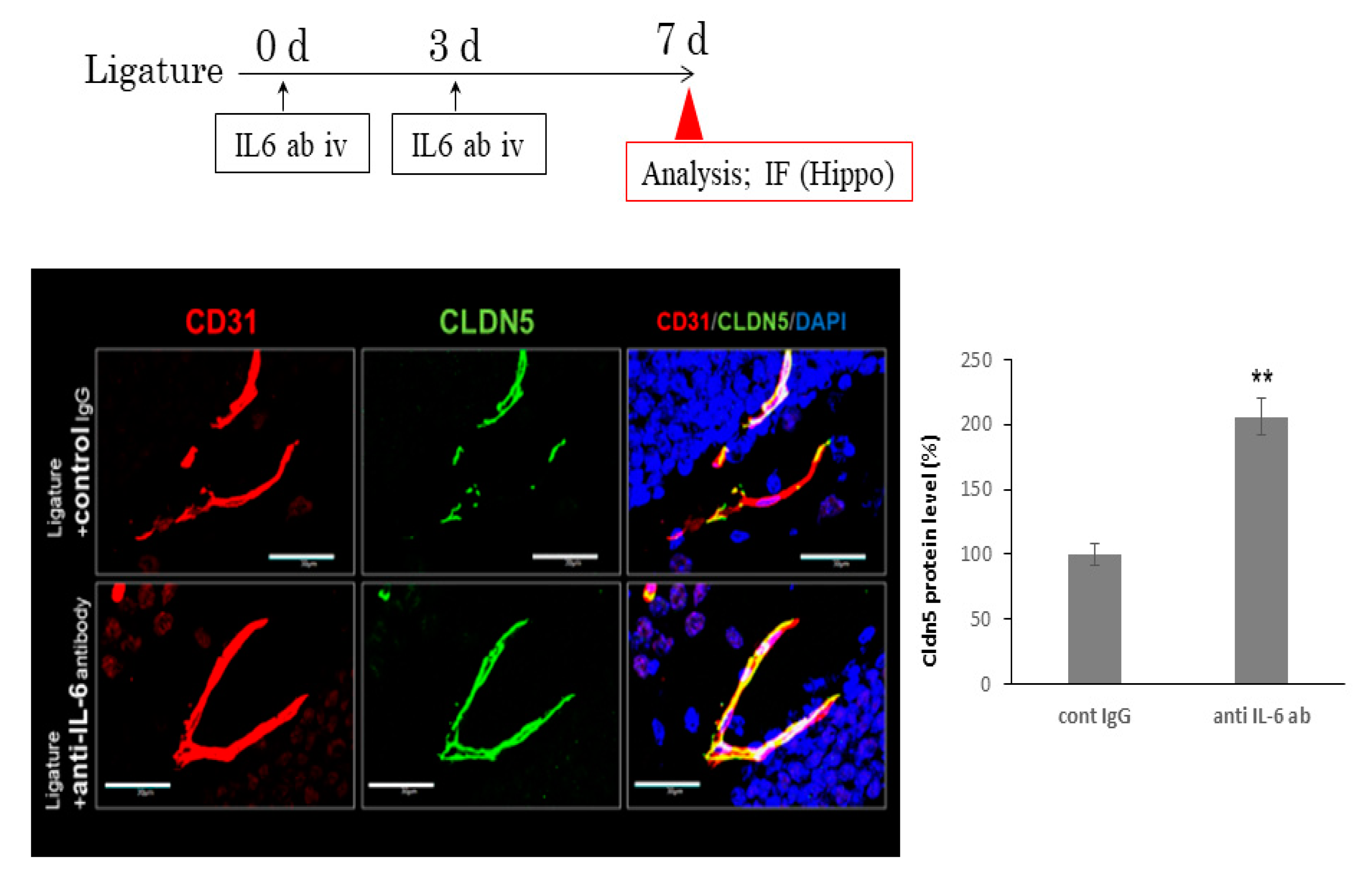
3.2. The Role of IL-6 in PD-Induced Increases in IL-1β mRNA Levels in the Hippocampus
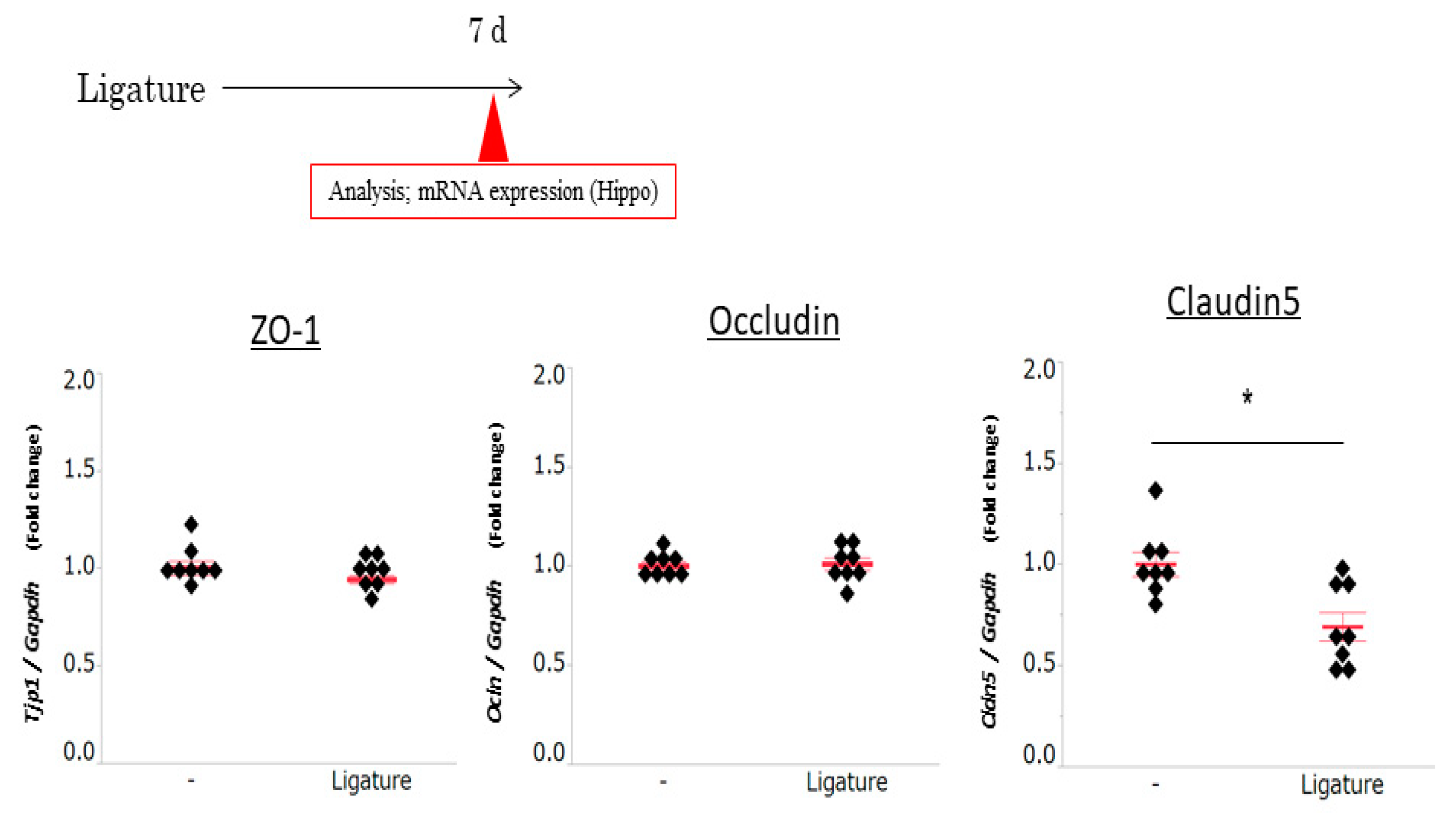
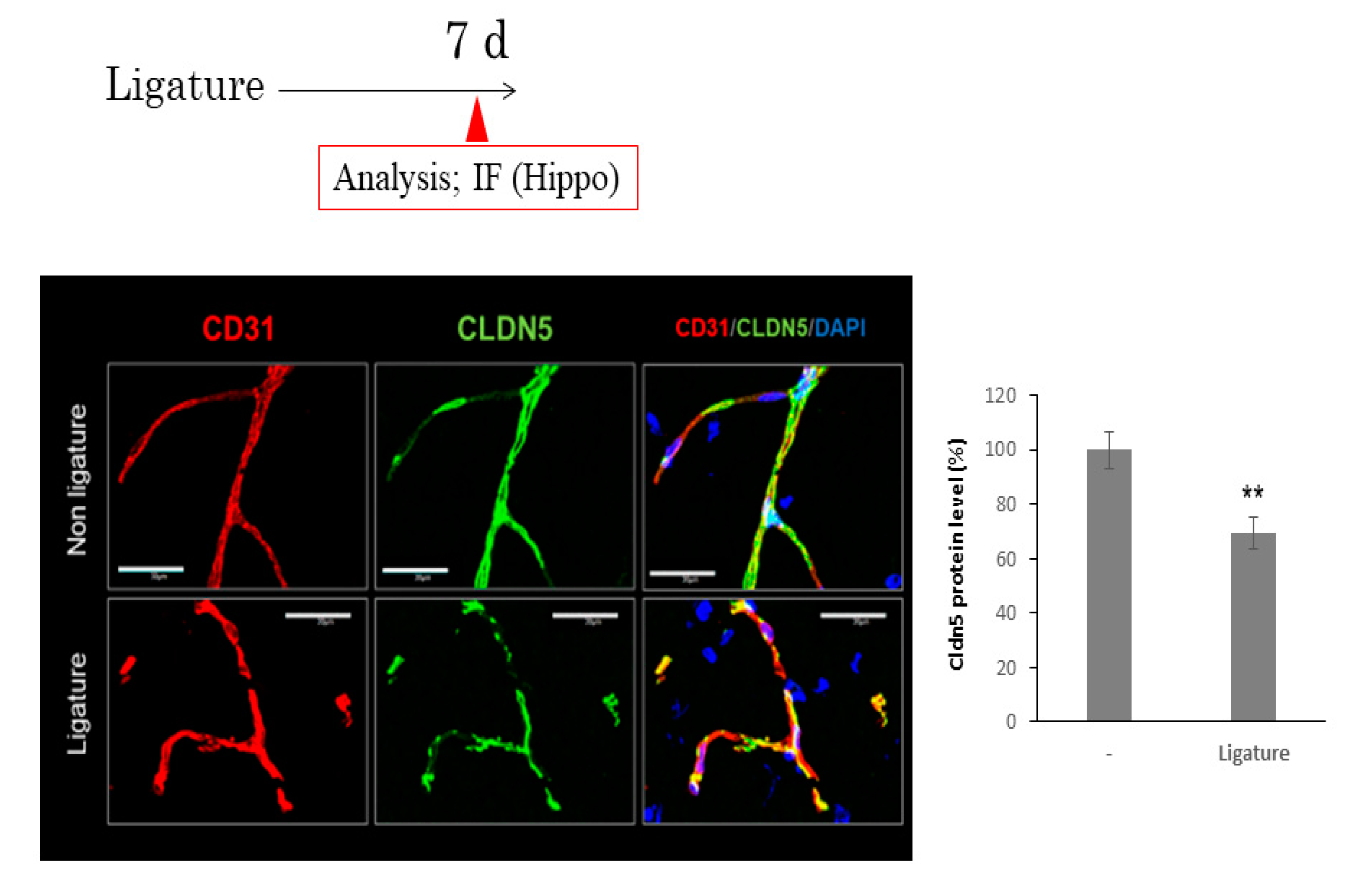
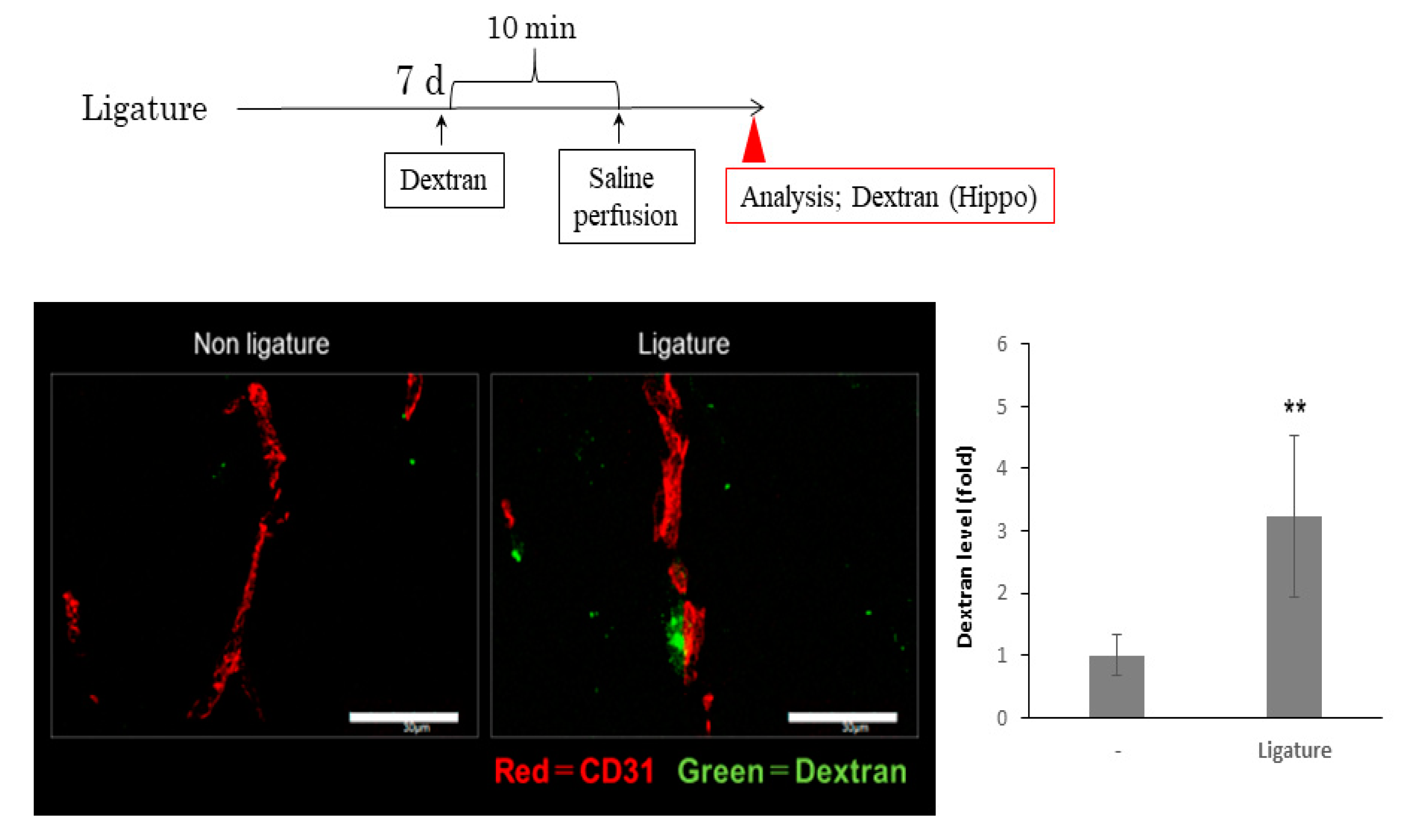
3.3. The Role of Ligature-Induced Periodontitis in BBB Disruption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaur, S.; White, S.; Bartold, P.M. Periodontal disease and rheumatoid arthritis: A systematic review. J. Dent. Res. 2013, 92, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Naka, S.; Nakano, K.; Wada, K.; Endo, H.; Mawatari, H.; Imajo, K.; Nomura, R.; Hokamura, K.; Ono, M.; et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012, 12, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chávarry, N.G.; Vettore, M.V.; Sansone, C.; Sheiham, A. The relationship between diabetes mellitus and destructive periodontal disease: A meta-analysis. Oral Health Prev. Dent. 2009, 7, 107–127. [Google Scholar] [PubMed]
- Humphrey, L.L.; Fu, R.; Buckley, D.I.; Freeman, M.; Helfand, M. Periodontal disease and coronary heart disease incidence: A systematic review and meta-analysis. J. Gen. Intern. Med. 2008, 23, 2079–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, K.; Ohara, T.; Furuta, M.; Takeshita, T.; Shibata, Y.; Hata, J.; Yoshida, D.; Yamashita, Y.; Ninomiya, T. Tooth Loss and Risk of Dementia in the Community: The Hisayama Study. J. Am. Geriatr. Soc. 2017, 65, e95–e100. [Google Scholar] [CrossRef]
- Okamoto, N.; Morikawa, M.; Yanagi, M.; Amano, N.; Tomioka, K.; Hazaki, K.; Harano, A.; Kurumatani, N. Association of Tooth Loss With Development of Swallowing Problems in Community-Dwelling Independent Elderly Population: The Fujiwara-kyo Study. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1548–1554. [Google Scholar] [CrossRef] [Green Version]
- Geng, J.; Wang, L.; Zhang, L.; Qin, C.; Song, Y.; Ma, Y.; Chen, Y.; Chen, S.; Wang, Y.; Zhang, Z.; et al. Blood-Brain Barrier Disruption Induced Cognitive Impairment Is Associated With Increase of Inflammatory Cytokine. Front. Aging Neurosci. 2018, 10, 129. [Google Scholar] [CrossRef]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef] [Green Version]
- Li, C.H.; Shyu, M.K.; Jhan, C.; Cheng, Y.W.; Tsai, C.H.; Liu, C.W.; Lee, C.C.; Chen, R.M.; Kang, J.J. Gold Nanoparticles Increase Endothelial Paracellular Permeability by Altering Components of Endothelial Tight Junctions, and Increase Blood-Brain Barrier Permeability in Mice. Toxicol. Sci. 2015, 148, 192–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegenthaler, J.A.; Sohet, F.; Daneman, R. ‘Sealing off the CNS’: Cellular and molecular regulation of blood-brain barriergenesis. Curr. Opin. Neurobiol. 2013, 23, 1057–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, N.R.; Liddelow, S.A.; Dziegielewska, K.M. Barrier mechanisms in the developing brain. Front. Pharm. 2012, 3, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayloo, S.; Gu, C. Transcytosis at the blood-brain barrier. Curr. Opin. Neurobiol. 2019, 57, 32–38. [Google Scholar] [CrossRef]
- Terrando, N.; Brzezinski, M.; Degos, V.; Eriksson, L.I.; Kramer, J.H.; Leung, J.M.; Miller, B.L.; Seeley, W.W.; Vacas, S.; Weiner, M.W.; et al. Perioperative cognitive decline in the aging population. Mayo Clin. Proc. 2011, 86, 885–893. [Google Scholar] [CrossRef] [Green Version]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Doubal, F.; Armitage, P.; Chappell, F.; Carpenter, T.; Muñoz Maniega, S.; Farrall, A.; Sudlow, C.; Dennis, M.; Dhillon, B. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann. Neurol. 2009, 65, 194–202. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Sandercock, P.A.; Dennis, M.S.; Starr, J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003, 34, 806–812. [Google Scholar] [CrossRef]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costea, L.; Mészáros, Á.; Bauer, H.; Bauer, H.C.; Traweger, A.; Wilhelm, I.; Farkas, A.E.; Krizbai, I.A. The Blood-Brain Barrier and Its Intercellular Junctions in Age-Related Brain Disorders. Int. J. Mol. Sci. 2019, 20, 5472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girard, T.D.; Jackson, J.C.; Pandharipande, P.P.; Pun, B.T.; Thompson, J.L.; Shintani, A.K.; Gordon, S.M.; Canonico, A.E.; Dittus, R.S.; Bernard, G.R.; et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit. Care Med. 2010, 38, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Guenther, U.; Popp, J.; Koecher, L.; Muders, T.; Wrigge, H.; Ely, E.W.; Putensen, C. Validity and reliability of the CAM-ICU Flowsheet to diagnose delirium in surgical ICU patients. J. Crit. Care 2010, 25, 144–151. [Google Scholar] [CrossRef]
- Ely, E.W.; Shintani, A.; Truman, B.; Speroff, T.; Gordon, S.M.; Harrell, F.E.; Inouye, S.K.; Bernard, G.R.; Dittus, R.S. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004, 291, 1753–1762. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, S.; Li, X.; Liu, E.; Wang, X.; Zhou, Q.; Ye, J.; Wang, J.Z. Peripheral inflammation promotes brain tau transmission via disrupting blood-brain barrier. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [Green Version]
- Begley, D.J.; Brightman, M.W. Structural and functional aspects of the blood-brain barrier. Prog. Drug Res. 2003, 61, 39–78. [Google Scholar] [CrossRef]
- Wolburg, H.; Lippoldt, A. Tight junctions of the blood-brain barrier: Development, composition and regulation. Vasc. Pharm. 2002, 38, 323–337. [Google Scholar] [CrossRef]
- Wolburg, H.; Noell, S.; Mack, A.; Wolburg-Buchholz, K.; Fallier-Becker, P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009, 335, 75–96. [Google Scholar] [CrossRef]
- Ilievski, V.; Zuchowska, P.K.; Green, S.J.; Toth, P.T.; Ragozzino, M.E.; Le, K.; Aljewari, H.W.; O’Brien-Simpson, N.M.; Reynolds, E.C.; Watanabe, K. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS ONE 2018, 13, e0204941. [Google Scholar] [CrossRef] [Green Version]
- Ishida, N.; Ishihara, Y.; Ishida, K.; Tada, H.; Funaki-Kato, Y.; Hagiwara, M.; Ferdous, T.; Abdullah, M.; Mitani, A.; Michikawa, M.; et al. Periodontitis induced by bacterial infection exacerbates features of Alzheimer’s disease in transgenic mice. NPJ Aging Mech. Dis. 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, Y.; Montgomery, J.M.; Cheyne, J.E. Hippocampal Deficits in Amyloid-β-Related Rodent Models of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 266. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 2013, 394, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okanobu, A.; Matsuda, S.; Kajiya, M.; Fujita, T.; Kittaka, M.; Shiba, H.; Kurihara, H. A novel gingival overgrowth mouse model induced by the combination of CsA and ligature-induced inflammation. J. Immunol. Methods 2017, 445, 31–36. [Google Scholar] [CrossRef]
- Yamawaki, Y.; Yoshioka, N.; Nozaki, K.; Ito, H.; Oda, K.; Harada, K.; Shirawachi, S.; Asano, S.; Aizawa, H.; Yamawaki, S.; et al. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res. 2018, 1680, 13–38. [Google Scholar] [CrossRef]
- Suh, J.S.; Lee, S.H.; Fouladian, Z.; Lee, J.Y.; Kim, T.; Kang, M.K.; Lusis, A.J.; Boström, K.I.; Kim, R.H.; Park, N.H. Rosuvastatin Prevents the Exacerbation of Atherosclerosis in Ligature-Induced Periodontal Disease Mouse Model. Sci. Rep. 2020, 10, 6383. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, Y.; Kato, T.; Takahashi, N.; Nakajima, M.; Arimatsu, K.; Minagawa, T.; Sato, K.; Ohno, H.; Yamazaki, K. Ligature-induced periodontitis in mice induces elevated levels of circulating interleukin-6 but shows only weak effects on adipose and liver tissues. J. Periodontal. Res. 2016, 51, 639–646. [Google Scholar] [CrossRef]
- Schuett, H.; Luchtefeld, M.; Grothusen, C.; Grote, K.; Schieffer, B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb. Haemost. 2009, 102, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Marcaccini, A.M.; Meschiari, C.A.; Sorgi, C.A.; Saraiva, M.C.; de Souza, A.M.; Faccioli, L.H.; Tanus-Santos, J.E.; Novaes, A.B.; Gerlach, R.F. Circulating interleukin-6 and high-sensitivity C-reactive protein decrease after periodontal therapy in otherwise healthy subjects. J. Periodontol. 2009, 80, 594–602. [Google Scholar] [CrossRef]
- Fritsch, W.C.; Maharry, R.R.; Clabaugh, W.A. Delayed purpuric reaction following superficial dermabrasion. Arch. Derm. 1976, 112, 83–85. [Google Scholar] [CrossRef]
- Machado, W.M.; Prestes, A.P.; Costa, T.P.; Mendes, R.T.; Olchanheski, L.R.; Sordi, R.; Otuki, M.F.; Fávero, G.M.; Vellosa, J.C.; Santos, F.A.; et al. The effect of simvastatin on systemic inflammation and endothelial dysfunction induced by periodontitis. J. Periodontal Res. 2014, 49, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Guo, J.; You, X.; Xia, W.; Yan, F. Expressions of interleukin-1β and interleukin-6 within aortas and uteri of rats with various severities of ligature-induced periodontitis. Inflammation 2011, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimers Dis 2013, 36, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Sparks Stein, P.; Steffen, M.J.; Smith, C.; Jicha, G.; Ebersole, J.L.; Abner, E.; Dawson, D. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement. 2012, 8, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.C.; Lin, C.C.; Chen, C.C.; Tzeng, N.S.; Liu, Y.P. Effects of Oxytocin on Fear Memory and Neuroinflammation in a Rodent Model of Posttraumatic Stress Disorder. Int. J. Mol. Sci. 2018, 19, 3848. [Google Scholar] [CrossRef] [Green Version]
- Shaftel, S.S.; Griffin, W.S.; O’Banion, M.K. The role of interleukin-1 in neuroinflammation and Alzheimer disease: An evolving perspective. J. Neuroinflamm. 2008, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Allan, S.M.; Tyrrell, P.J.; Rothwell, N.J. Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 2005, 5, 629–640. [Google Scholar] [CrossRef]
- Patel, H.C.; Boutin, H.; Allan, S.M. Interleukin-1 in the brain: Mechanisms of action in acute neurodegeneration. Ann. N. Y. Acad. Sci. 2003, 992, 39–47. [Google Scholar] [CrossRef]
- Griffin, W.S.; Sheng, J.G.; Gentleman, S.M.; Graham, D.I.; Mrak, R.E.; Roberts, G.W. Microglial interleukin-1 alpha expression in human head injury: Correlations with neuronal and neuritic beta-amyloid precursor protein expression. Neurosci. Lett. 1994, 176, 133–136. [Google Scholar] [CrossRef]
- Basu, A.; Krady, J.K.; Levison, S.W. Interleukin-1: A master regulator of neuroinflammation. J. Neurosci. Res. 2004, 78, 151–156. [Google Scholar] [CrossRef]
- Vela, J.M.; Molina-Holgado, E.; Arévalo-Martín, A.; Almazán, G.; Guaza, C. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol. Cell Neurosci. 2002, 20, 489–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, C.C.; Hu, S.; Sheng, W.S.; Bu, D.; Bukrinsky, M.I.; Peterson, P.K. Cytokine-stimulated astrocytes damage human neurons via a nitric oxide mechanism. Glia 1996, 16, 276–284. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sadowska, G.B.; Chen, X.; Park, S.Y.; Kim, J.E.; Bodge, C.A.; Cummings, E.; Lim, Y.P.; Makeyev, O.; Besio, W.G.; et al. Anti-IL-6 neutralizing antibody modulates blood-brain barrier function in the ovine fetus. FASEB J. 2015, 29, 1739–1753. [Google Scholar] [CrossRef] [Green Version]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef]
- Rochfort, K.D.; Collins, L.E.; McLoughlin, A.; Cummins, P.M. Tumour necrosis factor-α-mediated disruption of cerebrovascular endothelial barrier integrity in vitro involves the production of proinflammatory interleukin-6. J. Neurochem. 2016, 136, 564–572. [Google Scholar] [CrossRef]
- Rochfort, K.D.; Collins, L.E.; Murphy, R.P.; Cummins, P.M. Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: Consequences for interendothelial adherens and tight junctions. PLoS ONE 2014, 9, e101815. [Google Scholar] [CrossRef] [Green Version]
- Camire, R.B.; Beaulac, H.J.; Willis, C.L. Transitory loss of glia and the subsequent modulation in inflammatory cytokines/chemokines regulate paracellular claudin-5 expression in endothelial cells. J. Neuroimmunol. 2015, 284, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Keaney, J.; Walsh, D.M.; O’Malley, T.; Hudson, N.; Crosbie, D.E.; Loftus, T.; Sheehan, F.; McDaid, J.; Humphries, M.M.; Callanan, J.J.; et al. Autoregulated paracellular clearance of amyloid-β across the blood-brain barrier. Sci. Adv. 2015, 1, e1500472. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Gu, C.; Mandeville, E.T.; Dong, Y.; Esposito, E.; Zhang, Y.; Yang, G.; Shen, Y.; Fu, X.; Lo, E.H.; et al. Anesthesia and Surgery Impair Blood-Brain Barrier and Cognitive Function in Mice. Front. Immunol 2017, 8, 902. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furutama, D.; Matsuda, S.; Yamawaki, Y.; Hatano, S.; Okanobu, A.; Memida, T.; Oue, H.; Fujita, T.; Ouhara, K.; Kajiya, M.; et al. IL-6 Induced by Periodontal Inflammation Causes Neuroinflammation and Disrupts the Blood–Brain Barrier. Brain Sci. 2020, 10, 679. https://doi.org/10.3390/brainsci10100679
Furutama D, Matsuda S, Yamawaki Y, Hatano S, Okanobu A, Memida T, Oue H, Fujita T, Ouhara K, Kajiya M, et al. IL-6 Induced by Periodontal Inflammation Causes Neuroinflammation and Disrupts the Blood–Brain Barrier. Brain Sciences. 2020; 10(10):679. https://doi.org/10.3390/brainsci10100679
Chicago/Turabian StyleFurutama, Daisuke, Shinji Matsuda, Yosuke Yamawaki, Saki Hatano, Ai Okanobu, Takumi Memida, Hiroshi Oue, Tsuyoshi Fujita, Kazuhisa Ouhara, Mikihito Kajiya, and et al. 2020. "IL-6 Induced by Periodontal Inflammation Causes Neuroinflammation and Disrupts the Blood–Brain Barrier" Brain Sciences 10, no. 10: 679. https://doi.org/10.3390/brainsci10100679
APA StyleFurutama, D., Matsuda, S., Yamawaki, Y., Hatano, S., Okanobu, A., Memida, T., Oue, H., Fujita, T., Ouhara, K., Kajiya, M., Mizuno, N., Kanematsu, T., Tsuga, K., & Kurihara, H. (2020). IL-6 Induced by Periodontal Inflammation Causes Neuroinflammation and Disrupts the Blood–Brain Barrier. Brain Sciences, 10(10), 679. https://doi.org/10.3390/brainsci10100679