Brain Response Induced with Paired Associative Stimulation Is Related to Repetition Suppression of Motor Evoked Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
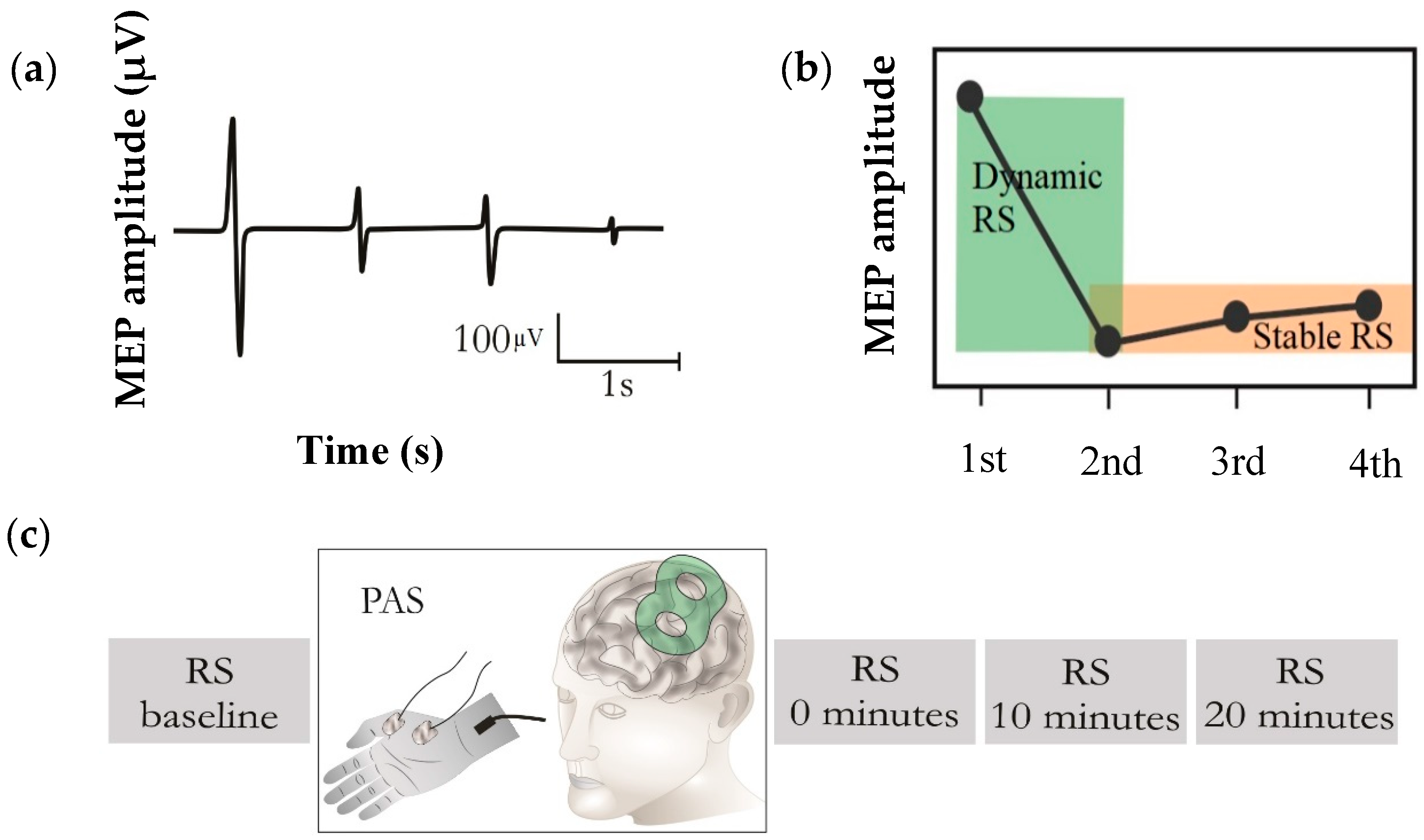
2.2. Transcranial Magnetic Stimulation (TMS)
2.3. Paired Associative Stimulation (PAS)
2.4. Statistical Analysis
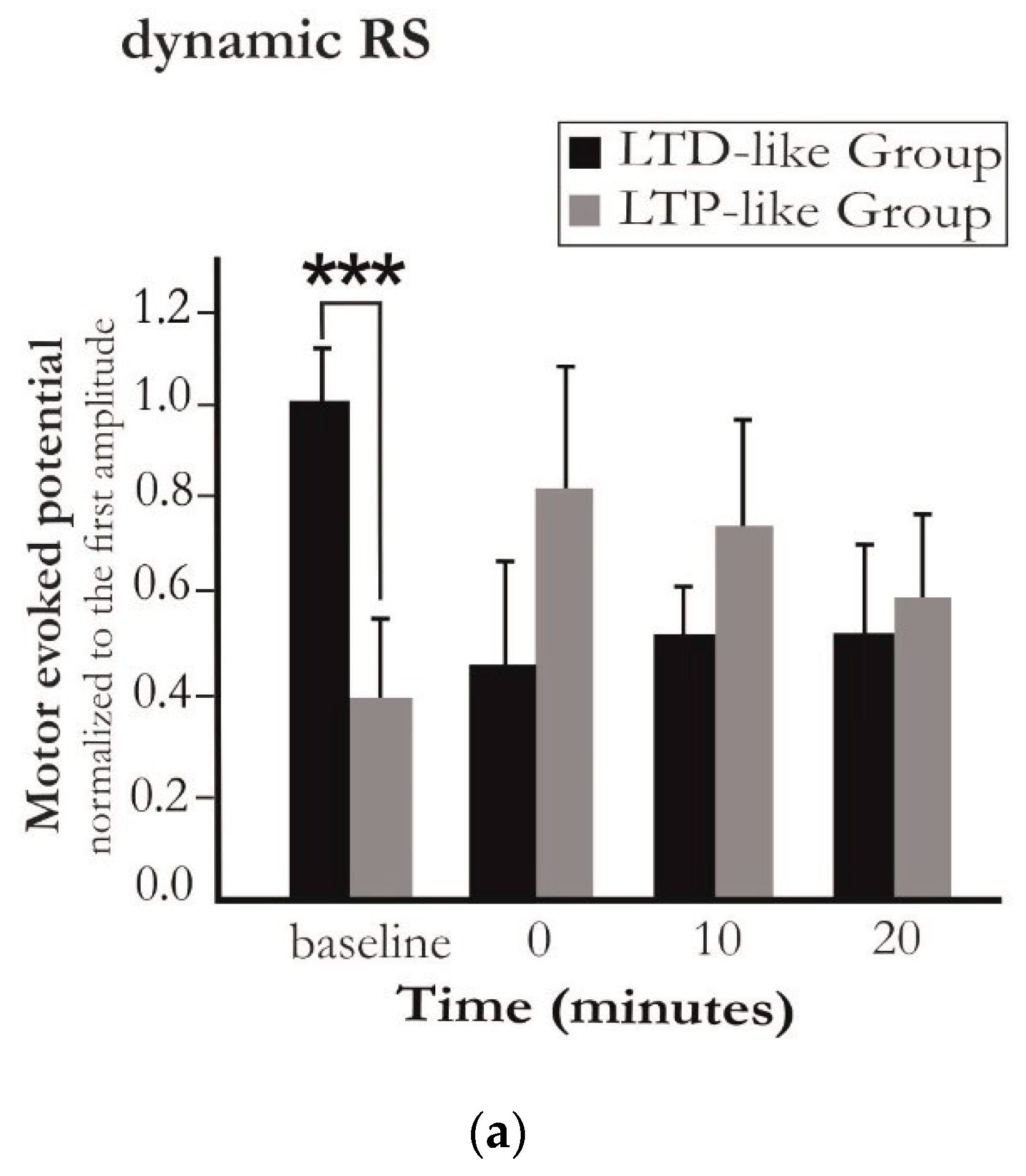
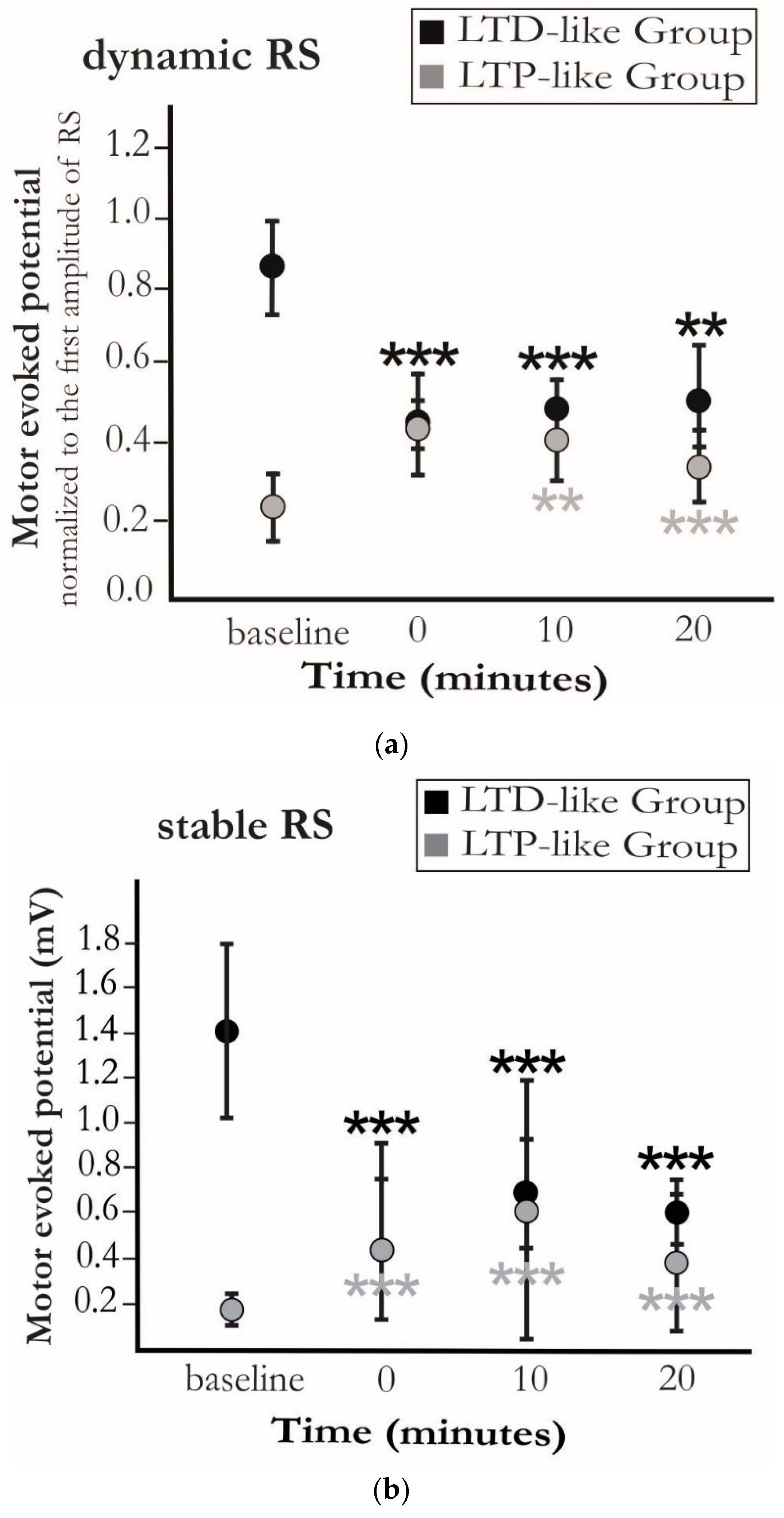
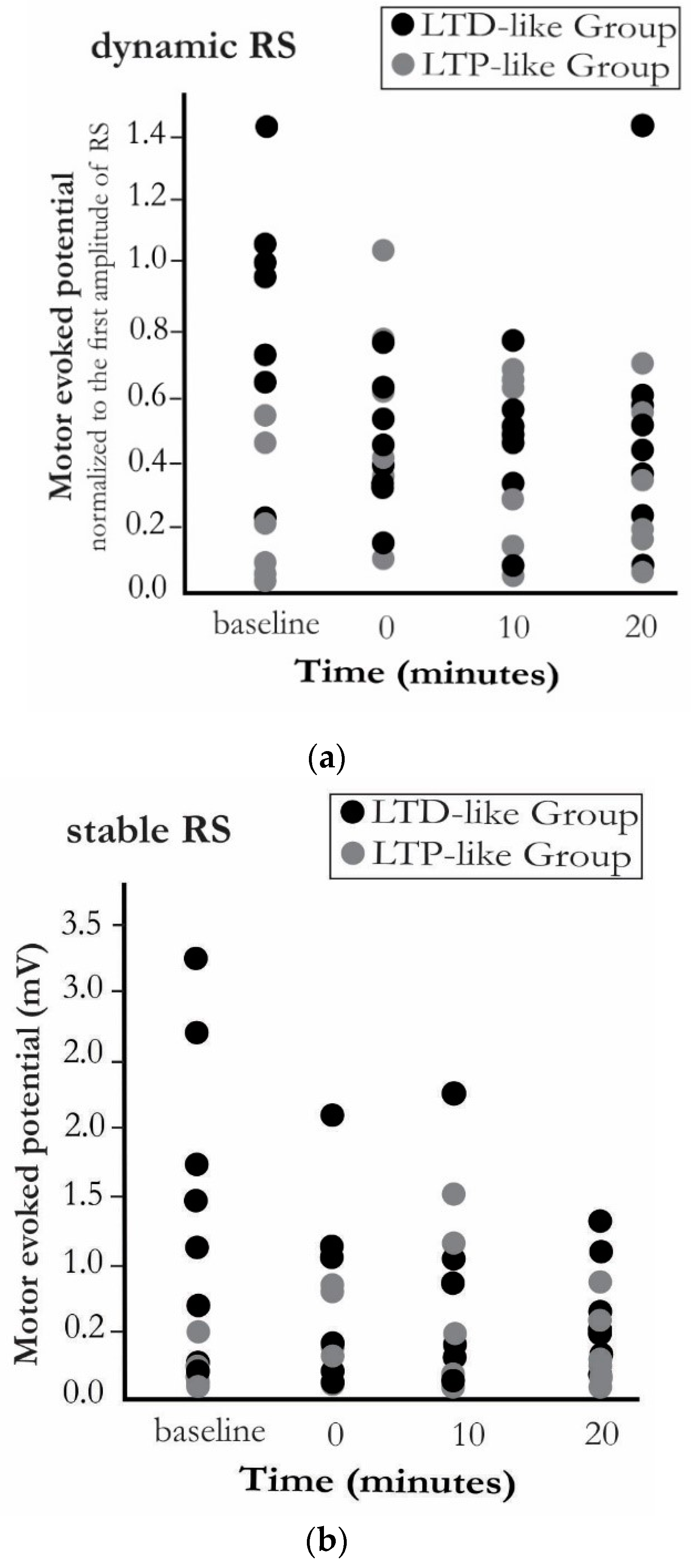
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A


References
- Moruzzi, G.; Magoun, H.W. Brain stem reticular formation and activation of the EEG. Electroencephalogr. Clin. Neurophysiol. 1949, 1, 455–473. [Google Scholar] [CrossRef]
- Grill-Spector, K.; Henson, R.; Martin, A. Repetition and the brain: Neural models of stimulus-specific effects. Trends Cogn. Sci. 2006, 10, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Desimone, R. Neural mechanisms for visual memory and their role in attention. Proc. Natl. Acad. Sci. USA 1996, 93, 13494–13499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, E.K.; Li, L.; Desimone, R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J. Neurosci. 1993, 13, 1460–1478. [Google Scholar] [CrossRef] [Green Version]
- Krekelberg, B.; Boynton, G.M.; van Wezel, R.J.A. Adaptation: From single cells to BOLD signals. Trends Neurosci. 2006, 29, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Picton, T. The N1 Wave of the Human Electric and Magnetic Response to Sound: A Review and an Analysis of the Component Structure. Psychophysiology 1987, 24, 375–425. [Google Scholar] [CrossRef]
- Löfberg, O.; Julkunen, P.; Tiihonen, P.; Pääkkönen, A.; Karhu, J. Repetition suppression in the cortical motor and auditory systems resemble each other—A combined TMS and evoked potential study. Neuroscience 2013, 243, 40–45. [Google Scholar] [CrossRef]
- Löfberg, O.; Julkunen, P.; Pääkkönen, A.; Karhu, J. The auditory-evoked arousal modulates motor cortex excitability. Neuroscience 2014, 274, 403–408. [Google Scholar] [CrossRef]
- Friston, K.; Kilner, J.; Harrison, L. A free energy principle for the brain. J. Physiol. Paris 2006, 100, 70–87. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Miller, E.K.; Desimone, R. The representation of stimulus familiarity in anterior inferior temporal cortex. J. Neurophysiol. 1993, 69, 1918–1929. [Google Scholar] [CrossRef]
- Sobotka, S.; Ringo, J.L. Mnemonic responses of single units recorded from monkey inferotemporal cortex, accessed via transcommissural versus direct pathways: A dissociation between unit activity and behavior. J. Neurosci. 1996, 16, 4222–4230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friston, K. A theory of cortical responses. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2005, 360, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Julkunen, P.; Löfberg, O.; Kallioniemi, E.; Hyppönen, J.; Kälviäinen, R.; Mervaala, E. Abnormal motor cortical adaptation to external stimulus in Unverricht-Lundborg disease (progressive myoclonus type 1, EPM1). J. Neurophysiol. 2018, 120, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Amedi, A.; Fregni, F.; Merabet, L.B. The plastic human brain cortex. Annu. Rev. Neurosci. 2005, 28, 377–401. [Google Scholar] [CrossRef] [Green Version]
- Rioult-Pedotti, M.-S.; Friedman, D.; Hess, G.; Donoghue, J.P. Strengthening of horizontal cortical connections following skill learning. Nat. Neurosci. 1998, 1, 230–234. [Google Scholar] [CrossRef]
- Stefan, K.; Wycislo, M.; Gentner, R.; Schramm, A.; Naumann, M.; Reiners, K.; Classen, J. Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training. Cereb. Cortex 2006, 16, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Rossini, P.M.; Dal Forno, G. Neuronal post-stroke plasticity in the adult. Restor. Neurol. Neurosci. 2004, 22, 193–206. [Google Scholar]
- Daskalakis, Z.J.; Christensen, B.K.; Fitzgerald, P.B.; Chen, R. Dysfunctional neural plasticity in patients with Schizophrenia. Arch. Gen. Psychiatry 2008, 65, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Player, M.J.; Taylor, J.L.; Weickert, C.S.; Alonzo, A.; Sachdev, P.; Martin, D.; Mitchell, P.B.; Loo, C.K. Neuroplasticity in depressed individuals compared with healthy controls. Neuropsychopharmacology 2013, 38, 2101–2108. [Google Scholar] [CrossRef] [Green Version]
- Flor, H. Cortical reorganisation and chronic pain: Implications for rehabilitation. J. Rehabil. Med. 2003, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Voronin, L.L. Long-term potentiation in the hippocampus. Neuroscience 1983, 10, 1051–1069. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Tarazona, F.; Keenan, J.; Tormos, J.M.; Hamilton, R.; Catala, M.D. Transcranial magnetic stimulation and neuroplasticity. Neuropsychologia 1999, 37, 207–217. [Google Scholar] [CrossRef]
- Chen, R.; Cohen, L.G.; Hallett, M. Nervous system reorganization following injury. Neuroscience 2002, 111, 761–773. [Google Scholar] [CrossRef]
- Stefan, K.; Kunesch, E.; Cohen, L.G.; Benecke, R.; Classen, J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 2000, 123 Pt 3, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Tolmacheva, A.; Savolainen, S.; Kirveskari, E.; Brandstack, N.; Mäkelä, J.P.; Shulga, A. Paired associative stimulation improves hand function after non-traumatic spinal cord injury: A case series. Clin. Neurophysiol. Pract. 2019, 4, 178–183. [Google Scholar] [CrossRef]
- Wolters, A.; Sandbrink, F.; Schlottmann, A.; Kunesch, E.; Stefan, K.; Cohen, L.G.; Benecke, R.; Classen, J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J. Neurophysiol. 2003, 89, 2339–2345. [Google Scholar] [CrossRef]
- Stefan, K.; Kunesch, E.; Benecke, R.; Cohen, L.G.; Classen, J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J. Physiol. 2002, 543, 699–708. [Google Scholar] [CrossRef]
- Müller-Dahlhaus, F.; Ziemann, U.; Classen, J. Plasticity resembling spike-timing dependent synaptic plasticity: The evidence in human cortex. Front. Synaptic Neurosci. 2010, 2, 34. [Google Scholar] [CrossRef] [Green Version]
- Awiszus, F.; Borckardt, J. TMS Motor Threshold Assessment Tool 2.0. 2012. Available online: http://clinicalresearcher.org/software.htm (accessed on 19 October 2012).
- Pitkänen, M.; Kallioniemi, E.; Julkunen, P. Effect of inter-train interval on the induction of repetition suppression of motor-evoked potentials using transcranial magnetic stimulation. PLoS ONE 2017, 12, e0181663. [Google Scholar] [CrossRef] [Green Version]
- Hamada, M.; Strigaro, G.; Murase, N.; Sadnicka, A.; Galea, J.M.; Edwards, M.J.; Rothwell, J.C. Cerebellar modulation of human associative plasticity. J. Physiol. 2012, 590, 2365–2374. [Google Scholar] [CrossRef]
- Friston, K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Ranganath, C.; Rainer, G. Neural mechanisms for detecting and remembering novel events. Nat. Rev. Neurosci. 2003, 4, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Goldman, M.S. Balanced cortical microcircuitry for maintaining information in working memory. Nat. Neurosci. 2013, 16, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J.; Loughry, B.; O’reilly, R.C. Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cogn. Affect. Behav. Neurosci. 2001, 1, 137–160. [Google Scholar] [CrossRef] [Green Version]
- Koskenkorva, P.; Khyuppenen, J.; Niskanen, E.; Könönen, M.; Bendel, P.; Mervaala, E.; Lehesjoki, A.E.; Kälviäinen, R.; Vanninen, R. Motor cortex and thalamic atrophy in Unverricht-Lundborg disease: Voxel-based morphometric study. Neurology 2009, 73, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Turrigiano, G.G.; Leslie, K.R.; Desai, N.S.; Rutherford, L.C.; Nelson, S.B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 1998, 391, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Bienenstock, E.L.; Cooper, L.N.; Munro, P.W. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J. Neurosci. 1982, 2, 32–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Alonso, V.; Cheeran, B.; Río-Rodríguez, D.; Fernández-del-Olmo, M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014, 7, 372–380. [Google Scholar] [CrossRef]
- Campana, M.; Papazova, I.; Pross, B.; Hasan, A.; Strube, W. Motor-cortex excitability and response variability following paired-associative stimulation: A proof-of-concept study comparing individualized and fixed inter-stimulus intervals. Exp. Brain Res. 2019, 237, 1727–1734. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kariminezhad, S.; Karhu, J.; Säisänen, L.; Reijonen, J.; Könönen, M.; Julkunen, P. Brain Response Induced with Paired Associative Stimulation Is Related to Repetition Suppression of Motor Evoked Potential. Brain Sci. 2020, 10, 674. https://doi.org/10.3390/brainsci10100674
Kariminezhad S, Karhu J, Säisänen L, Reijonen J, Könönen M, Julkunen P. Brain Response Induced with Paired Associative Stimulation Is Related to Repetition Suppression of Motor Evoked Potential. Brain Sciences. 2020; 10(10):674. https://doi.org/10.3390/brainsci10100674
Chicago/Turabian StyleKariminezhad, Shohreh, Jari Karhu, Laura Säisänen, Jusa Reijonen, Mervi Könönen, and Petro Julkunen. 2020. "Brain Response Induced with Paired Associative Stimulation Is Related to Repetition Suppression of Motor Evoked Potential" Brain Sciences 10, no. 10: 674. https://doi.org/10.3390/brainsci10100674
APA StyleKariminezhad, S., Karhu, J., Säisänen, L., Reijonen, J., Könönen, M., & Julkunen, P. (2020). Brain Response Induced with Paired Associative Stimulation Is Related to Repetition Suppression of Motor Evoked Potential. Brain Sciences, 10(10), 674. https://doi.org/10.3390/brainsci10100674






