Mental Health and Family Functioning in Patients and Their Family Members after Traumatic Brain Injury: A Cross-Sectional Study
Abstract
:1. Introduction
- (a)
- Describe and compare aspects of mental health and family functioning in home-dwelling patients with TBI and their family members at 6–18 months post-injury (i.e., the study inclusion time)
- (b)
- Explore individual- and family functioning-related factors that are associated with mental health.
2. Materials and Methods
2.1. Study Design and Settings
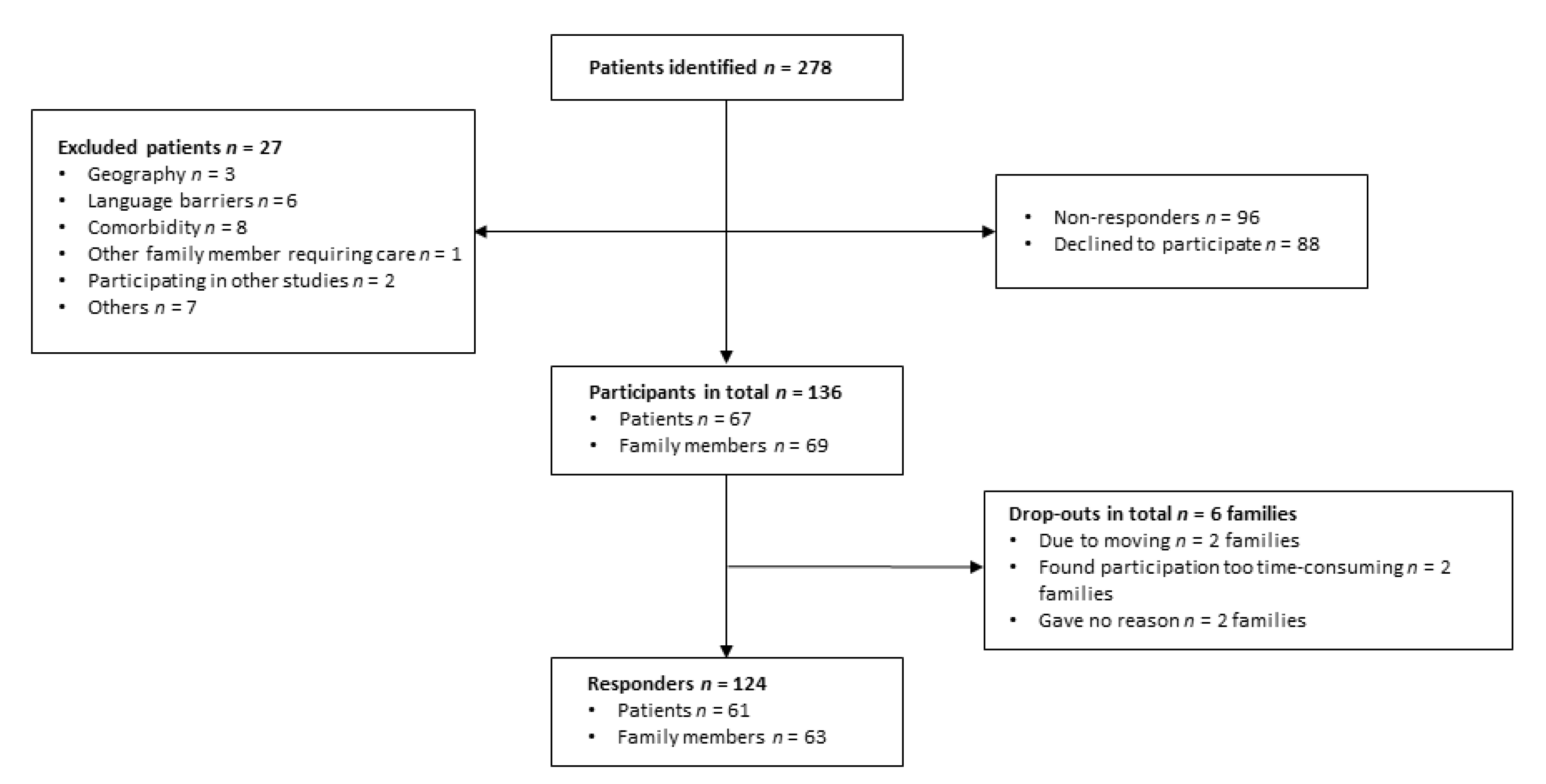
2.2. Participants
2.3. Measures
2.4. Data Sources
2.5. Study Sample Size
2.6. Data Analysis and Statistics
3. Results
Injury Characteristics
4. Discussion
4.1. Post-Injury Functioning
4.2. Family Functioning
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roozenbeek, B.; Maas, A.I.R.; Menon, D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghe, S.; Defloor, T.; Grypdonck, M. Stress and coping among families of patients with traumatic brain injury: A review of the literature. J. Clin. Nurs. 2005, 14, 1004–1012. [Google Scholar] [CrossRef]
- Stocchetti, N.; Zanier, E.R. Chronic impact of traumatic brain injury on outcome and quality of life: A narrative review. Crit. Care 2016, 20, 148. [Google Scholar] [CrossRef] [Green Version]
- Sigurdardottir, S.; Andelic, N.; Røe, C.; Jerstad, T.; Schanke, A.-K. Post-concussion symptoms after traumatic brain injury at 3 and 12 months post-injury: A prospective study. Brain Inj. 2009, 23, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Scholten, A.C.; Haagsma, J.; Andriessen, T.; Vos, P.; Steyerberg, E.; Van Beeck, E.; Polinder, S. Health-related quality of life after mild, moderate and severe traumatic brain injury: Patterns and predictors of suboptimal functioning during the first year after injury. Injury 2015, 46, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Polinder, S.; Haagsma, J.A.; Van Klaveren, D.; Steyerberg, E.W.; Van Beeck, E.F. Health-related quality of life after TBI: A systematic review of study design, instruments, measurement properties, and outcome. Popul. Health Metrics 2015, 13, 4. [Google Scholar] [CrossRef] [Green Version]
- Kreitzer, N.; Kurowski, B.G.; Bakas, T. Systematic Review of Caregiver and Dyad Interventions after Adult Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2018, 99, 2342–2354. [Google Scholar] [CrossRef]
- Baker, A.; Barker, S.; Sampson, A.; Martin, C. Caregiver outcomes and interventions: A systematic scoping review of the traumatic brain injury and spinal cord injury literature. Clin. Rehabil. 2016, 31, 45–60. [Google Scholar] [CrossRef]
- Manskow, U.S.; Friborg, O.; Røe, C.; Braine, M.; Damsgård, E.; Anke, A. Patterns of change and stability in caregiver burden and life satisfaction from 1 to 2 years after severe traumatic brain injury: A Norwegian longitudinal study. Neurorehabilitation 2017, 40, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Bayen, E.; Jourdan, C.; Ghout, I.; Darnoux, E.; Azerad, S.; Vallat-Azouvi, C.; Weiss, J.-J.; Aegerter, P.; Pradat-Diehl, P.; Joël, M.-E.; et al. Objective and Subjective Burden of Informal Caregivers 4 Years After a Severe Traumatic Brain Injury. J. Head Trauma Rehabil. 2016, 31, E59–E67. [Google Scholar] [CrossRef] [Green Version]
- Arango-Lasprilla, J.C.; Nicholls, E.; Cabrera, T.V.; Drew, A.; Jimenez-Maldonado, M.; Martinez-Cortes, M. Health-related quality of life in caregivers of individuals with traumatic brain injury from Guadalajara, Mexico. J. Rehabil. Med. 2011, 43, 983–986. [Google Scholar] [CrossRef] [Green Version]
- Norup, A.; Snipes, D.J.; Siert, L.; Mortensen, E.L.; Perrin, P.B.; Arango-Lasprilla, J.C. Longitudinal Trajectories of Health Related Quality of Life in Danish Family Members of Individuals with Severe Brain Injury. Aust. J. Rehabil. Couns. 2013, 19, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Ennis, N.; Rosenbloom, B.N.; Canzian, S.; Topolovec-Vranic, J. Depression and anxiety in parent versus spouse caregivers of adult patients with traumatic brain injury: A systematic review. Neuropsychol. Rehabil. 2013, 23, 1–18. [Google Scholar] [CrossRef]
- Vangel, S.J.; Rapport, L.J.; Hanks, R.A. Effects of Family and Caregiver Psychosocial Functioning on Outcomes in Persons with Traumatic Brain Injury. J. Head Trauma Rehabil. 2011, 26, 20–29. [Google Scholar] [CrossRef]
- Schönberger, M.; Ponsford, J.; Olver, J.; Ponsford, M. A longitudinal study of family functioning after TBI and relatives’ emotional status. Neuropsychol. Rehabil. 2010, 20, 813–829. [Google Scholar] [CrossRef]
- Testa, J.A.; Malec, J.F.; Moessner, A.M.; Brown, A.W. Predicting Family Functioning After TBI. J. Head Trauma Rehabil. 2006, 21, 236–247. [Google Scholar] [CrossRef]
- Perlesz, A.; Kinsella, G.J.; Crowe, S.F. Psychological Distress and Family Satisfaction Following Traumatic Brain Injury: Injured Individuals and Their Primary, Secondary, and Tertiary Carers. J. Head Trauma Rehabil. 2000, 15, 909–929. [Google Scholar] [CrossRef]
- Ponsford, J.; Olver, J.; Ponsford, M.; Nelms, R. Long-term adjustment of families following traumatic brain injury where comprehensive rehabilitation has been provided. Brain Inj. 2003, 17, 453–468. [Google Scholar] [CrossRef]
- Lehan, T.J.; Arango-Lasprilla, J.C.; Reyes, C.J.D.L.; Quijano, M.C. The ties that bind: The relationship between caregiver burden and the neuropsychological functioning of TBI survivors. Neurorehabilitation 2012, 30, 87–95. [Google Scholar] [CrossRef]
- Stevens, L.F.; Perrin, P.B.; Hubbard, R.; Sosa, D.M.D.; Jove, I.G.E.; Arango-Lasprilla, J.C. Using multiple views of family dynamics to predict the mental health of individuals with TBI and their caregivers in Mexico. Neurorehabilitation 2013, 33, 273–283. [Google Scholar] [CrossRef]
- Andelic, N.; Howe, E.I.; Hellstrøm, T.; Sánchez, M.F.; Lu, J.; Løvstad, M.; Røe, C. Disability and quality of life 20 years after traumatic brain injury. Brain Behav. 2018, 8, e01018. [Google Scholar] [CrossRef] [PubMed]
- Hanks, R.A.; Rapport, L.J.; Vangel, S. Caregiving appraisal after traumatic brain injury: The effects of functional status, coping style, social support and family functioning. Neurorehabilitation 2007, 22, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Tsyben, A.; Guilfoyle, M.; Timofeev, I.; Anwar, F.; Allanson, J.; Outtrim, J.; Menon, D.; Hutchinson, P.; Helmy, A. Spectrum of outcomes following traumatic brain injury—Relationship between functional impairment and health-related quality of life. Acta Neurochir. 2017, 160, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.F.; Lehan, T.; Duran, M.A.S.; Plaza, S.L.O.; Arango-Lasprilla, J.C. Pilot study of a newly developed intervention for families facing serious injury. Top. Spinal Cord Inj. Rehabil. 2016, 22, 49–59. [Google Scholar] [CrossRef]
- Rasmussen, M.S.; Andelic, N.; Nordenmark, T.H.; Arango-Lasprilla, J.C.; Soberg, H.L. The family as a resource for improving patient and family functioning after traumatic brain injury: A descriptive nonrandomized feasibility study of a family-centered intervention. Cogent Med. 2019, 6. [Google Scholar] [CrossRef]
- Hagen, C.; Malkmus, D.; Durham, P. Levels of cognitive functioning, Rehabilitation of the Head Injured Adult; Comprehensive Physical Management: Downey, CA: Professional Staff Association of Rancho Los Amigos National Rehabilitation Center. 1979. Available online: http://file.lacounty.gov/SDSInter/dhs/218111_RLOCFFamilyGuideEnglish.pdf (accessed on 30 June 2020).
- Jennett, B. Assessment of Outcome after Severe Brain Damage: A Practical Scale. Lancet 1975, 305, 480–484. [Google Scholar] [CrossRef]
- Association for the Advancement of Automotive Medicine. The Abbreviated Injury Scale (AIS) 2005: 2008. Available online: https://www.aaam.org/abbreviated-injury-scale-ais/ (accessed on 30 June 2020).
- King, N.S.; Crawford, S.; Wenden, F.J.; Moss, N.E.G.; Wade, D.T. The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 1995, 242, 587–592. [Google Scholar] [CrossRef]
- Ingebrigtsen, T.; Waterloo, K.; Marup-Jensen, S.; Attner, E.; Romner, B. Quantification of post-concussion symptoms 3 months after minor head injury in 100 consecutive patients. J. Neurol. 1998, 245, 609–612. [Google Scholar] [CrossRef]
- Findler, M.; Cantor, J.; Haddad, L.; Gordon, W.; Ashman, T. The reliability and validity of the SF-36 health survey questionnaire for use with individuals with traumatic brain injury. Brain Inj. 2001, 15, 715–723. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Bjorner, J.B.; Turner-Bowker, D.M.; Gandek, B.; Maruish, M.E. User’s Manual for the SF-36v2 Health Survey, 2nd ed.; QualityMetric Incorporated: Lincoln, RI, USA, 2007. [Google Scholar]
- Olson, D. FACES IV and the Circumplex Model: Validation Study. J. Marital. Fam. Ther. 2011, 37, 64–80. [Google Scholar] [CrossRef]
- Hamilton, E.; Carr, A. Systematic Review of Self-Report Family Assessment Measures. Fam. Process. 2015, 55, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luszczynska, A.; Scholz, U.; Schwarzer, R. The General Self-Efficacy Scale: Multicultural Validation Studies. J. Psychol. 2005, 139, 439–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friborg, O.; Barlaug, D.; Martinussen, M.; Rosenvinge, J.H.; Hjemdal, O. Resilience in relation to personality and intelligence. Int. J. Methods Psychiatr. Res. 2005, 14, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Von Steinbüchel, N.; Wilson, L.; Gibbons, H.; Hawthorne, G.; Höfer, S.; Schmidt, S.; Bullinger, M.; Maas, A.I.R.; Neugebauer, E.; Powell, J.; et al. Quality of Life after Brain Injury (QOLIBRI): Scale Validity and Correlates of Quality of Life. J. Neurotrauma 2010, 27, 1157–1165. [Google Scholar] [CrossRef]
- Wilson, L.; Marsden-Loftus, I.; Koskinen, S.; Bakx, W.; Bullinger, M.; Formisano, R.; Maas, A.I.R.; Neugebauer, E.A.M.; Powell, J.; Sarajuuri, J.; et al. Interpreting Quality of Life after Brain Injury Scores: Cross-Walk with the Short Form-36. J. Neurotrauma 2017, 34, 59–65. [Google Scholar] [CrossRef]
- Soberg, H.L.; Røe, C.; Brunborg, C.; Von Steinbuechel, N.; Andelic, N. The Norwegian version of the QOLIBRI—A study of metric properties based on a 12 month follow-up of persons with traumatic brain injury. Health Qual. Life Outcomes 2017, 15, 14. [Google Scholar] [CrossRef] [Green Version]
- Andelic, N.; Hammergren, N.; Bautz-Holter, E.; Sveen, U.; Brunborg, C.; Røe, C. Functional outcome and health-related quality of life 10 years after moderate-to-severe traumatic brain injury. Acta Neurol. Scand. 2009, 120, 16–23. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Field, A. Discovering Statistics Using IBM SPSS Statistics and Sex and Drugs and Rock ’n’ Roll, 4th ed.; SAGE: Los Angeles, CA, USA, 2013. [Google Scholar]
- Smits, M.; Hunink, M.; Van Rijssel, D.; Dekker, H.; Vos, P.; Kool, D.; Nederkoorn, P.; Hofman, P.; Twijnstra, A.; Tanghe, H.; et al. Outcome after Complicated Minor Head Injury. Am. J. Neuroradiol. 2007, 29, 506–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garratt, A.M.; Stavem, K. Measurement properties and normative data for the Norwegian SF-36: Results from a general population survey. Health Qual. Life Outcomes 2017, 15, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chronister, J.; Chan, F. A stress process model of caregiving for individuals with traumatic brain injury. Rehabilit. Psychol. 2006, 51, 190–201. [Google Scholar] [CrossRef]
- Vogler, J.; Klein, A.-M.; Bender, A. Long-term health-related quality-of-life in patients with acquired brain injury and their caregivers. Brain Inj. 2014, 28, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Von Steinbuechel, N.; Meeuwsen, M.; Zeldovich, M.; Vester, J.C.; Maas, A.; Koskinen, S.; Covic, M.A. Differences in Health-Related Quality of Life after Traumatic Brain Injury between Varying Patient Groups: Sensitivity of a Disease-Specific (QOLIBRI) and a Generic (SF-36) Instrument. J. Neurotrauma 2020, 37, 1242–1254. [Google Scholar] [CrossRef]
- Bayen, E.; Pradat-Diehl, P.; Jourdan, C.; Ghout, I.; Bosserelle, V.; Azerad, S.; Weiss, J.-J.; Joël, M.-E.; Aegerter, P.; Azouvi, P. Predictors of Informal Care Burden 1 Year After a Severe Traumatic Brain Injury. J. Head Trauma Rehabil. 2013, 28, 408–418. [Google Scholar] [CrossRef]
- Jones, K.; Theadom, A.; Prah, P.; Starkey, N.J.; Barker-Collo, S.; Ameratunga, S.; Feigin, V.L.; For the BIONIC Study. Changes over time in family members of adults with mild traumatic brain injury. Brain Impair. 2019, 21, 154–172. [Google Scholar] [CrossRef]
- Draper, K.; Ponsford, J.; Schönberger, M. Psychosocial and Emotional Outcomes 10 Years Following Traumatic Brain Injury. J. Head Trauma Rehabil. 2007, 22, 278–287. [Google Scholar] [CrossRef]
- Kreutzer, J.S.; Rapport, L.J.; Marwitz, J.H.; Harrison-Felix, C.; Hart, T.; Glenn, M.; Hammond, F. Caregivers’ Well-Being after Traumatic Brain Injury: A Multicenter Prospective Investigation. Arch. Phys. Med. Rehabil. 2009, 90, 939–946. [Google Scholar] [CrossRef]
- Stevens, L.F.; Arango-Lasprilla, J.C.; Deng, X.; Schaaf, K.W.; Aragon, C.J.D.L.R.; Quijano, M.C.; Kreutzer, J. Factors associated with depression and burden in Spanish speaking caregivers of individuals with traumatic brain injury. Neurorehabilitation 2012, 31, 443–452. [Google Scholar] [CrossRef]
- Williamson, M.L.C.; Elliott, T.R.; Berry, J.W.; Underhill, A.T.; Stavrinos, D.; Fine, P.R. Predictors of health-related quality-of-life following traumatic brain injury. Brain Inj. 2013, 27, 992–999. [Google Scholar] [CrossRef]
- Forslund, M.V.; Roe, C.; Sigurdardóttir, S.; Andelic, N. Predicting health-related quality of life 2 years after moderate-to-severe traumatic brain injury. Acta Neurol. Scand. 2013, 128, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Norup, A.; Kristensen, K.S.; Poulsen, I.; Mortensen, E.L. Evaluating clinically significant changes in health-related quality of life: A sample of relatives of patients with severe traumatic brain injury. Neuropsychol. Rehabil. 2015, 27, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gulin, S.L.; Perrin, P.B.; Stevens, L.F.; Villaseñor-Cabrera, T.J.; Jiménez-Maldonado, M.; Martínez-Cortes, M.L.; Arango-Lasprilla, J.C. Health-related quality of life and mental health outcomes in Mexican TBI caregivers. Fam. Syst. Health 2014, 32, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Daher, M.; Simpson, G.K. A predictive model of resilience among family caregivers supporting relatives with traumatic brain injury (TBI): A structural equation modelling approach. Neuropsychol. Rehabil. 2019, 2019, 1–22. [Google Scholar] [CrossRef]
- Bermejo-Toro, L.; Sánchez-Izquierdo, M.; Calvete, E.; Roldán, M.A. Quality of life, psychological well-being, and resilience in caregivers of people with acquired brain injury (ABI). Brain Inj. 2020, 34, 480–488. [Google Scholar] [CrossRef]
- Lukow, H.R.; Godwin, E.E.; Marwitz, J.H.; Mills, A.; Hsu, N.H.; Kreutzer, J.S. Relationship Between Resilience, Adjustment, and Psychological Functioning After Traumatic Brain Injury. J. Head Trauma Rehabil. 2015, 30, 241–248. [Google Scholar] [CrossRef]
- Sullivan, K.A.; Kempe, C.B.; Edmed, S.L.; Bonanno, G.A. Resilience and Other Possible Outcomes after Mild Traumatic Brain Injury: A Systematic Review. Neuropsychol. Rev. 2016, 26, 173–185. [Google Scholar] [CrossRef]
- Sander, A.M.; Caroselli, J.S.; High, W.M., Jr.; Becker, C.; Neese, L.; Scheibel, R. Relationship of family functioning to progress in a post-acute rehabilitation programme following traumatic brain injury. Brain Inj. 2002, 16, 649–657. [Google Scholar] [CrossRef]
- Perrin, P.B.; Stevens, L.F.; Sutter, M.E.; Hubbard, R.; Sosa, D.M.D.; Jove, I.G.E.; Arango-Lasprilla, J.C. Exploring the Connections Between Traumatic Brain Injury Caregiver Mental Health and Family Dynamics in Mexico City, Mexico. PM&R 2013, 5, 839–849. [Google Scholar] [CrossRef]
- Lehan, T.J.; Stevens, L.F.; Arango-Lasprilla, J.C.; Sosa, D.M.D.; Jove, I.G.E. Balancing act: The influence of adaptability and cohesion on satisfaction and communication in families facing TBI in Mexico. Neurorehabilitation 2012, 30, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Reher, D.S. Family Ties in Western Europe: Persistent Contrasts. Popul. Dev. Rev. 1998, 24, 203. [Google Scholar] [CrossRef] [Green Version]
- Quinn, C.; Dunbar, S.B.; Clark, P.C.; Strickland, O.L. Challenges and strategies of dyad research: Cardiovascular examples. Appl. Nurs. Res. 2010, 23, e15–e20. [Google Scholar] [CrossRef] [Green Version]
- Sibley, L.M.; Weiner, J.P. An evaluation of access to health care services along the rural-urban continuum in Canada. BMC Health Serv. Res. 2011, 11, 20. [Google Scholar] [CrossRef] [PubMed]
| Patients (n = 61) | Family Members (n = 63) | |||
|---|---|---|---|---|
| Frequency (%) | Mean (SD) | Frequency (%) | Mean (SD) | |
| Age, mean (SD) | 43.8 (11.2) | 42.6 (11.3) | ||
| Sex (% females) | 33 (54.1) | 33 (52.4) | ||
| Comorbidity | 12 (19.6) | |||
| Marital status | ||||
| Married | 35 (57.4) | 37 (58.7) | ||
| Partner/cohabitant | 24 (39.3) | 24 (38.1) | ||
| Single | 2 (3.3) | 2 (3.2) | ||
| Length of relationship | ||||
| <1 year | 3 (5.0) | 3 (4.9) | ||
| 1–5 years | 7 (11.9) | 8 (13.1) | ||
| >5 years | 49 (83.1) | 50 (82.0) | ||
| Living arrangement | ||||
| Living in the same household as the patient | 57 (90.5) | |||
| Number of people living in the patient’s household | 3.1 (1.2) | |||
| Education | ||||
| Low | 16 (26.2) | 15 (23.8) | ||
| High | 45 (73.8) | 48 (76.2) | ||
| Patients’ pre-injury work status | ||||
| Not working | 4 (6.6) | |||
| Working | 57 (93.4) | |||
| Current work status | ||||
| Full-time work | 5 (8.2) | 53 (84.1) | ||
| Partial sick-leave | 33 (54.1) | 4 (6.3) | ||
| Sick-leave | 23 (37.7) | 6 (9.5) | ||
| Type of relation to the injured | ||||
| Partner/spouse | 58 (92.1) | |||
| Parent | 1 (1.6) | |||
| Child | 4 (6.3) | |||
| Injury Characteristics (n = 61) | Frequency (%) | Mean (SD)/Median (IQR) |
|---|---|---|
| Glasgow Coma Scale score | 15 (14, 15) | |
| Mild TBI | 50 (82.0) | |
| Moderate TBI | 3 (4.9) | |
| Severe TBI | 8 (13.1) | |
| AIS head score | 1.0 (1, 3) | |
| Intracranial injury | 18 (29.5) | |
| Surgical procedure | 8 (13.1) | |
| Falls | 23 (37.7) | |
| Traffic accidents | 19 (31.1) | |
| Mechanical object | 14 (23.0) | |
| Violence/Assault | 2 (3.3) | |
| Others | 3 (4.9) | |
| Time since injury (weeks) | 49.4 (36, 69) | |
| Length of stay (days) | 5.4 (range 0–37) | |
| RPQ total score (n = 56) | 27.7 (11.1) | |
| Self-reported comorbidities (n = 59) | 11 (18.6%) |
| Outcome | Patients | Family Members | ||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean difference | p-Values | |
| SF-36 MCS | 41.8 (9.9) | 47.7 (9.0) | 5.9 | 0.001 |
| GH (SF-36) | 45.8 (10.6) | 54.4 (10.0) | 8.6 | <0.001 |
| QOLIBRI overall scale | 58.1 (16.1) | - | - | - |
| FACES IV circumplex ratio | 3.0 (1.1) | 3.1 (1.2) | 0.1 | 0.692 |
| FCS | 65.7 (26.3) | 66.4 (25.0) | 0.7 | 0.884 |
| FSS | 55.6 (28.7) | 55.3 (26.4) | 0.3 | 0.946 |
| PHQ-9 | 9.6 (5.1) | 6.3 (4.3) | 3.3 | <0.001 |
| GAD-7 | 6.0 (4.2) | 4.9 (3.7) | 1.1 | 0.193 |
| RSA | 107.2 (16.6) | 113.9 (16.2) | 6.7 | 0.025 |
| GSE | 30.1 (5.1) | 31.8 (4.5) | 1.7 | 0.044 |
| Outcome Variable MCS | Univariate Regression | Multiple Regression Backward | |||
|---|---|---|---|---|---|
| B (C.I.) | p-Value | B | p-Value | 95% C.I. | |
| Age | 0.09 (−0.68, 0.25) | 0.265 | |||
| Sex (female/male) | 3.09 (−0.42, 6.58) | 0.084 | 2.56 | 0.038 | (0.14, 5.0) |
| Relation (patient/family) | 5.85 (2.47, 9.23) | 0.001 | |||
| FACES IV circumplex ratio | 2.37 (0.91, 3.84) | 0.002 | |||
| FCS | 0.08 (0.02, 0.15) | 0.018 | |||
| FSS | 0.11 (0.05, 0.17) | <0.001 | |||
| PHQ-9 | −1.41 (−1.67, −1.16) | <0.001 | −0.79 | <0.001 | (−1.16, −0.43) |
| GAD-7 | −1.59 (−1.94, −1.25) | <0.001 | −0.64 | 0.003 | (−1.06, −0.22) |
| RSA | 0.32 (0.23, 0.41) | <0.001 | 0.12 | 0.007 | (0.04, 0.21) |
| GSE | 0.96 (0.63, 1.28) | <0.001 | |||
| GH (SF-36) | 0.43 (0.29, 0.57) | <0.001 | |||
| R2 | 0.576 | ||||
| Adjusted R2 | 0.562 | ||||
| F value | 39.76 | <0.001 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmussen, M.S.; Arango-Lasprilla, J.C.; Andelic, N.; Nordenmark, T.H.; Soberg, H.L. Mental Health and Family Functioning in Patients and Their Family Members after Traumatic Brain Injury: A Cross-Sectional Study. Brain Sci. 2020, 10, 670. https://doi.org/10.3390/brainsci10100670
Rasmussen MS, Arango-Lasprilla JC, Andelic N, Nordenmark TH, Soberg HL. Mental Health and Family Functioning in Patients and Their Family Members after Traumatic Brain Injury: A Cross-Sectional Study. Brain Sciences. 2020; 10(10):670. https://doi.org/10.3390/brainsci10100670
Chicago/Turabian StyleRasmussen, Mari S., Juan Carlos Arango-Lasprilla, Nada Andelic, Tonje H. Nordenmark, and Helene L. Soberg. 2020. "Mental Health and Family Functioning in Patients and Their Family Members after Traumatic Brain Injury: A Cross-Sectional Study" Brain Sciences 10, no. 10: 670. https://doi.org/10.3390/brainsci10100670
APA StyleRasmussen, M. S., Arango-Lasprilla, J. C., Andelic, N., Nordenmark, T. H., & Soberg, H. L. (2020). Mental Health and Family Functioning in Patients and Their Family Members after Traumatic Brain Injury: A Cross-Sectional Study. Brain Sciences, 10(10), 670. https://doi.org/10.3390/brainsci10100670






