Cognitive Impairments and Self-Reported Sleep in Early-Stage Parkinson’s Disease with Versus without Probable REM Sleep Behavior Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Overview
2.2.2. Cognition Measures
2.2.3. Sleep Measures
2.3. Analyses
3. Results
3.1. Probable RBD and Cognition Analyses
3.2. Probable RBD and Demographic, Sleep, Mood, and PD Severity Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dubois, B.; Pillon, B. Cognitive deficits in Parkinson’s disease. J. Neurol. 1996, 244, 2–8. [Google Scholar] [CrossRef]
- Foltynie, T.; Brayne, C.E.G.; Robbins, T.W.; Barker, R.A. The cognitive ability of an incident cohort of Parkinson’s patients in the UK. The CamPaIGN study. Brain 2004, 127, 550–560. [Google Scholar] [CrossRef]
- Galvin, J.E. Cognitive change in Parkinson Disease. Alzheimer Dis. Assoc. Disord. 2006, 20, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; Moberg, P.J.; Culbertson, W.C.; Duda, J.E.; Katz, I.R.; Stern, M.B. Dimensions of executive function in Parkinson’s disease. Dement. Geriatr. Cogn. Disord. 2005, 20, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, X.; Wang, J.; Tang, L.; Xie, A. Prevalence of rapid eye movement sleep behavior disorder (RBD) in Parkinson’s disease: A meta and meta-regression analysis. Neurol. Sci. 2017, 38, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Kurz, M.W. The epidemiology of dementia associated with Parkinson Disease. J. Neurol. Sci. 2010, 289, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Hely, M.A.; Reid, W.G.; Adena, M.A.; Halliday, G.M.; Morris, J.G.L. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Gunn, D.G.; Naismith, S.L.; Lewis, S.J. Sleep disturbances in Parkinson Disease and their potential role in heterogeneity. J. Geriatr. Psychiatry Neurol. 2010, 23, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Seugnet, L.; Galvin, J.E.; Suzuki, Y.; Gottschalk, L.; Shaw, P.J. Persistent short-term memory defects following sleep deprivation in a drosophila model of Parkinson Disease. Sleep 2009, 32, 984–992. [Google Scholar] [CrossRef]
- Bergonzi, P.; Chiurulla, C.; Gambi, D.; Mennuni, G.; Pinto, F. L-dopa plus dopa-decarboxylase inhibitor: Sleep organization in Parkinson’s syndrome before and after treatment. Acta Neurol. Belg. 1975, 75, 5–10. [Google Scholar]
- Mitra, T.; Chaudhuri, K.R. Sleep dysfunction and role of dysautonomia in Parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15, S93–S95. [Google Scholar] [CrossRef]
- Alatriste-Booth, V.; Rodriguez-Violante, M.; Camacho-Ordonez, A.; Cervantes-Arriaga, C. Prevalence and correlates of sleep disorders in Parkinson’s disease: A polysomnographic study. Arq. Neuro-Psiquiatr. 2015, 73, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Gomutbutra, P.; Kanjanaratanakorn, K.; Tiyapun, N. Prevalence and clinical characteristics of Probable REM Behavior Disorder in Thai Parkinson’s disease patients. Parkinson’s Dis. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- De Cock, V.C.; Vidailhet, M.; Leu, S.; Texeira, A.; Apartis, E.; Elbaz, A.; Roze, E.; Willer, J.C.; Derenne, J.P.; Agid, Y.; et al. Restoration of normal motor control in Parkinson’s disease during REM sleep. Brain 2007, 130, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Manni, R.; Sinforiani, E.; Pacchetti, C.; Zucchella, C.; Cremascoli, R.; Terzaghi, M. Cognitive dysfunction and REM sleep behavior disorder: Key findings in the literature and preliminary longitudinal findings. Int. J. Psychophysiol. 2013, 89, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Massicotte-Marquez, J.; Decary, A.; Gagnon, J.F.; Vendette, M.; Mathieu, A.; Postuma, R.B.; Carrier, J.; Montplaisir, J. Executive dysfunction and memory impairment in idiopathic REM sleep behavior disorder. Neurology 2008, 70, 1250–1257. [Google Scholar] [CrossRef]
- Nomura, T.; Kishi, M.; Nakashima, K. Differences in clinical characteristics when REM sleep behavior disorder precedes or comes after the onset of Parkinson’s disease. J. Neurol. Sci. 2017, 382, 58–60. [Google Scholar] [CrossRef]
- Postuma, R.B.; Bertrand, J.-A.; Montplaisir, J.; Desjardins, C.; Vendette, M.; Rios Romenets, S.; Panisset, M.; Gagnon, J.-F. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: A prospective study. Mov. Disord. 2012, 27, 720–726. [Google Scholar] [CrossRef]
- Chahine, L.M.; Xie, S.X.; Simuni, T.; Tran, B.; Postuma, R.; Amara, A.; Oertel, W.H.; Iranzo, A.; Scordia, C.; Fullard, M.; et al. Longitudinal changes in cognition in early Parkinson’s disease patients with REM sleep behavior disorder. Parkinsonism Relat. Disord. 2016, 27, 102–106. [Google Scholar] [CrossRef]
- Jozwiak, N.; Postuma, R.B.; Montplaisir, J.; Latreille, V.; Panisset, M.; Chouinard, S.; Bourgouin, P.-A.; Gagnon, J.-F. REM sleep behavior disorder and cognitive impairment in Parkinson’s Disease. Sleep 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Kamble, N.; Yadav, R.; Lenka, A.; Kumar, K.; Nagaraju, B.C.; Pal, P.K. Impaired sleep quality and cognition in patients of Parkinson’s disease with REM sleep behavior disorder: A comparative study. Sleep Med. 2019, 62, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Lei, K.; Li, Y.; Liu, X.F.; Chang, Y. The correlation between possible RBD and cognitive function in Parkinson’s disease patients in China. Ann. Clin. Transl. Neurol. 2019, 6, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.-F.; Vendette, M.; Postuma, R.B.; Desjardins, C.; Massicotte-Marquez, J.; Panisset, M.; Montplaisir, J. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson’s disease. Ann. Neurol. 2009, 66, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Vendette, M.; Gagnon, J.-F.; Decary, A.; Massicotte-Marquez, J.; Postuma, R.B.; Doyon, J.; Panisset, M.; Montplaisir, J. REM sleep behavior disorder predicts cognitive impairment in Parkinson Disease without dementia. Neurology 2007, 69, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Neikrug, A.B.; Maglione, J.E.; Liu, L.; Natarajan, L.; Avanzino, J.A.; Corey-Bloom, J.; Palmer, B.W.; Loredo, J.S.; Ancoli-Israel, S. Effects of sleep disorders on the non-motor symptoms of Parkinson Disease. J. Clin. Sleep Med. 2013, 9, 1119–1129. [Google Scholar] [PubMed]
- Poryazova, R.; Oberholzer, M.; Baumann, C.R.; Bassetti, C.L. REM sleep behavior disorder in Parkinson’s disease: A questionnaire-based survey. J. Clin. Sleep Med. 2013, 9, 55–59. [Google Scholar] [CrossRef]
- Postuma, R.B.; Gagnon, J.-F.; Pelletier, A.; Montplaisir, J.Y. Insomnia and somnolence in idiopathic RBD: A prospective cohort study. NPJ Parkinson’s Dis. 2017, 3, 9. [Google Scholar] [CrossRef]
- Stiasny-Kolster, K.; Sixel-Doring, F.; Trenkwalder, C.; Heinzel-Gutenbrunner, M.; Seppi, K.; Poewe, W.; Hogl, B.; Frauscher, B. Diagnostic value of the REM sleep behavior disorder screening questionnaire in Parkinson’s disease. Sleep Med. 2015, 16, 186–189. [Google Scholar] [CrossRef]
- Kay, D.B.; Tanner, J.J.; Bowers, D. Sleep disturbances and depression severity in patients with Parkinson’s disease. Brain Behav. 2018, 8, e00967. [Google Scholar] [CrossRef]
- Fahn, S.; Elton, R.L. Recent Developments in Parkinson’s Disease; Fahn, S., Marsden, C.D., Calne, D.B., Goldstein, M., Eds.; Macmillan Healthcare Information: Florham Park, NJ, USA, 1987; pp. 153–163. [Google Scholar]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Kukull, W.A.; Larson, E.B.; Teri, L.; Bowen, J.; McCormick, W.; Pfanschmidt, M.L. The Mini-Mental State Examination score and the clinical diagnosis of dementia. J. Clin. Epidemiol. 1994, 47, 1061–1067. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R. STAI Manual; Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Delis, D.C.; Kaplan, E.; Kramer, J.H. The Delis-Kaplan Executive Function System; The Psychological Corporation: San Antonio, TX, USA, 2001. [Google Scholar]
- Benton, A.L.; Hamsher, K.D.; Varney, N.R.; Spreen, O. Contributions to Neuropsychological Assessment: A Clinical Manual; Oxford University Press, Inc.: Oxford, NY, USA, 1983. [Google Scholar]
- Ivnik, R.J.; Malec, J.F.; Smith, G.E.; Tangalos, E.G.; Petersen, R.C. Neuropsychological tests’ norms above age 55: COWAT, BNT, MAE token, WRAT-R reading, AMNART, STROOP, TMT, and JLO. Clin. Neuropsychol. 1996, 10, 262–278. [Google Scholar] [CrossRef]
- Kirchner, W.K. Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 1958, 55, 352–358. [Google Scholar] [CrossRef]
- Altena, E.; van Der Werf, Y.D.; Strijers, R.L.; van Someren, E.J. Sleep loss affects vigilance: Effects of chronic insomnia and sleep therapy. J. Sleep Res. 2008, 17, 335–343. [Google Scholar] [CrossRef]
- Newcomer, J.W.; Selke, G.; Melson, A.K.; Hershey, T.; Craft, S.; Richards, K.; Alderson, A.L. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch. Gen. Psychiatry 1999, 56, 527–533. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Hoch, C.C.; Yeager, A.L.; Kupfer, D.J. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep 1991, 14, 331–338. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallieres, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Cella, D.; Riley, W.; Stone, A.; Rothrock, N.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Chen, K.J.; Wang, C.C.; Lai, C.L. Cognitive and motor components of response speed in the stroop test in Parkinson’s disease patients. Kaohsiung J. Med. Sci. 2008, 24, 197–203. [Google Scholar] [CrossRef]
- Obeso, I.; Wilkinson, L.; Casabona, E.; Bringas, M.L.; Alvarez, M.; Alvarez, L.; Alvarez, L.; Pavon, N.; Rodriguez-Oroz, M.-C.; Macias, R.; et al. Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson’s disease. Exp. Brain Res. 2011, 212, 371–384. [Google Scholar] [CrossRef]
- Sinforiani, E.; Zangaglia, R.; Manni, R.; Cristina, S.; Marchioni, E.; Nappi, G.; Mancini, F.; Pacchetti, C. REM Sleep Behavior Disorder, hallucinations, and cognitive impairment in Parkinson’s Disease. Mov. Disord. 2006, 21, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Arnulf, I.; Neutel, D.; Herlin, B.; Golmard, J.L.; Leu-Semenescu, S.; Cochen de Cock, V.; Vidailhet, M. Sleepiness in idiopathic REM sleep behavior disorder and Parkinson Disease. Sleep 2015, 38, 1529–1535. [Google Scholar] [CrossRef]
- Mahale, R.; Yadav, R.; Pal, P.K. Rapid eye movement sleep behaviour disorder in young- and older-onset Parkinson Disease: A questionnaire-based study. Sleep Med. 2014, 15, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.D.; Crawford, J.R. A Meta-Analytic Review of verbal fluency performance in patients with traumatic brain injury. Neuropsychology 2004, 18, 621–628. [Google Scholar] [CrossRef]
- El-Nazer, R.; Adler, C.H.; Beach, T.G.; Belden, C.M.; Artz, J.; Shill, H.A.; Driver-Dunckley, E.; Mehta, S.H.; Sabbagh, M.N.; Serrano, G.E.; et al. Regional neuropathology distribution and verbal fluency impairments in Parkinson’s Disease. Park Rel. Dis. 2019, 65, 73–78. [Google Scholar] [CrossRef]

| PD with pRBD (n = 19) | PD without pRBD (n = 31) | χ2/Z/t | df | p-Value | Effect Size (ϕ/V/rs/d) | |
|---|---|---|---|---|---|---|
| Demographic, Mood, and PD Measures | ||||||
| Age, y | 70.1 (7.4) | 65.7 (6.6) | −2.16 | 48 | 0.036 * | d = −0.62 |
| Male sex, n | 16 | 22 | 1.13 | 1 | 0.760 | ϕ = 0.15 |
| Education, y | 16 [13, 20] | 16 [12, 18] | −0.84 | 0.400 | rs = −0.12 | |
| White, n | 16 | 22 | 0.13 | 1 | 0.721 | ϕ = 0.05 |
| Married, n | 15 | 28 | 5.71 | 5 | 0.335 | V = 0.15 |
| State-Trait Anxiety Inventory, State | 25 [20, 41] | 35 [27, 43] | 1.69 | - | 0.091 | rs = 0.24 |
| Patient Health Questionnaire-9 | 5 [3, 8] | 6 [2, 10] | 0.20 | - | 0.841 | rs = 0.03 |
| Modified Hoehn-Yahr Scale † | 2 [2, 2] | 2 [2, 2] | 0.92 | - | 0.358 | rs = 0.13 |
| PD duration, mos ‡ | 84 [36, 120] | 96 [66, 135] | 0.73 | - | 0.330 | rs = 0.12 |
| Cognition Measures | ||||||
| D-KEFS Color-Word Interference Test | ||||||
| Color naming, ss | 8.7 (2.5) | 9.7 (2.4) | 1.42 | 48 | 0.162 | d = 0.41 |
| Word reading, ss | 8.7 (2.7) | 10.3 (2.6) | 2.06 | 48 | 0.044 * | d = 0.60 § |
| Inhibition, ss | 9.2 (3.1) | 10.0 (3.7) | 0.80 | 48 | 0.425 | d = 0.23 |
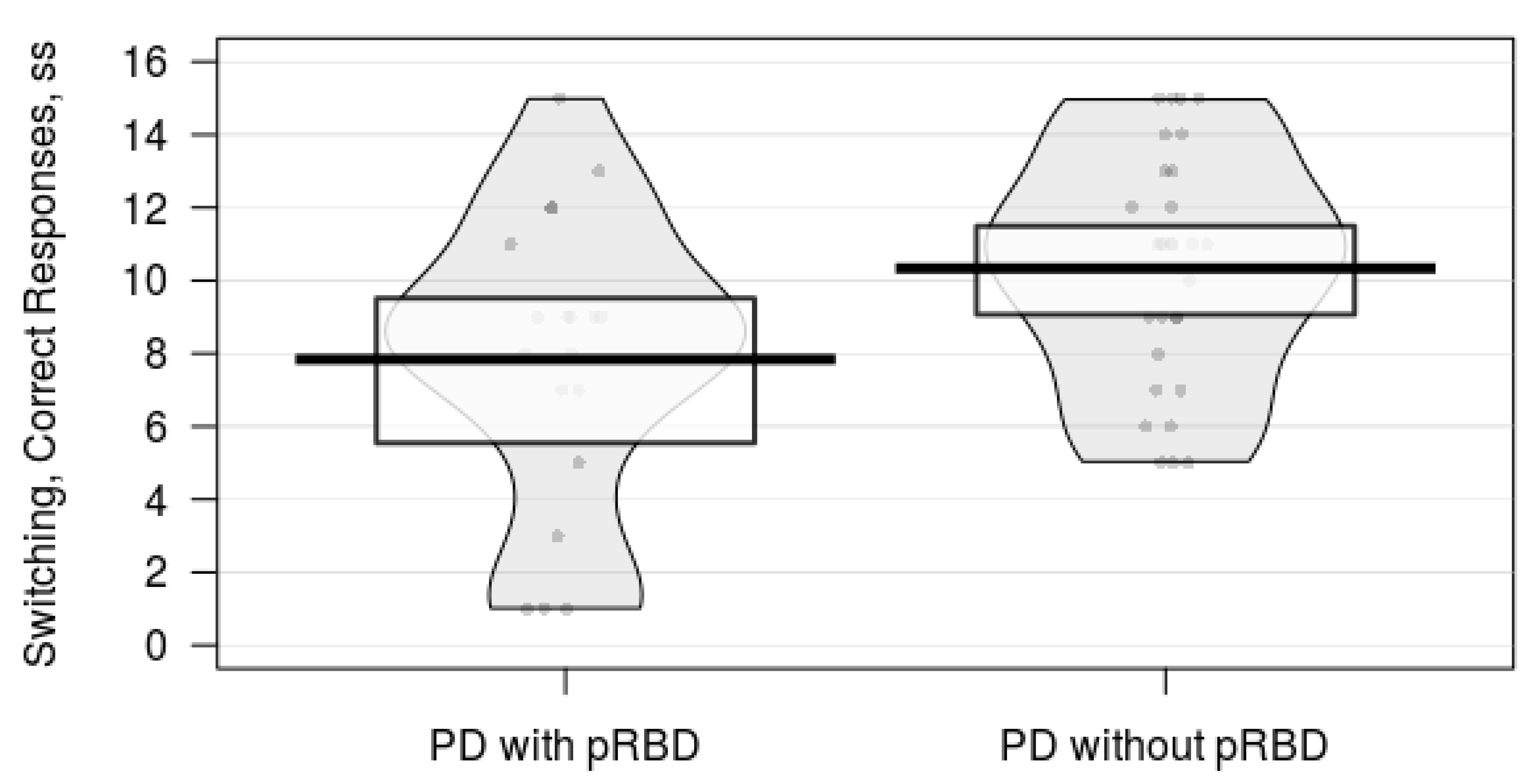
| Switching, ss ‡ | 9.4 (3.5) | 10.8 (2.4) | 1.65 | 46 | 0.106 | d = 0.48 |
| Visuospatial processing, JOLO, ss | 12.2 (2.8) | 12.2 (2.4) | 0.01 | 48 | 0.996 | d = 0.00 |
| Working Memory, 2- & 3-back | ||||||
| Average accuracy †‡ | 0.8 [0.8, 0.9] | 0.9 [0.8, 0.9] | −0.71 | 0.480 | rs = 0.10 | |
| Average RT, ms †‡ | 876 [781, 875] | 902 [768, 902] | −0.48 | 0.633 | rs = 0.07 | |
| Sustained Attention, CPT | ||||||
| Total accuracy † | 0.9 [0.6, 1.0] | 0.9 [0.8, 1.0] | −0.51 | - | 0.608 | rs = 0.04 |
| Total RT, ms † | 399 [374, 476] | 394.0 [357, 455] | −0.26 | - | 0.797 | rs = −0.07 |
| D-KEFS Verbal Fluency Test | ||||||
| Letter, ss † | 10.4 (3.4) | 11.2 (3.7) | 0.70 | 47 | 0.485 | d = 0.20 |
| Category, ss † | 9.6 (2.3) | 11.0 (3.3) | 1.66 | 47 | 0.104 | d = 0.48 |
| Switching, ss † | 7.8 (4.1) | 10.3 (3.2) | 2.38 | 47 | 0.021 * | d = 0.69 § |
| Episodic memory, stories | ||||||
| Immediate recall † | 20.0 (6.1) | 21.0 (4.8) | 0.59 | 47 | 0.561 | d = 0.17 |
| Delayed recall † | 17.0 (5.4) | 17.0 (4.5) | 0.19 | 47 | 0.854 | d = 0.06 |
| Sleep Measures | ||||||
| Pittsburgh Sleep Quality Index total score | 6.9 (5.2) | 7.1 (3.2) | 0.15 | 48 | 0.878 | d = 0.04 |
| Total sleep time, min | 396 (105) | 401 (65) | 0.23 | 48 | 0.816 | d = 0.24 |
| Insomnia Severity Index total score | 8 [3, 15] | 6 [3, 12] | −0.84 | - | 0.399 | rs = −0.12 |
| Epworth Sleepiness Scale total score | 10 [7, 16] | 8 [4, 9] | −1.80 | - | 0.073 | rs = −0.26 |
| Sleep disturbance, PROMIS-SD † | 53 [41, 77] | 52 [45, 59] | −0.51 | - | 0.608 | rs = −0.07 |
| Sleep-related impairment, PROMIS-SRI † | 31 [27, 49] | 34 [26, 38] | −0.81 | - | 0.417 | rs = 0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trout, J.; Christiansen, T.; Bulkley, M.B.; Tanner, J.J.; Sozda, C.N.; Bowers, D.; Kay, D.B. Cognitive Impairments and Self-Reported Sleep in Early-Stage Parkinson’s Disease with Versus without Probable REM Sleep Behavior Disorder. Brain Sci. 2020, 10, 9. https://doi.org/10.3390/brainsci10010009
Trout J, Christiansen T, Bulkley MB, Tanner JJ, Sozda CN, Bowers D, Kay DB. Cognitive Impairments and Self-Reported Sleep in Early-Stage Parkinson’s Disease with Versus without Probable REM Sleep Behavior Disorder. Brain Sciences. 2020; 10(1):9. https://doi.org/10.3390/brainsci10010009
Chicago/Turabian StyleTrout, Jonathan, Taylor Christiansen, M. Brooks Bulkley, Jared J. Tanner, Christopher N. Sozda, Dawn Bowers, and Daniel B. Kay. 2020. "Cognitive Impairments and Self-Reported Sleep in Early-Stage Parkinson’s Disease with Versus without Probable REM Sleep Behavior Disorder" Brain Sciences 10, no. 1: 9. https://doi.org/10.3390/brainsci10010009
APA StyleTrout, J., Christiansen, T., Bulkley, M. B., Tanner, J. J., Sozda, C. N., Bowers, D., & Kay, D. B. (2020). Cognitive Impairments and Self-Reported Sleep in Early-Stage Parkinson’s Disease with Versus without Probable REM Sleep Behavior Disorder. Brain Sciences, 10(1), 9. https://doi.org/10.3390/brainsci10010009




