Calcitonin Gene-Related Peptide (CGRP) and Cluster Headache
Abstract
1. Introduction
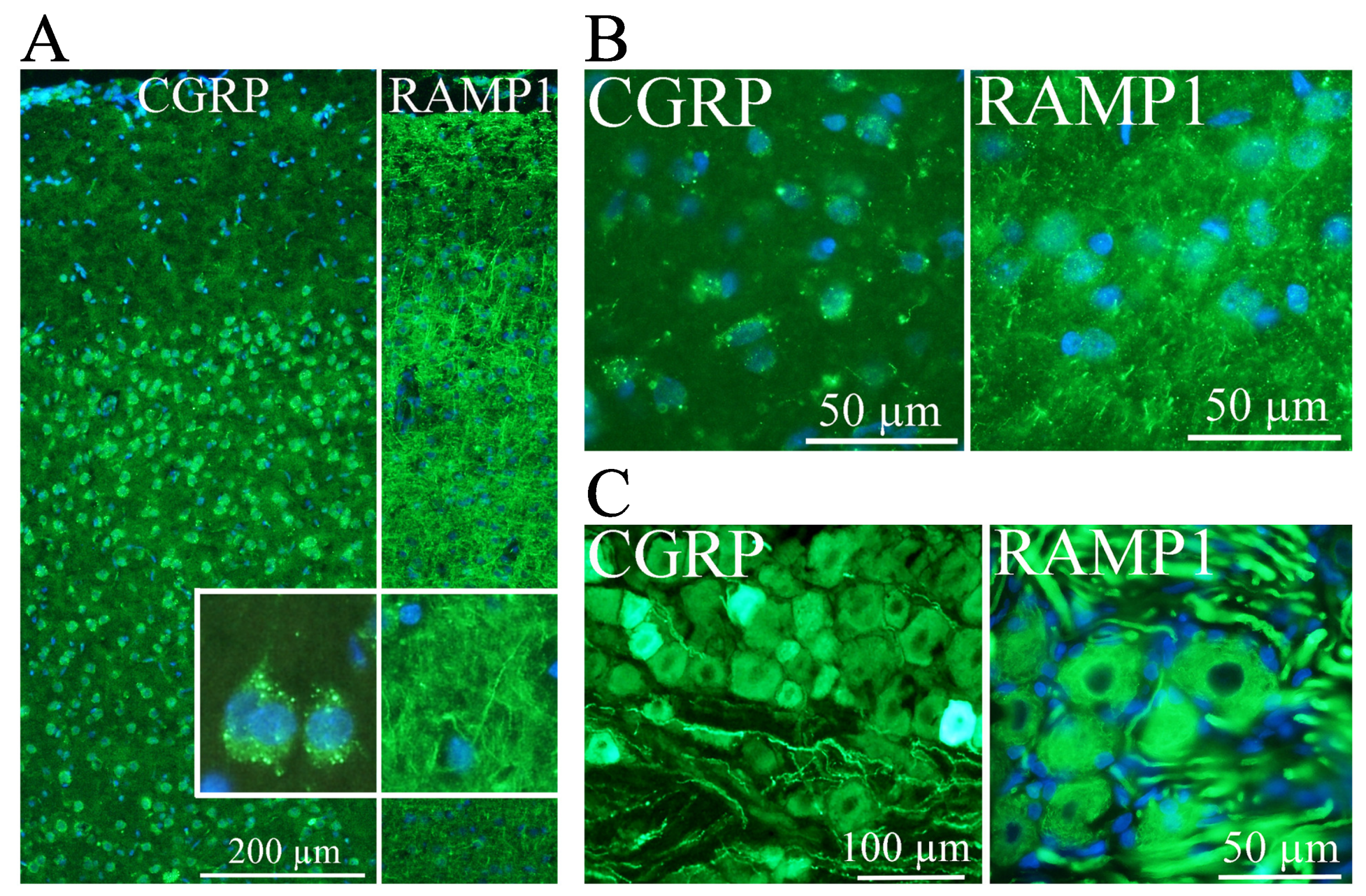
2. CGRP Localization
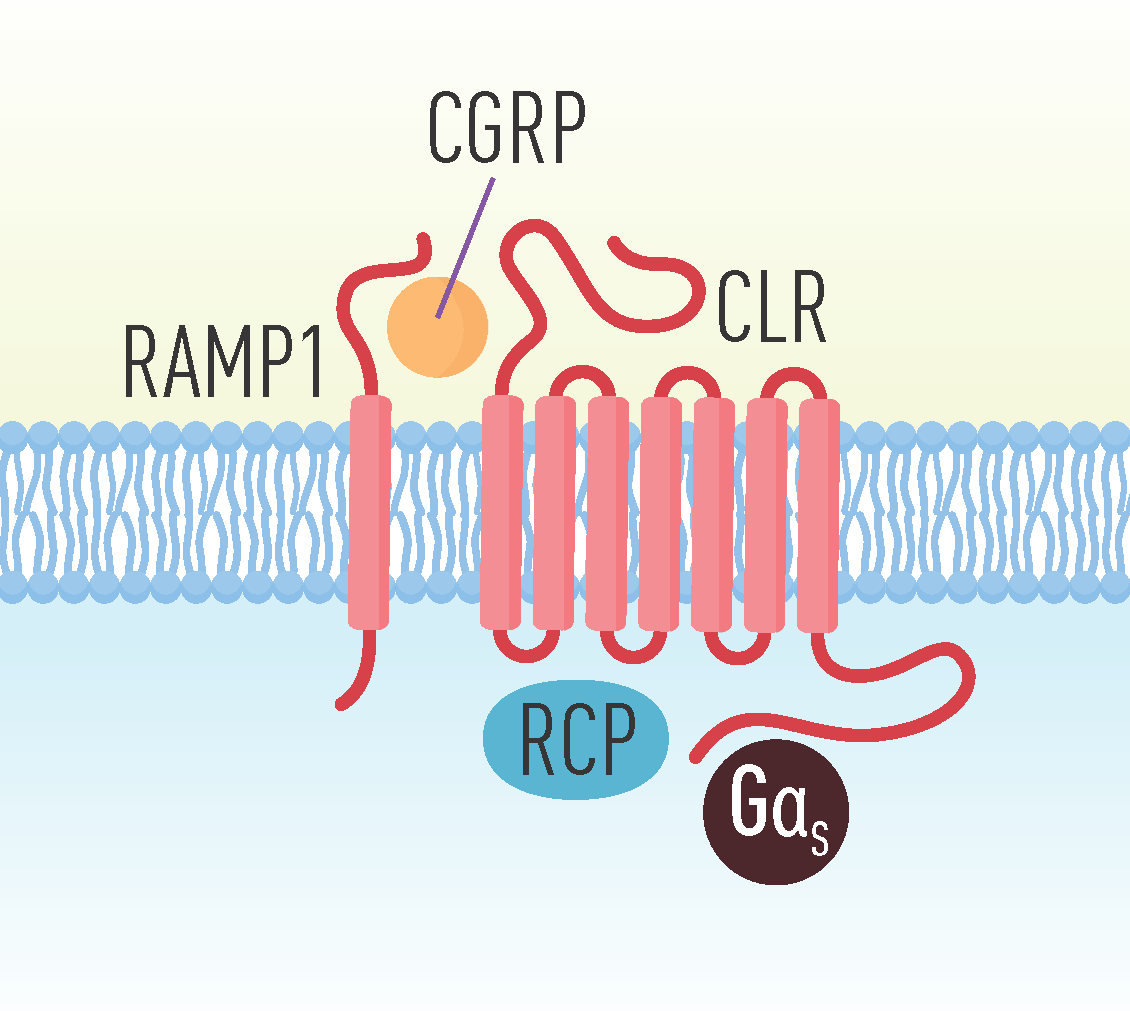
3. CGRP Receptors
4. CGRP Function
5. CGRP and Primary Headache
5.1. Migraine Pathophysiology and CGRP
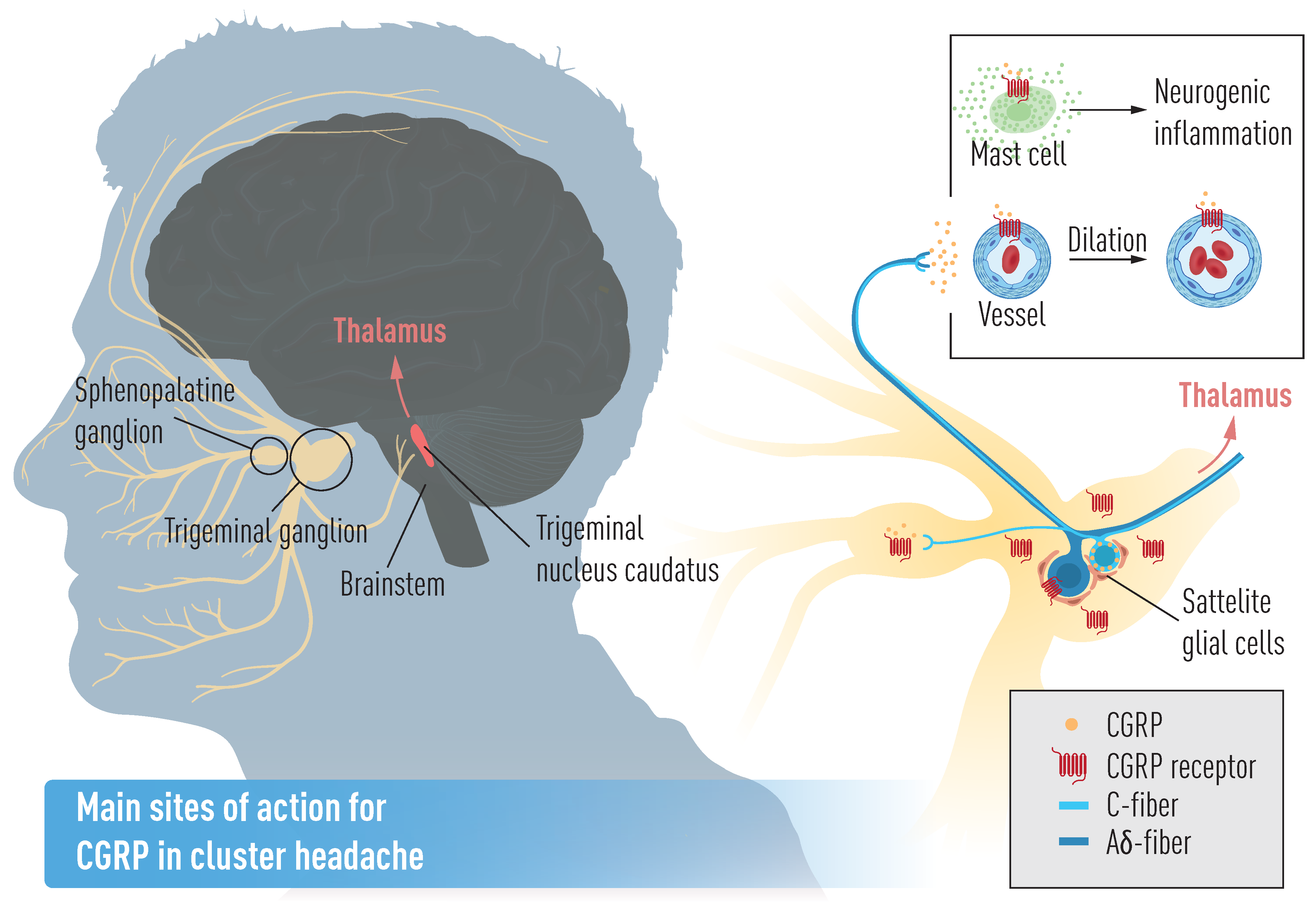
5.2. Cluster Headache Pathophysiology and CGRP
6. CGRP and Genetics
7. CGRP and Treatment
7.1. Migraine Treatment
7.2. Future CH Treatment
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marcel, A. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders. Cephalalgia 2018, 38, 808–811. [Google Scholar] [CrossRef]
- Fischera, M.; Marziniak, M.; Gralow, I.; Evers, S. The incidence and prevalence of cluster headache: A meta-analysis of population-based studies. Cephalalgia 2008, 28, 614–618. [Google Scholar] [CrossRef]
- Steinberg, A.; Josefsson, P.; Alexanderson, K.; Sjöstrand, C. Cluster headache. Neurology 2019, 93, e404–e413. [Google Scholar] [CrossRef]
- Seng, E.K.; Singer, A.; Metts, C.; Grinberg, A.S.; Patel, Z.; Marzouk, M.; Rosenberg, L.; Day, M.; Minen, M.; Lipton, R.B.; et al. 61st Annual Scientific Meeting American Headache Society® July 11–14 2019 Pennsylvania Convention Center Philadelphia, PA. Headache J. Head Face Pain 2019, 59, 1–208. [Google Scholar] [CrossRef]
- Schor, L.I. IHC Electronic Posters - Saturday and Sunday: Cluster Headache: Investigating severity of pain, suicidality, personal burden, access to effective treatment, and demographics among a large International survey sample. Cephalalgia 2017, 37, 172–208. [Google Scholar] [CrossRef]
- Koehler, P.J. Prevalence of headache in Tulp’s Observationes Medicae (1641) with a description of cluster headache. Cephalalgia 1993, 13, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Ekman, R.; Jansen, I.; Ottosson, A.; Uddman, R. Peptide-containing nerve fibers in human cerebral arteries: Immunocytochemistry, radioimmunoassay and in vitro pharmacology. Ann. Neurol. 1987, 21, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.D.; Williams, T.J.; Tippins, J.R.; Morris, H.R.; MacIntyre, I. Calcitonin gene-related peptide is a potent vasodilator. Nature 1985, 313, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Fredholm, B.B.; Hamel, E.; Jansen, I.; Verrecchia, C. Perivascular peptides relax cerebral arteries concomitant with stimulation of cyclic adenosine monophosphate accumulation or release of an endothelium-derived relaxing factor in the cat. Neurosci. Lett. 1985, 58, 213–217. [Google Scholar] [CrossRef]
- Amara, S.G.; Jonas, V.; Rosenfeld, M.G.; Ong, E.S.; Evans, R.M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 1982, 298, 240–244. [Google Scholar] [CrossRef]
- Cooper, G.J.S.; Willis, A.C.; Clark, A.; Turner, R.C.; Sim, R.B.; Reid, K.B.M. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc. Natl. Acad. Sci. USA 1987, 84, 8628–8632. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Kangawa, K.; Kawamoto, M.; Ichiki, Y.; Nakamura, S.; Matsuo, H.; Eto, T. Adrenomedullin: A novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993, 192, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Steenbergh, P.H.; Höppener, J.W.; Zandberg, J.; Lips, C.J.; Jansz, H.S. A second human calcitonin/CGRP gene. FEBS Lett. 1985, 183, 403–407. [Google Scholar] [CrossRef]
- Underwood, E. FDA just approved the first drug to prevent migraines. Here’s the story of its discovery—and its limitations. Science 2018. [Google Scholar] [CrossRef]
- Haanes, K.A.; Edvinsson, L. Pathophysiological Mechanisms in Migraine and the Identification of New Therapeutic Targets. CNS Drugs 2019, 33, 525–537. [Google Scholar] [CrossRef]
- Edvinsson, L. Functional role of perivascular peptides in the control of cerebral circulation. Trends Neurosci. 1985, 8, 126–131. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988, 23, 193–196. [Google Scholar] [CrossRef]
- Rosenfeld, M.G.; Mermod, J.-J.; Amara, S.G.; Swanson, L.W.; Sawchenko, P.E.; Rivier, J.; Vale, W.W.; Evans, R.M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 1983, 304, 129–135. [Google Scholar] [CrossRef]
- Eftekhari, S.; Warfvinge, K.; Blixt, F.; Edvinsson, L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J. Headache Pain 2013, 14, P89. [Google Scholar] [CrossRef][Green Version]
- Goadsby, P.J.; Edvinsson, L. Human in vivo evidence for trigeminovascular activation in cluster headache Neuropeptide changes and effects of acute attacks therapies. Brain 1994, 117, 427–434. [Google Scholar] [CrossRef]
- Vollesen, A.L.H.; Snoer, A.; Beske, R.P.; Guo, S.; Hoffmann, J.; Jensen, R.H.; Ashina, M. Effect of Infusion of Calcitonin Gene-Related Peptide on Cluster Headache Attacks: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 1187–1197. [Google Scholar] [CrossRef]
- Fanciullacci, M.; Alessandri, M.; Figini, M.; Geppetti, P.; Michelacci, S. Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain 1995, 60, 119–123. [Google Scholar] [CrossRef]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies—Successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Warfvinge, K.; Edvinsson, L. Distribution of CGRP and CGRP receptor components in the rat brain. Cephalalgia 2019, 39, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Leff, S.E.; Evans, R.M.; Rosenfeld, M.G. Splice commitment dictates neuron-specific alternative RNA processing in calcitonin/CGRP gene expression. Cell 1987, 48, 517–524. [Google Scholar] [CrossRef]
- Van Rossum, D.; Hanisch, U.K.; Quirion, R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci. Biobehav. Rev. 1997, 21, 649–678. [Google Scholar] [CrossRef]
- Maggi, C.A. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog. Neurobiol. 1995, 45, 1–98. [Google Scholar] [CrossRef]
- Feuerstein, G.; Willette, R.; Aiyar, N. Clinical perspectives of calcitonin gene related peptide pharmacology. Can J. Physiol. Pharmacol. 1995, 73, 1070–1074. [Google Scholar] [CrossRef]
- Holzer, P. Chemosensitive afferent nerves in the regulation of gastric blood flow and protection. Adv. Exp. Med. Biol. 1995, 371B, 891–895. [Google Scholar]
- Muff, R.; Born, W.; Fischer, J.A. Calcitonin, calcitonin gene-related peptide, adrenomedullin and amylin: Homologous peptides, separate receptors and overlapping biological actions. Eur. J. Endocrinol. 1995, 133, 17–20. [Google Scholar] [CrossRef]
- Poyner, D. Pharmacology of receptors for calcitonin gene-related peptide and amylin. Trends Pharmacol. Sci. 1995, 16, 424–428. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Calcitonin gene-related peptide and its receptors: Molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr. Rev. 1996, 17, 533–585. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Amylin, calcitonin gene-related peptide, calcitonin, and adrenomedullin: A peptide superfamily. Crit. Rev. Neurobiol. 1997, 11, 167–239. [Google Scholar] [CrossRef] [PubMed]
- Hendrikse, E.R.; Bower, R.L.; Hay, D.L.; Walker, C.S. Molecular studies of CGRP and the CGRP family of peptides in the central nervous system. Cephalalgia 2019, 39, 403–419. [Google Scholar] [CrossRef]
- Zaidi, M.; Breimer, L.H.; MacIntyre, I. Biology of peptides from the calcitonin genes. Q. J. Exp. Physiol. 1987, 72, 371–408. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, T.; Boecker, H.; Tolle, T.R.; Bussone, G.; May, A.; Leone, M. Specific hypothalamic activation during a spontaneous cluster headache attack. Neurology 2004, 62, 516–517. [Google Scholar] [CrossRef]
- May, A.; Bahra, A.; Büchel, C.; Frackowiak, R.S.; Goadsby, P.J. Hypothalamic activation in cluster headache attacks. Lancet 1998, 352, 275–278. [Google Scholar] [CrossRef]
- Steinberg, A.; Fourier, C.; Ran, C.; Waldenlind, E.; Sjöstrand, C.; Belin, A.C. Cluster headache–clinical pattern and a new severity scale in a Swedish cohort. Cephalalgia 2018, 38, 1286–1295. [Google Scholar] [CrossRef]
- Lund, N.L.; Snoer, A.H.; Jennum, P.J.; Jensen, R.H.; Barloese, M.C.J. Sleep in cluster headache revisited: Results from a controlled actigraphic study. Cephalalgia 2018. [Google Scholar] [CrossRef]
- Leone, M.; Bussone, G. A review of hormonal findings in cluster headache. Evidence for hypothalamic involvement. Cephalalgia 1993, 13, 309–317. [Google Scholar] [CrossRef]
- Takahashi, K.; Mouri, T.; Sone, M.; Murakami, O.; Itoi, K.; Imai, Y.; Ohneda, M.; Yoshinaga, K.; Sasano, N. Calcitonin Gene-Related Peptide in the Human Hypothalamus. Endocrinol. Jpn. 1989, 36, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Tajti, J.; Uddman, R.; Möller, S.; Sundler, F.; Edvinsson, L. Messenger molecules and receptor mRNA in the human trigeminal ganglion. J. Auton. Nerv. Syst. 1999, 76, 176–183. [Google Scholar] [CrossRef]
- Eftekhari, S.; Salvatore, C.A.; Calamari, A.; Kane, S.A.; Tajti, J.; Edvinsson, L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 2010, 169, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, S.; Salvatore, C.A.; Johansson, S.; Chen, T.; Zeng, Z.; Edvinsson, L. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood–brain barrier. Brain Res. 2015, 1600, 93–109. [Google Scholar] [CrossRef]
- Thalakoti, S.; Patil, V.V.; Damodaram, S.; Vause, C.V.; Langford, L.E.; Freeman, S.E.; Durham, P.L. Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache J. Head Face Pain 2007, 47, 1008–1025. [Google Scholar] [CrossRef]
- Purves, D. Neuroscience, 3rd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2004. [Google Scholar]
- Goadsby, P.J. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends Mol. Med. 2007, 13, 39–44. [Google Scholar] [CrossRef]
- Eftekhari, S.; Edvinsson, L. Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level. BMC Neurosci. 2011, 12, 112. [Google Scholar] [CrossRef]
- Edvinsson, L.; Chan, K.Y.; Eftekhari, S.; Nilsson, E.; De Vries, R.; Säveland, H.; Dirven, C.M.; Danser, A.J.; MaassenVanDenBrink, A. Effect of the calcitonin gene-related peptide (CGRP) receptor antagonist telcagepant in human cranial arteries. Cephalalgia 2010, 30, 1233–1240. [Google Scholar] [CrossRef]
- Hagner, S.; Stahl, U.; Knoblauch, B.; McGregor, G.; Lang, R. Calcitonin receptor-like receptor: Identification and distribution in human peripheral tissues. Cell Tissue Res. 2002, 310, 41–50. [Google Scholar] [CrossRef]
- Edvinsson, L.; Ahnstedt, H.; Larsen, R.; Sheykhzade, M. Differential localization and characterization of functional calcitonin gene-related peptide receptors in human subcutaneous arteries. Acta Physiol. 2014, 210. [Google Scholar] [CrossRef]
- O’Connor, T.P.; van der Kooy, D. Enrichment of a vasoactive neuropeptide (calcitonin gene related peptide) in the trigeminal sensory projection to the intracranial arteries. J. Neurosci. 1988, 8, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Lennerz, J.K.; Rühle, V.; Ceppa, E.P.; Neuhuber, W.L.; Bunnett, N.W.; Grady, E.F.; Messlinger, K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: Differences between peripheral and central CGRP receptor distribution. J. Comp. Neurol. 2008, 507, 1277–1299. [Google Scholar] [CrossRef] [PubMed]
- Poyner, D.R.; Sexton, P.M.; Marshall, I.; Smith, D.M.; Quirion, R.; Born, W.; Muff, R.; Fischer, J.A.; Foord, S.M. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002, 54, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Foord, S.M.; Marshall, F.H. RAMPs: Accessory proteins for seven transmembrane domain receptors. Trends Pharmacol. Sci. 1999, 20, 184–187. [Google Scholar] [CrossRef]
- Hay, D.L.; Garelja, M.L.; Poyner, D.R.; Walker, C.S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 2018, 175, 3–17. [Google Scholar] [CrossRef] [PubMed]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. {RAMPs} regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998, 393, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Muff, R.; Gujer, R.; Fischer, J.A.; Born, W. The transmembrane domain of receptor-activity-modifying protein 1 is essential for the functional expression of a calcitonin gene-related peptide receptor. Biochemistry 2002, 41, 11398–11404. [Google Scholar] [CrossRef]
- Brain, S.D.; Grant, A.D. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004, 84, 903–934. [Google Scholar] [CrossRef]
- Sutherland, H.G.; Buteri, J.; Menon, S.; Haupt, L.M.; Macgregor, E.A.; Lea, R.A. Association study of the calcitonin gene-related polypeptide-alpha (CALCA) and the receptor activity modifying 1 (RAMP1) genes with migraine. Gene 2013, 515, 187–192. [Google Scholar] [CrossRef]
- Cargnin, S.; Pautasso, C.; Viana, M.; Sances, G.; Mittino, D.; Cantello, R.; Tassorelli, C.; Nappi, G.; Terrazzino, S. Association of RAMP1 rs7590387 with the risk of migraine transformation into medication overuse headache. Headache 2015, 55, 658–668. [Google Scholar] [CrossRef]
- Fraser, N.J.; Wise, A.; Brown, J.; McLatchie, L.M.; Main, M.J.; Foord, S.M. The Amino Terminus of Receptor Activity Modifying Proteins Is a Critical Determinant of Glycosylation State and Ligand Binding of Calcitonin Receptor-Like Receptor. Mol. Pharmacol. 1999, 55, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.S.; Eftekhari, S.; Bower, R.L.; Wilderman, A.; Insel, P.A.; Edvinsson, L. A second trigeminal CGRP receptor: Function and expression of the AMY1 receptor. Ann. Clin. Transl. Neurol. 2015, 2, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Marquez de Prado, B.; Hammond, D.L.; Russo, A.F. Genetic enhancement of calcitonin gene-related Peptide-induced central sensitization to mechanical stimuli in mice. J. Pain 2009, 10, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Recober, A.; Kuburas, A.; Zhang, Z.; Wemmie, J.A.; Anderson, M.G.; Russo, A.F. Role of calcitonin gene-related peptide in light-aversive behavior: Implications for migraine. J. Neurosci. 2009, 29, 8798–8804. [Google Scholar] [CrossRef]
- Evans, B.N.; Rosenblatt, M.I.; Mnayer, L.O.; Oliver, K.R.; Dickerson, I.M. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J. Biol. Chem. 2000, 275, 31438–31443. [Google Scholar] [CrossRef]
- Padilla, B.E.; Cottrell, G.S.; Roosterman, D.; Pikios, S.; Muller, L.; Steinhoff, M. Endothelin-converting enzyme-1 regulates endosomal sorting of calcitonin receptor-like receptor and β-arrestins. J. Cell Biol. 2007, 179, 981–997. [Google Scholar] [CrossRef]
- Hilairet, S.; Bélanger, C.; Bertrand, J.; Laperrière, A.; Foord, S.M.; Bouvier, M. Agonist-promoted internalization of a ternary complex between calcitonin receptor-like receptor, receptor activity-modifying protein 1 ({RAMP}1), and beta-arrestin. J. Biol. Chem. 2001, 276, 42182–42190. [Google Scholar] [CrossRef]
- Cottrell, G.S.; Padilla, B.; Pikios, S.; Roosterman, D.; Steinhoff, M.; Grady, E.F. Post-endocytic sorting of calcitonin receptor-like receptor and receptor activity-modifying protein 1. J. Biol. Chem. 2007, 282, 12260–12271. [Google Scholar] [CrossRef]
- Manoukian, R.; Sun, H.; Miller, S.; Shi, D.; Chan, B.; Xu, C. Effects of monoclonal antagonist antibodies on calcitonin gene-related peptide receptor function and trafficking. J. Headache Pain 2019, 20. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990, 28, 183–187. [Google Scholar] [CrossRef]
- Mair, J.; Lechleitner, P.; Längle, T.; Wiedermann, C.; Dienstl, F.; Saria, A. Plasma CGRP in acute myocardial infarction. Lancet (Lond. Engl.) 1990, 335, 168. [Google Scholar] [CrossRef]
- Eysselein, V.E.; Reinshagen, M.; Patel, A.; Davis, W.; Nast, C.; Sternini, C. Calcitonin Gene—Related Peptide in Inflammatory Bowel Disease and Experimentally Induced Colitis. Ann. N. Y. Acad. Sci. 1992, 657, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.D.; Williams, T.J. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br. J. Pharmacol. 1985, 86, 855–860. [Google Scholar] [CrossRef]
- Anthony, M.; Lance, J.W. Histamine and Serotonin in Cluster Headache. Arch. Neurol. 1971, 25, 225–231. [Google Scholar] [CrossRef]
- Ottosson, A.; Edvinsson, L. Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia 1997, 17, 166–174. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Donelan, J.; Kandere-Grzybowska, K.; Konstantinidou, A. The role of mast cells in migraine pathophysiology. Brain Res. Rev. 2005, 49, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, J.C.A.; Warfvinge, K.; Krause, D.N.; Blixt, F.W.; Sheykhzade, M.; Edvinsson, L. C-fibers may modulate adjacent Aδ-fibers through axon-axon CGRP signaling at nodes of Ranvier in the trigeminal system. J. Headache Pain 2019, 20, 105. [Google Scholar] [CrossRef]
- Li, J.; Vause, C.V.; Durham, P.L. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res. 2008, 1196, 22–32. [Google Scholar] [CrossRef]
- Lingueglia, E. Acid-sensing ion channels in sensory perception. J. Biol. Chem. 2007, 282, 17325–17329. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L. Joint 1994 Wolff Award Presentation. Peripheral and central trigeminovascular activation in cat is blocked by the serotonin (5HT)-1D receptor agonist 311C90. Headache 1994, 34, 394–399. [Google Scholar] [CrossRef]
- Walker, C.S.; Conner, A.C.; Poyner, D.R.; Hay, D.L. Regulation of signal transduction by calcitonin gene-related peptide receptors. Trends Pharmacol. Sci. 2010, 31, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Disa, J.; Parameswaran, N.; Nambi, P.; Aiyar, N. Involvement of cAMP-dependent protein kinase and pertussis toxin-sensitive G-proteins in CGRP mediated JNK activation in human neuroblastoma cell line. Neuropeptides 2000, 34, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.P.; Kim, J. Exogenous CGRP upregulates profibrogenic growth factors through PKC/JNK signaling pathway in kidney proximal tubular cells. Cell Biol. Toxicol. 2018, 34, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Drissi, H.; Lasmoles, F.; Le Mellay, V.; Marie, P.J.; Lieberherr, M. Activation of phospholipase C-beta1 via Galphaq/11 during calcium mobilization by calcitonin gene-related peptide. J. Biol. Chem. 1998, 273, 20168–20174. [Google Scholar] [CrossRef]
- Nikitenko, L.L.; Blucher, N.; Fox, S.B.; Bicknell, R.; Smith, D.M.; Rees, M.C.P. Adrenomedullin and CGRP interact with endogenous calcitonin-receptor-like receptor in endothial cells and induce its desensitisation by different mechanism. J. Cell Sci. 2006, 119, 910–922. [Google Scholar] [CrossRef]
- van Dongen, R.M.; Zielman, R.; Noga, M.; Dekkers, O.M.; Hankemeier, T.; van den Maagdenberg, A.M. Migraine biomarkers in cerebrospinal fluid: A systematic review and meta-analysis. Cephalalgia 2017, 37, 49–63. [Google Scholar] [CrossRef]
- Nicolodi, M.; Del Bianco, E. Sensory neuropeptides (substance P, calcitonin gene-related peptide) and vasoactive intestinal polypeptide in human saliva: Their pattern in migraine and cluster headache. Cephalalgia Int. J. Headache 1990, 10, 39–50. [Google Scholar] [CrossRef]
- Bellamy, J.L.; Cady, R.K.; Durham, P.L. Salivary levels of CGRP and VIP in rhinosinusitis and migraine patients. Headache 2006, 46, 24–33. [Google Scholar] [CrossRef]
- Snoer, A.; Vollesen, A.L.H.; Beske, R.P.; Guo, S.; Hoffmann, J.; Fahrenkrug, J. Calcitonin-gene related peptide and disease activity in cluster headache. Cephalalgia 2019, 39, 575–584. [Google Scholar] [CrossRef]
- Asghar, M.S.; Hansen, A.E.; Amin, F.M.; van der Geest, R.J.; Koning, P.; van der Larsson, H.B.W. Evidence for a vascular factor in migraine. Ann. Neurol. 2011, 69, 635–645. [Google Scholar] [CrossRef]
- Lassen, L.; Haderslev, P.; Jacobsen, V.; Iversen, H.; Sperling, B.; Olesen, J. Cgrp May Play A Causative Role in Migraine. Cephalalgia 2002, 22, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Hauge, A.W.; Olesen, J.; Ashina, M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 2010, 30, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, M.L.; Edvinsson, L. Comparison of CGRP and NO responses in the human peripheral microcirculation of migraine and control subjects. Cephalalgia 2008, 28, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Pedersen-Bjergaard, U.; Nielsen, L.B.; Jensen, K.; Edvinsson, L.; Jansen, I.; Olesen, J. Calcitonin gene-related peptide, neurokinin A and substance P: Effects on Nociception and neurogenic inflammation in human skin and temporal muscle. Peptides 1991, 12, 333–337. [Google Scholar] [CrossRef]
- Jensen, K.; Tuxen, C.; Pedersen-Bjergaard, U.; Jansen, I. Pain, tenderness, wheal and flare induced by substance-P, bradykinin and 5-hydroxytryptamine in humans. Cephalalgia 1991, 11, 175–182. [Google Scholar] [CrossRef]
- Edvinsson, L.; Ekman, R.; Goadsby, P.J. Measurement of vasoactive neuropeptides in biological materials: Problems and pitfalls from 30 years of experience and novel future approaches. Cephalalgia 2010, 30, 761–766. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L. The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann. Neurol. 1993, 33, 48–56. [Google Scholar] [CrossRef]
- Ashina, M.; Bendtsen, L.; Jensen, R.; Schifter, S.; Olesen, J. Evidence for increased plasma levels of calcitonin gene-related peptide in migraine outside of attacks. Pain 2000, 86, 133–138. [Google Scholar] [CrossRef]
- Kamm, K.; Straube, A.; Ruscheweyh, R. Calcitonin gene-related peptide levels in tear fluid are elevated in migraine patients compared to healthy controls. Cephalalgia 2019, 39, 1535–1543. [Google Scholar] [CrossRef]
- Cady, R.K.; Vause, C.V.; Ho, T.W.; Bigal, M.E.; Durham, P.L. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache 2009, 49, 1258–1266. [Google Scholar] [CrossRef]
- Durham, P.L.; Russo, A.F. Stimulation of the calcitonin gene-related peptide enhancer by mitogen-activated protein kinases and repression by an antimigraine drug in trigeminal ganglia neurons. J. Neurosci. 2003, 23, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Messlinger, K.; Lennerz, J.K.; Eberhardt, M.; Fischer, M.J.M. CGRP and NO in the Trigeminal System: Mechanisms and Role in Headache Generation. Headache J. Head Face Pain 2012, 52, 1411–1427. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J. Pathophysiology of cluster headache: A trigeminal autonomic cephalgia. Lancet Neurol. 2002, 1, 251–257. [Google Scholar] [CrossRef]
- Bacchelli, E.; Cainazzo, M.M.; Cameli, C.; Guerzoni, S.; Martinelli, A.; Zoli, M. A genome-wide analysis in cluster headache points to neprilysin and PACAP receptor gene variants. J. Headache Pain 2016, 17, 114. [Google Scholar] [CrossRef]
- Edvinsson, L. Sensory Nerves in Man and Their Role in Primary Headaches. Cephalalgia 2001, 21, 761–764. [Google Scholar] [CrossRef]
- Csati, A.; Tajti, J.; Kuris, A.; Tuka, B.; Edvinsson, L.; Warfvinge, K. Distribution of vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, nitric oxide synthase, and their receptors in human and rat sphenopalatine ganglion. Neuroscience 2012, 202, 158–168. [Google Scholar] [CrossRef]
- Csati, A.; Tajti, J.; Tuka, B.; Edvinsson, L.; Warfvinge, K. Calcitonin gene-related peptide and its receptor components in the human sphenopalatine ganglion-Interaction with the sensory system. Brain Res. 2012, 1435, 29–39. [Google Scholar] [CrossRef]
- Ran, C.; Fourier, C.; Michalska, J.M.; Steinberg, A.; Sjöstrand, C.; Waldenlind, E. Screening of genetic variants in ADCYAP1R1, MME and 14q21 in a Swedish cluster headache cohort. J. Headache Pain 2017, 18. [Google Scholar] [CrossRef]
- Michalska, J.M.; Ran, C.; Fourier, C.; Steinberg, A.; Sjöstrand, C.; Waldenlind, E. Involvement of CGRP receptor RAMP1 in cluster headache: A Swedish case-control study. Cephalalgia Rep. 2019, 2. [Google Scholar] [CrossRef]
- Nakazato, T.; Nakayama, T.; Naganuma, T.; Sato, N.; Fu, Z.; Wang, Z. Haplotype-based case-control study of receptor (calcitonin) activity-modifying protein-1 gene in cerebral infarction. J. Hum. Hypertens 2010, 24, 351–358. [Google Scholar] [CrossRef]
- Costa, M.; Squassina, A.; Piras, I.S.; Pisanu, C.; Congiu, D.; Niola, P. Preliminary Transcriptome Analysis in Lymphoblasts from Cluster Headache and Bipolar Disorder Patients Implicates Dysregulation of Circadian and Serotonergic Genes. J. Mol. Neurosci. 2015, 56, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Sjöstrand, C.; Duvefelt, K.; Steinberg, A.; Remahl, I.N.; Waldenlind, E.; Hillert, J. Gene expression profiling in cluster headache: A pilot microarray study. Headache 2006, 46, 1518–1534. [Google Scholar] [CrossRef] [PubMed]
- Eising, E.; Pelzer, N.; Vijfhuizen, L.S.; Vries, B.; de Ferrari, M.D.; Hoen, P.A.C. Identifying a gene expression signature of cluster headache in blood. Sci. Rep. 2017, 7, 40218. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, P.; Aurilia, C.; Fofi, L.; Egeo, G.; Ferroni, P. The role of anti-CGRP antibodies in the pathophysiology of primary headaches. Neurol. Sci. 2017, 38, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Tso, A.R.; Goadsby, P.J. Anti-CGRP Monoclonal Antibodies: The Next Era of Migraine Prevention? Curr. Treat. Options Neurol. 2017, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Ashina, H.; Newman, L.; Ashina, S. Calcitonin gene-related peptide antagonism and cluster headache: An emerging new treatment. Neurol. Sci. 2017, 1–5. [Google Scholar] [CrossRef]
- Vikelis, M.; Spingos, K.C.; Rapoport, A.M. A new era in headache treatment. Neurol. Sci. 2018, 39, 47–58. [Google Scholar] [CrossRef]
- Charles, A.; Pozo-Rosich, P. Targeting calcitonin gene-related peptide: A new era in migraine therapy. Lancet 2019, 394, 1765–1774. [Google Scholar] [CrossRef]
- Ohlsson, L.; Haanes, K.A.; Kronvall, E.; Xu, C.; Snellman, J.; Edvinsson, L. Erenumab (AMG 334), a monoclonal antagonist antibody against the canonical CGRP receptor, does not impair vasodilatory or contractile responses to other vasoactive agents in human isolated cranial arteries. Cephalalgia 2019, 333102419867282. [Google Scholar] [CrossRef]
- Goadsby, P.J. Primary headache disorders: Five new things. Neurol. Clin. Pract. 2019, 9, 233–240. [Google Scholar] [CrossRef]
- Gupta, S.; Mehrotra, S.; Avezaat, C.J.J.; Villalón, C.M.; Saxena, P.R.; MaassenVanDenBrink, A. Characterisation of CGRP receptors in the human isolated middle meningeal artery. Life Sci. 2006, 79, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Edvinsson, L.; Eftekhari, S.; Kimblad, P.O.; Kane, S.A.; Lynch, J. Characterization of the calcitonin gene-related peptide receptor antagonist telcagepant (MK-0974) in human isolated coronary arteries. J. Pharmacol. Exp. Ther. 2010, 334, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Beltran, E.; Chan, K.Y.; Danser, A.J.; MaassenVanDenBrink, A.; Edvinsson, L. Characterisation of the calcitonin gene-related peptide receptor antagonists ubrogepant and atogepant in human isolated coronary, cerebral and middle meningeal arteries. Cephalalgia 2019. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Dodick, D.W.; Leone, M.; Bardos, J.N.; Oakes, T.M.; Millen, B.A. Trial of Galcanezumab in Prevention of Episodic Cluster Headache. N. Engl. J. Med. 2019, 381, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Lupi, C.; Guerzoni, S.; Negro, A.; Benemei, S. Once-monthly galcanezumab for the prevention of migraine in adults: An evidence-based descriptive review and potential place in therapy. Ther. Clin. Risk Manag. 2019, 15, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Felgenhauer, K. Protein size and cerebrospinal fluid composition. Klin. Wochenschr. 1974, 52, 1158–1164. [Google Scholar] [CrossRef]
- Lundblad, C.; Haanes, K.A.; Grände, G.; Edvinsson, L. Experimental inflammation following dural application of complete Freund’s adjuvant or inflammatory soup does not alter brain and trigeminal microvascular passage. J. Headache Pain 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Slowiczek, L. Aimovig: Dosage, side effects, uses, and more. Medical News Today. 21 March 2019. Available online: https://www.medicalnewstoday.com/articles/326056.php (accessed on 25 October 2019).
- Payesko, J. Teva Halts Development of Fremanezumab|NeurologyLive. NeurologyLive. 2019. Available online: https://www.neurologylive.com/clinical-focus/teva-halts-clinical-development-of-fremanezumab-for-cluster-headache (accessed on 25 October 2019).
- Yuan, H.; Spare, N.M.; Silberstein, S.D. Targeting CGRP for the Prevention of Migraine and Cluster Headache: A Narrative Review. Headache 2019, 59 (Suppl. 2), 20–32. [Google Scholar] [CrossRef]
- Teva Pharmaceutical Industries Ltd. Teva Announces Update on Fremanezumab Clinical Development for use in Episodic Cluster Headache n.d. Available online: https://ir.tevapharm.com/investors/press-releases/press-release-details/2019/Teva-Announces-Update-on-Fremanezumab-Clinical-Development-for-use-in-Episodic-Cluster-Headache/default.aspx (accessed on 15 November 2019).
- Giani, L.; Proietti Cecchini, A.; Leone, M. Anti-CGRP in cluster headache therapy. Neurol. Sci. 2019, 40, 129–135. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmine Belin, A.; Ran, C.; Edvinsson, L. Calcitonin Gene-Related Peptide (CGRP) and Cluster Headache. Brain Sci. 2020, 10, 30. https://doi.org/10.3390/brainsci10010030
Carmine Belin A, Ran C, Edvinsson L. Calcitonin Gene-Related Peptide (CGRP) and Cluster Headache. Brain Sciences. 2020; 10(1):30. https://doi.org/10.3390/brainsci10010030
Chicago/Turabian StyleCarmine Belin, Andrea, Caroline Ran, and Lars Edvinsson. 2020. "Calcitonin Gene-Related Peptide (CGRP) and Cluster Headache" Brain Sciences 10, no. 1: 30. https://doi.org/10.3390/brainsci10010030
APA StyleCarmine Belin, A., Ran, C., & Edvinsson, L. (2020). Calcitonin Gene-Related Peptide (CGRP) and Cluster Headache. Brain Sciences, 10(1), 30. https://doi.org/10.3390/brainsci10010030






