Anoctamin 3: A Possible Link between Cluster Headache and Ca2+ Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Genotyping
2.3. Establishment of Fibroblast Cell Lines
2.4. cDNA Preparation
2.5. Reverse Transcription qPCR (qRT-PCR)
2.6. Statistical Analysis
3. Results
3.1. Genetic Analysis
3.2. Formal Selection of Reference Genes for Studying CH in Fibroblasts
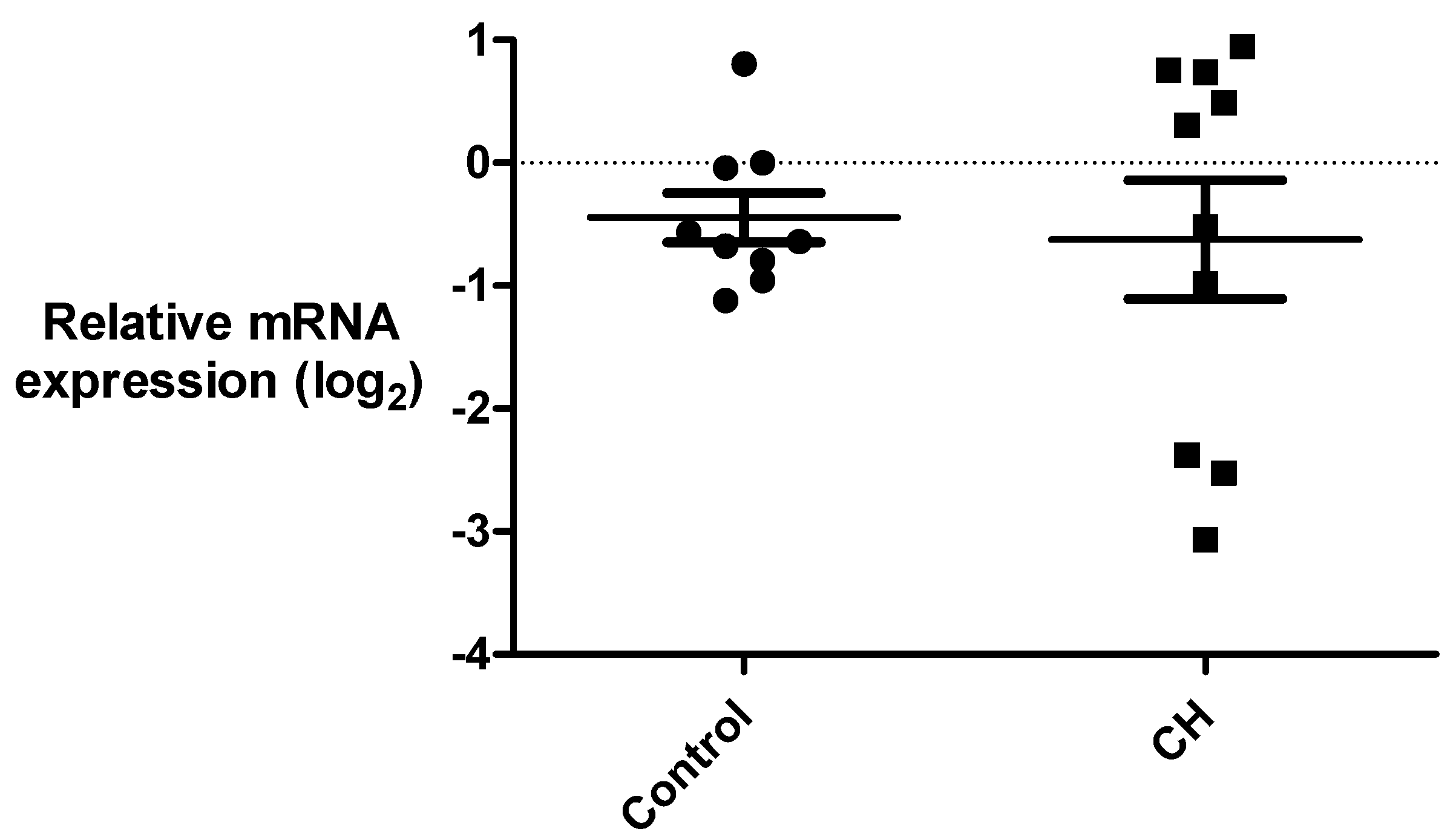
3.3. Gene Expression Analysis of ANO3 and ITGAL
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- The International Headache Society (IHS). Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Schor, L.I. IHC Electronic Posters—Saturday and Sunday: Cluster Headache: Investigating severity of pain, suicidality, personal burden, access to effective treatment, and demographics among a large International survey sample. Cephalalgia 2017, 37, 172–208. [Google Scholar] [CrossRef]
- Manzoni, G.C.; Terzano, M.G.; Bono, G.; Micieli, G.; Martucci, N.; Nappi, G. Cluster Headache—Clinical Findings in 180 Patients. Cephalalgia 1983, 3, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, A.; Fourier, C.; Ran, C.; Waldenlind, E.; Sjöstrand, C.; Belin, A.C. Cluster headache—Clinical pattern and a new severity scale in a Swedish cohort. Cephalalgia 2018, 38, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Fischera, M.; Marziniak, M.; Gralow, I.; Evers, S. The Incidence and Prevalence of Cluster Headache: A Meta-Analysis of Population-Based Studies. Cephalalgia 2008, 28, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Edvinsson, L. Human in vivo evidence for trigeminovascular activation in cluster headache Neuropeptide changes and effects of acute attacks therapies. Brain 1994, 117, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Vollesen, A.L.H.; Snoer, A.; Beske, R.P.; Guo, S.; Hoffmann, J.; Jensen, R.H. Effect of Infusion of Calcitonin Gene-Related Peptide on Cluster Headache Attacks: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 1187–1197. [Google Scholar] [CrossRef]
- Hoffmann, J.; May, A. Diagnosis, pathophysiology, and management of cluster headache. Lancet Neurol. 2018, 17, 75–83. [Google Scholar] [CrossRef]
- May, A.; Bahra, A.; Büchel, C.; Frackowiak, R.S.; Goadsby, P.J. Hypothalamic activation in cluster headache attacks. Lancet 1998, 352, 275–278. [Google Scholar] [CrossRef]
- Gibson, K.F.; Dos Santos, A.; Lund, N.; Jensen, R.; Stylianou, I.M. Genetics of cluster headache. Cephalalgia 2019. [Google Scholar] [CrossRef]
- Rainero, I.; Gallone, S.; Valfrè, W.; Ferrero, M.; Angilella, G.; Rivoiro, C.; Rubino, E.; De Martino, P.; Savi, L.; Ferrone, M.; et al. A polymorphism of the hypocretin receptor 2 gene is associated with cluster headache. Neurology 2004, 63, 1286–1288. [Google Scholar] [CrossRef] [PubMed]
- Rainero, I.; Rubino, E.; Gallone, S.; Fenoglio, P.; Negro, E.; De Martino, P.; Savi, L.; Pinessi, L. Cluster Headache is Associated With theAlcohol Dehydrogenase 4(ADH4) Gene. Headache J. Head Face Pain 2010, 50, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Fourier, C.; Ran, C.; Zinnegger, M.; Johansson, A.S.; Sjöstrand, C.; Waldenlind, E.; Steinberg, A.; Belin, A.C. A genetic CLOCK variant associated with cluster headache causing increased mRNA levels. Cephalalgia 2018, 38, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Ran, C.; Fourier, C.; Zinnegger, M.; Steinberg, A.; Sjöstrand, C.; Waldenlind, E.; Belin, A.C. Implications for the migraine SNP rs1835740 in a Swedish cluster headache population. J. Headache Pain 2018, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Sjöstrand, C.; Modin, H.; Masterman, T.; Ekbom, K.; Waldenlind, E.; Hillert, J. Analysis of Nitric Oxide Synthase Genes in Cluster Headache. Cephalalgia 2002, 22, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Weller, C.M.; Wilbrink, L.A.; Houwing-Duistermaat, J.J.; Koelewijn, S.C.; Vijfhuizen, L.S.; Haan, J.; Ferrari, M.D.; Terwindt, G.M.; van den Maagdenberg, A.M.; de Vries, B. Cluster headache and the hypocretin receptor 2 reconsidered: A genetic association study and meta-analysis. Cephalalgia 2015, 35, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Rainero, I.; Rivoiro, C.; Gallone, S.; Valfrè, W.; Ferrero, M.; Angilella, G.; Rubino, E.; De Martino, P.; Savi, L.; Giudice, R.L.; et al. Lack of Association between the 3092 T°C Clock Gene Polymorphism and Cluster Headache. Cephalalgia 2005, 25, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Cevoli, S.; Mochi, M.; Pierangeli, G.; Zanigni, S.; Grimaldi, D.; Bonavina, G.; Torelli, P.; Manzoni, G.C.; Cortelli, P.; Montagna, P. Investigation of the T3111C CLOCK gene polymorphism in cluster headache. J. Neurol. 2008, 255, 299–300. [Google Scholar] [CrossRef]
- Fourier, C.; Ran, C.; Steinberg, A.; Sjöstrand, C.; Waldenlind, E.; Belin, A.C. Screening of Two ADH4 Variations in a Swedish Cluster Headache Case–Control Material. Headache J. Head Face Pain 2016, 56, 835–840. [Google Scholar] [CrossRef]
- Fan, Z.; Hou, L.; Wan, D.; Ao, R.; Zhao, D.; Yu, S. Genetic association of HCRTR2, ADH4 and CLOCK genes with cluster headache: A Chinese population-based case-control study. J. Headache Pain 2018, 19, 1. [Google Scholar] [CrossRef]
- Fourier, C.; Ran, C.; Steinberg, A.; Sjöstrand, C.; Waldenlind, E.; Belin, A.C. Analysis of HCRTR2 Gene Variants and Cluster Headache in Sweden. Headache J. Head Face Pain 2019, 59, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Tuka, B.; Szabó, N.; Tóth, E.; Kincses, Z.T.; Párdutz, Á.; Szok, D.; Körtési, T.; Bagoly, T.; Helyes, Z.; Edvinsson, L.; et al. Release of PACAP-38 in episodic cluster headache patients – an exploratory study. J. Headache Pain 2016, 17, 251. [Google Scholar] [CrossRef] [PubMed]
- Bacchelli, E.; Cainazzo, M.M.; Cameli, C.; Guerzoni, S.; Martinelli, A.; Zoli, M.; Maestrini, E.; Pini, L.A. A genome-wide analysis in cluster headache points to neprilysin and PACAP receptor gene variants. J. Headache Pain 2016, 17, 279. [Google Scholar] [CrossRef] [PubMed]
- Ran, C.; Fourier, C.; Michalska, J.M.; Steinberg, A.; Sjöstrand, C.; Waldenlind, E.; Belin, A.C. Screening of genetic variants in ADCYAP1R1, MME and 14q21 in a Swedish cluster headache cohort. J. Headache Pain 2017, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Tfelt-Hansen, P.C.; Tfelt-Hansen, D.P.C.; Jensen, R.H. Management of Cluster Headache. CNS Drugs 2012, 26, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Markley, H.G. Verapamil and migraine prophylaxis: Mechanisms and efficacy. Am. J. Med. 1991, 90, S48–S53. [Google Scholar] [CrossRef]
- Elliott, W.J.; Ram, C.V.S. Calcium Channel Blockers. J. Clin. Hypertens. 2011, 13, 687–689. [Google Scholar] [CrossRef]
- Kuga, T.; Sadoshima, J.; Tomoike, H.; Kanaide, H.; Akaike, N.; Nakamura, M. Actions of Ca2+ antagonists on two types of Ca2+ channels in rat aorta smooth muscle cells in primary culture. Circ. Res. 1990, 67, 469–480. [Google Scholar] [CrossRef]
- Freeze, B.S.; McNulty, M.M.; Hanck, D.A. State-dependent verapamil block of the cloned human Ca(v)3.1 T-type Ca(2+) channel. Mol. Pharmacol. 2006, 70, 718–726. [Google Scholar] [CrossRef]
- Diochot, S.; Richard, S.; Baldy-Moulinier, M.; Nargeot, J.; Valmier, J. Dihydropyridines, phenylalkylamines and benzothiazepines block N-, P/Q- and R-type calcium currents. Pflugers. Arch. 1995, 431, 10–19. [Google Scholar] [CrossRef]
- Miranda, H.F.; Bustamante, D.; Kramer, V.; Pelissier, T.; Saavedra, H.; Paeile, C.; Fernandez, E.; Pinardi, G. Antinociceptive effects of Ca2+ channel blockers. Eur. J. Pharmacol. 1992, 217, 137–141. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Z.; Gong, Q.; Makielski, J.C.; January, C.T. Mechanism of Block and Identification of the Verapamil Binding Domain to HERG Potassium Channels. Circ. Res. 1999, 84, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, P.; Antoine, M.-H.; Ouedraogo, R.; Pirotte, B.; Herchuelz, A.; Cosgrove, K.E.; Fernandez, E.; Pinardi, G. Verapamil, a phenylalkylamine Ca2+ channel blocker, inhibits ATP-sensitive K+ channels in insulin-secreting cells from rats. Diabetologia 1997, 40, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- DeCoursey, T.E. Mechanism of K+ channel block by verapamil and related compounds in rat alveolar epithelial cells. J. Gen. Physiol. 1995, 106, 745–779. [Google Scholar] [CrossRef] [PubMed]
- Huffaker, S.J.; Chen, J.; Nicodemus, K.K.; Sambataro, F.; Yang, F.; Mattay, V.; Lipska, B.K.; Hyde, T.M.; Song, J.; Rujescu, D.; et al. A novel, primate-specific, brain isoform of KCNH2 impacts cortical physiology, cognition, neuronal repolarization and risk for schizophrenia. Nat. Med. 2009, 15, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Cao, Y.-Q. Over-Expression of TRESK K+ Channels Reduces the Excitability of Trigeminal Ganglion Nociceptors. PLoS ONE 2014, 9, e87029. [Google Scholar] [CrossRef] [PubMed]
- Lafrenière, R.G.; Cader, M.Z.; Poulin, J.-F.; Andres-Enguix, I.; Simoneau, M.; Gupta, N.; Boisvert, K.; Lafrenière, F.; McLaughlan, S.; Dubé, M.-P.; et al. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat. Med. 2010, 16, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Karch, R.; Zeitlinger, M.; Philippe, C.; Römermann, K.; Stanek, J.; Maier-Salamon, A.; Wadsak, W.; Jäger, W.; Hacker, M.; et al. Approaching complete inhibition of P-glycoprotein at the human blood–brain barrier: An (R)-[11C]verapamil PET study. Br. J. Pharmacol. 2015, 35, 743–746. [Google Scholar] [CrossRef]
- Römermann, K.; Wanek, T.; Bankstahl, M.; Bankstahl, J.P.; Fedrowitz, M.; Müller, M.; Löscher, W.; Kuntner, C.; Langer, O. (R)-[11C]verapamil is selectively transported by murine and human P-glycoprotein at the blood–brain barrier, and not by MRP1 and BCRP. Nucl. Med. Boil. 2013, 40, 873–878. [Google Scholar] [CrossRef]
- Safa, A.R. Photoaffinity labeling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proc. Natl. Acad. Sci. USA 1988, 85, 7187–7191. [Google Scholar] [CrossRef]
- Tsuruo, T.; Iida, H.; Tsukagoshi, S.; Sakurai, Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982, 42, 4730–4733. [Google Scholar]
- Yusa, K.; Tsuruo, T. Reversal mechanism of multidrug resistance by verapamil: Direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Res. 1989, 49, 5002–5006. [Google Scholar]
- Cutrer, F.M.; Klein, C.J.; Atkinson, E.J. IHC Oral Abstracts: Genomic variants related to Verapamil response in the treatment of Migraine. Cephalalgia 2017, 37, 1–24. [Google Scholar] [CrossRef]
- The International Headache Society (IHS). The international classification of headache disorders: 2nd edition. Cephalalgia 2004, 24. [Google Scholar] [CrossRef]
- Takashima, A. Establishment of Fibroblast Cultures. Curr. Protoc. Cell Boil. 1998, 2, 2.1.1–2.1.12. [Google Scholar] [CrossRef]
- Johansson, A.-S.; Owe-Larsson, B.; Hetta, J.; Lundkvist, G.B. Altered circadian clock gene expression in patients with schizophrenia. Schizophr. Res. 2016, 174, 17–23. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Dupont, W.D.; Plummer, W.D., Jr.; Plummer, W.D. Power and sample size calculations. A review and computer program. Control. Clin. Trials 1990, 11, 116–128. [Google Scholar] [CrossRef]
- Rodriguez, S.; Gaunt, T.R.; Day, I.N.M. Hardy-Weinberg Equilibrium Testing of Biological Ascertainment for Mendelian Randomization Studies. Am. J. Epidemiol. 2008, 169, 505–514. [Google Scholar] [CrossRef]
- Kumar, S.; Ambrosini, G.; Bucher, P. SNP2TFBS—A database of regulatory SNPs affecting predicted transcription factor binding site affinity. Nucleic. Acids Res. 2017, 45, D139–D144. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Boil. 2002, 3, 0034-1. [Google Scholar]
- Sjöstrand, C.; Giedratis, V.; Ekbom, K.; Waldenlind, E.; Hillert, J. CACNA1A Gene Polymorphisms in Cluster Headache. Cephalalgia 2001, 21, 953–958. [Google Scholar] [CrossRef]
- Haan, J.; Van Vliet, J.; Kors, E.; Terwindt, G.; Vermeulen, F.; Maagdenberg, A.V.D.; Frants, R.; Ferrari, M. No Involvement of the Calcium Channel Gene (CACNA1A) in a Family with Cluster Headache. Cephalalgia 2001, 21, 959–962. [Google Scholar] [CrossRef]
- Charlesworth, G.; Plagnol, V.; Holmström, K.M.; Bras, J.; Sheerin, U.-M.; Preza, E.; Rubio-Agusti, I.; Ryten, M.; Schneider, S.A.; Stamelou, M.; et al. Mutations in ANO3 Cause Dominant Craniocervical Dystonia: Ion Channel Implicated in Pathogenesis. Am. J. Hum. Genet. 2012, 91, 1041–1050. [Google Scholar] [CrossRef]
- Lohmann, K.; Klein, C. Update on the Genetics of Dystonia. Curr. Neurol. Neurosci. Rep. 2017, 17, 1090. [Google Scholar] [CrossRef]
- Picollo, A.; Malvezzi, M.; Accardi, A. TMEM16 Proteins: Unknown Structure and Confusing Functions. J. Mol. Biol. 2015, 427, 94–105. [Google Scholar] [CrossRef]
- Duran, C.; Qu, Z.; Osunkoya, A.O.; Cui, Y.; Hartzell, H.C. ANOs 3–7 in the anoctamin/Tmem16 Cl-channel family are intracellular proteins. Am. J. Physiol. Physiol. 2012, 302, C482–C493. [Google Scholar] [CrossRef]
- Huang, F.; Wang, X.; Ostertag, E.M.; Nuwal, T.; Huang, B.; Jan, Y.-N.; Basbaum, A.I.; Jan, L.Y. TMEM16C facilitates Na+-activated K+ currents in rat sensory neurons and regulates pain processing. Nat. Neurosci. 2013, 16, 1284–1290. [Google Scholar] [CrossRef]

| Controls | CH Patients | |
|---|---|---|
| Individuals | 586 | 628 |
| Average age in years ± SD | n/a | 52.1 ± 14.6 |
| Male % (n) | 60.6 (355) | 68.3 (429) |
| Chronic CH % (n) | n/a | 10.6 (66) |
| Average age at onset ± SD | n/a | 32.0 ± 13.6 |
| Positive family history % (n) | n/a | 10.8 § (61) |
| Prophylactic verapamil use % (n) | n/a | 30.4 (191) |
| Diurnal rhythmicity % (n) | n/a | 68.0 † (380) |
| Gene | SNP rs Number (HGVS) | Consequence | TaqMan Assay |
|---|---|---|---|
| PCDHB6 | rs17844444 (NC_000005.10:g.141152584G>A) | Missense Gly→Asp | C__32960006_10 |
| PLCE1 | rs10882386 (NC_000010.10:g.95790669G>A) | Unknown (5′UTR) | C___1946626_10 |
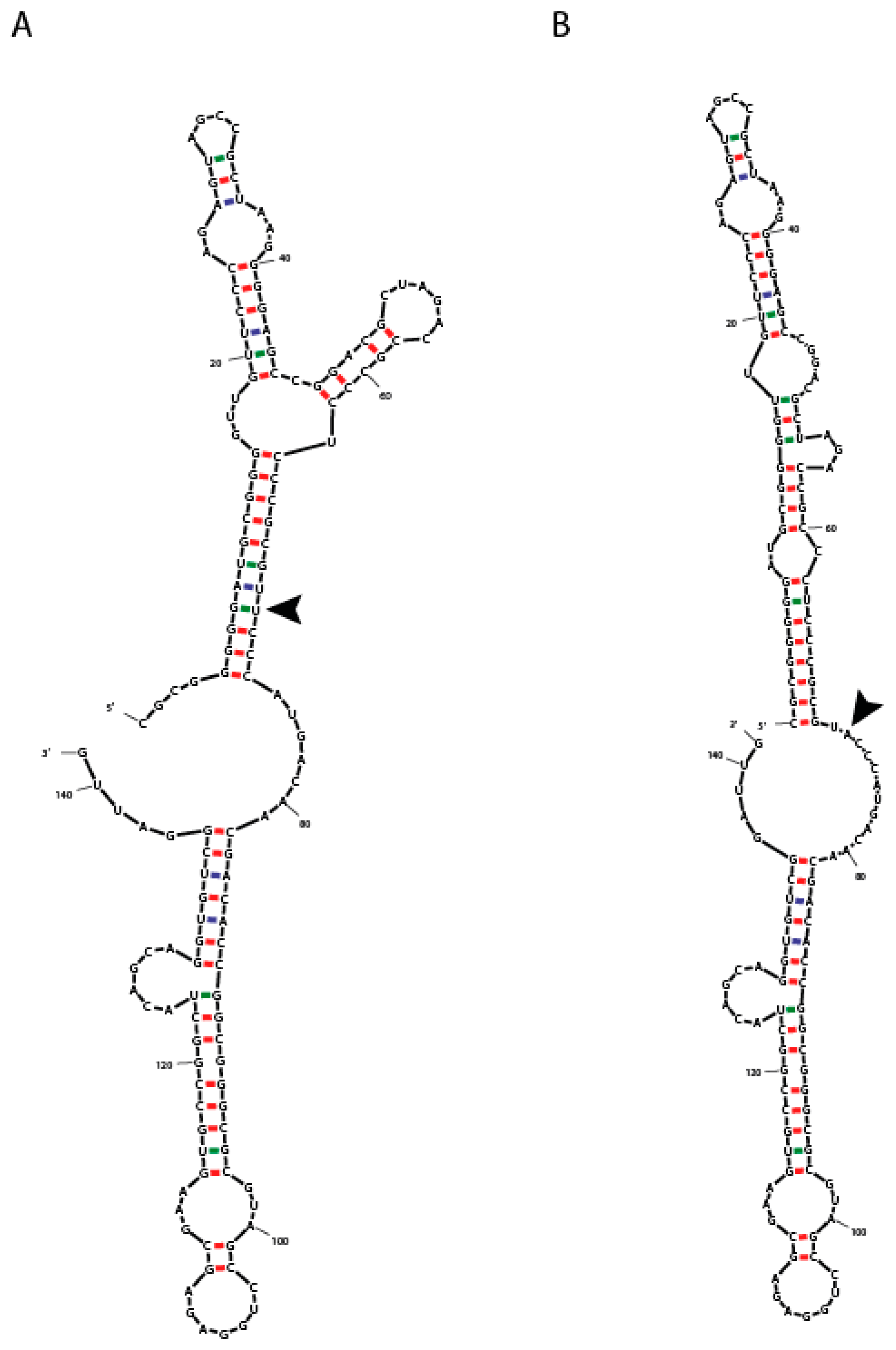
| ANO3 | rs1531394 (NC_000011.10:g.26332096T>A) | Unknown (5′UTR) | C___1616984_10 |
| ITGAL | rs2230433 (NC_000016.10:g.30506720G>C) | Missense Arg→Thr | C__11789692_10 |
| Gene of Interest | Forward Primer 5′-3′ | Reverse Primer 5′-3′ | |
| Anoctamin 3 | ANO3 | CCTGGAGTTTTGGAAAAGGAGA | CTTGGCTTCAAACTGGGGAC |
| Integrin Subunit α L | ITGAL | CACTCTATGTCAGTTTCACCCC | GTTGTGGTCGTGGATGGAAG |
| Reference Gene | Forward Primer 5′-3′ | Reverse Primer 5′-3′ | |
| Glyceraldehyde-3-Phosphate Dehydrogenase | GAPDH | AGCCACATCGCTCAGACAC | GCCCAATACGACCAAATCC |
| Importin 8 | IPO8 | ATTGGAAGAAACCGCGCTTG | TGTGTACACCTCCTGCAGTG |
| Peptidylprolyl Isomerase A | PPIA | GACCCAACACAAATGGTTCC | GGCCTCCACAATATTCATGC |
| Pyruvate Dehydrogenase E1 β Subunit | PDHB | GGTTTCCCATTCAAGACCTG | TGGTTTCCATGTCCATTGGT |
| TATA-Box Binding Protein | TBP | AGGCAACACAGGGAACCTC | TTGCAGCTGCGGTACAATCC |
| SNP Name | Genotype/Allele | Control % (n) | CH % (n) | χ2 (df) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| rs17844444 | GG | 75.30 (439) | 75.41 (466) | |||
| GA | 22.64 (132) | 21.52 (133) | ||||
| AA | 2.06 (12) | 3.07 (19) | 1.37 (2) | 0.50 | ||
| G | 86.62 (1010) | 86.17 (1065) | 1.04 | |||
| A | 13.38 (156) | 13.84 (171) | 0.071 (1) | (1.82–1.31) | 0.79 | |
| rs10882386 | GG | 55.36 (320) | 56.64 (354) | |||
| GA | 39.62 (229) | 36.80 (230) | ||||
| AA | 5.02 (29) | 6.56 (41) | 1.94 (2) | 0.38 | ||
| G | 75.17 (869) | 75.04 (938) | 1.007 | |||
| A | 24.83 (287) | 24.96 (312) | 0.0008 (1) | (0.84–1.21) | 0.98 | |
| rs1531394 | TT | 37.29 (217) | 38.28 (240) | |||
| TA | 48.97 (285) | 42.27 (265) | ||||
| AA | 13.75 (80) | 19.46 (122) | 9.28 (2) | 0.0097 * | ||
| T | 61.77 (719) | 59.41 (745) | 1.10 | |||
| A | 38.23 (445) | 40.59 (509) | 1.31 (1) | (0.94–1.30) | 0.25 | |
| rs2230433 | GG | 51.55 (299) | 57.44 (359) | |||
| GC | 40.69 (236) | 35.52 (222) | ||||
| CC | 7.76 (45) | 7.04 (44) | 4.24 (2) | 0.12 | ||
| G | 71.90 (834) | 75.2 (940) | 0.84 | |||
| C | 28.10 (326) | 24.8 (310) | 3.38 (1) | (0.70–1.011) | 0.066 |
| Genotype | Control % (n) | All CH Patients % (n) | CH Using Verapamil % (n) | CH not Using Verapamil % (n) | OR (95%CI) | p-Value |
|---|---|---|---|---|---|---|
| TT + TA | 86.25 (502) | 80.54 (505) | 1.56 | |||
| AA | 13.75 (80) | 19.46 (122) | 1.11–2.06 | 0.0086 | ||
| Stratified Analysis | ||||||
| TT + TA | 79.06 (151) | 1.66 | ||||
| AA | 20.94 (40) | 1.09–2.53 | 0.021 | |||
| TT + TA | 81.19 (354) | 1.45 | ||||
| AA | 18.81 (82) | 1.04–2.04 | 0.031 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, C.; Fourier, C.; Arafa, D.; Liesecke, F.; Sjöstrand, C.; Waldenlind, E.; Steinberg, A.; Belin, A.C. Anoctamin 3: A Possible Link between Cluster Headache and Ca2+ Signaling. Brain Sci. 2019, 9, 184. https://doi.org/10.3390/brainsci9080184
Ran C, Fourier C, Arafa D, Liesecke F, Sjöstrand C, Waldenlind E, Steinberg A, Belin AC. Anoctamin 3: A Possible Link between Cluster Headache and Ca2+ Signaling. Brain Sciences. 2019; 9(8):184. https://doi.org/10.3390/brainsci9080184
Chicago/Turabian StyleRan, Caroline, Carmen Fourier, Donia Arafa, Franziska Liesecke, Christina Sjöstrand, Elisabet Waldenlind, Anna Steinberg, and Andrea Carmine Belin. 2019. "Anoctamin 3: A Possible Link between Cluster Headache and Ca2+ Signaling" Brain Sciences 9, no. 8: 184. https://doi.org/10.3390/brainsci9080184
APA StyleRan, C., Fourier, C., Arafa, D., Liesecke, F., Sjöstrand, C., Waldenlind, E., Steinberg, A., & Belin, A. C. (2019). Anoctamin 3: A Possible Link between Cluster Headache and Ca2+ Signaling. Brain Sciences, 9(8), 184. https://doi.org/10.3390/brainsci9080184








