TGF-β Promotes the Proliferation of Microglia In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell-Proliferation Assay
2.3. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.4. Cell Death-Detection Assay
2.5. Cell Viability Analysis
2.6. Statistical Analysis
3. Results
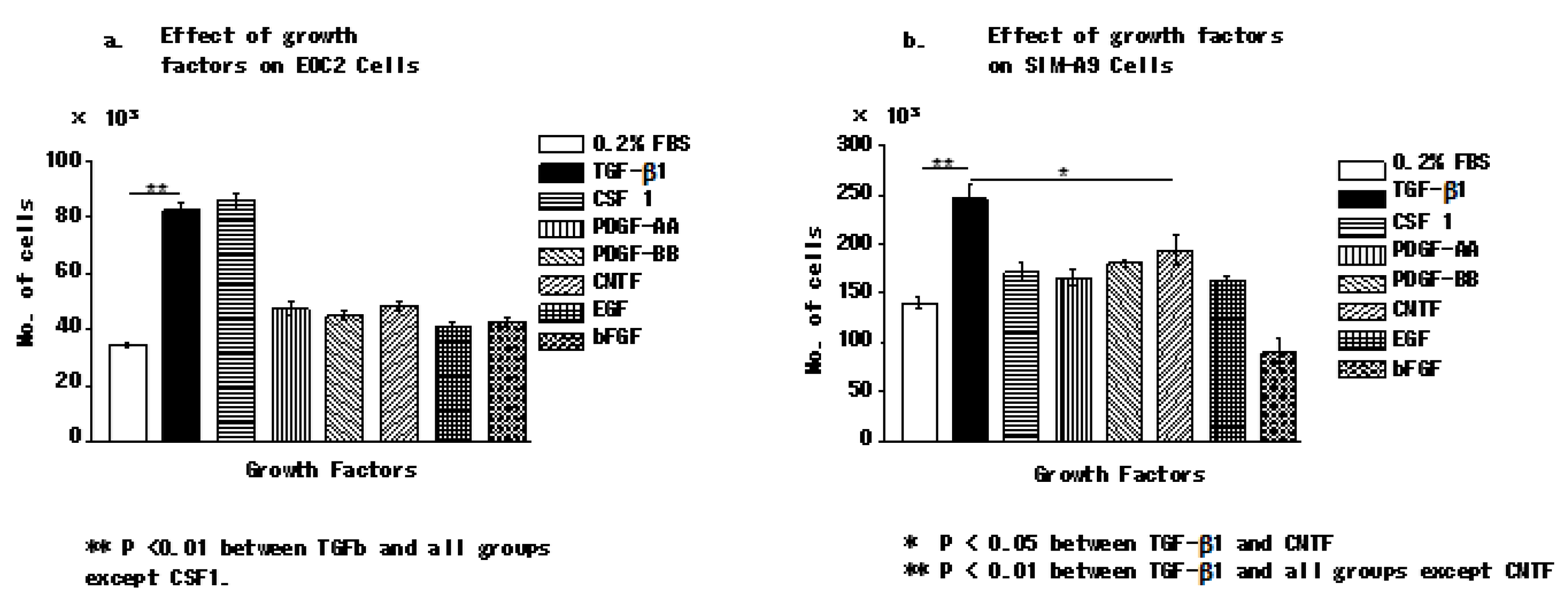
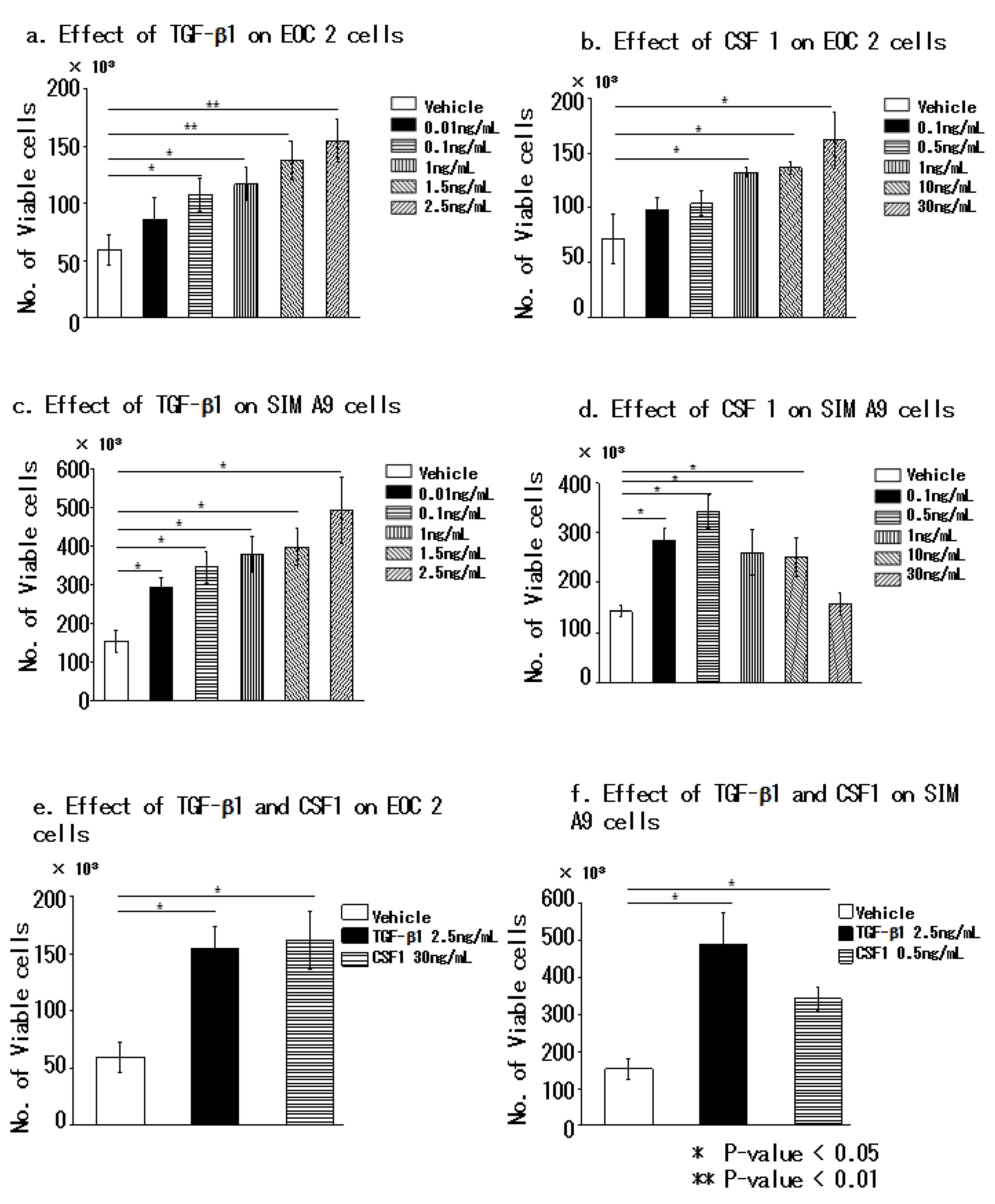
3.1. Transforming Growth Factor Beta 1 Increases the Number of Microglial Cells In Vitro
3.2. Transforming Growth Factor Beta 1 and Colony-Stimulating Factor 1 Effectively Promote the Proliferation of Microglial Cells
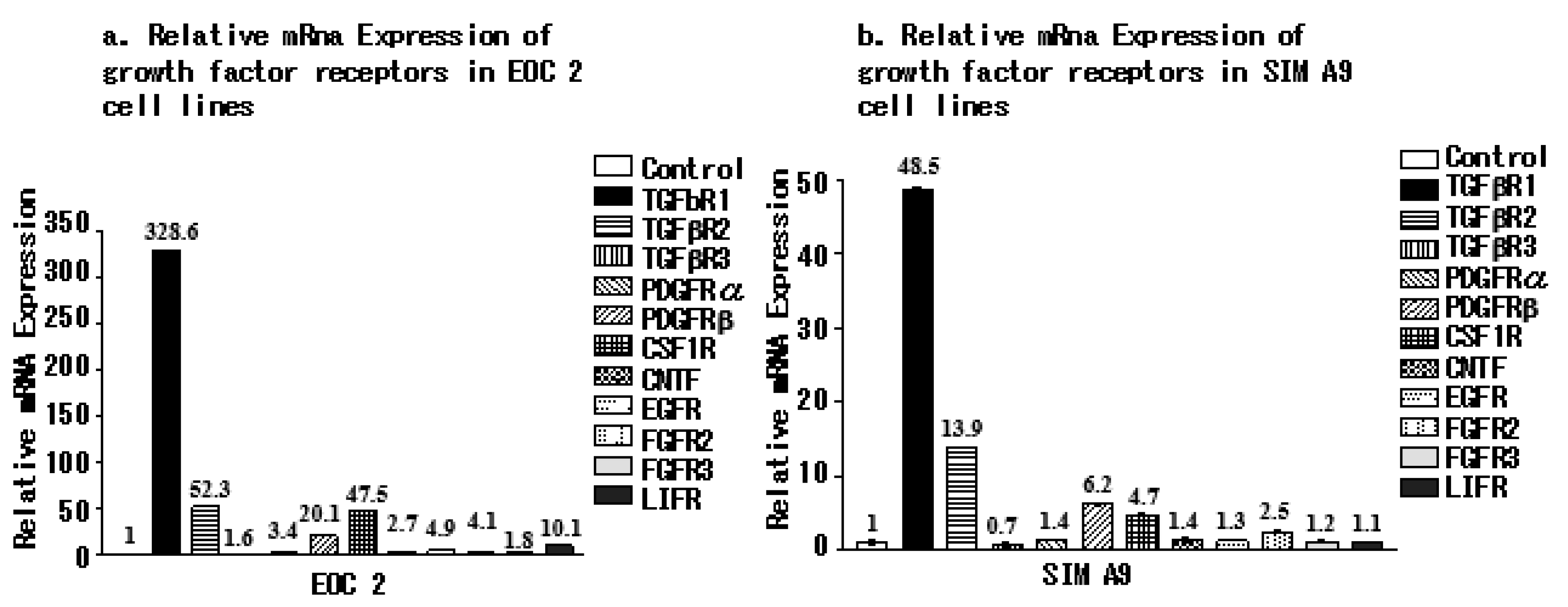
3.3. Microglial Cell Lines Express Different Growth Factor Receptors
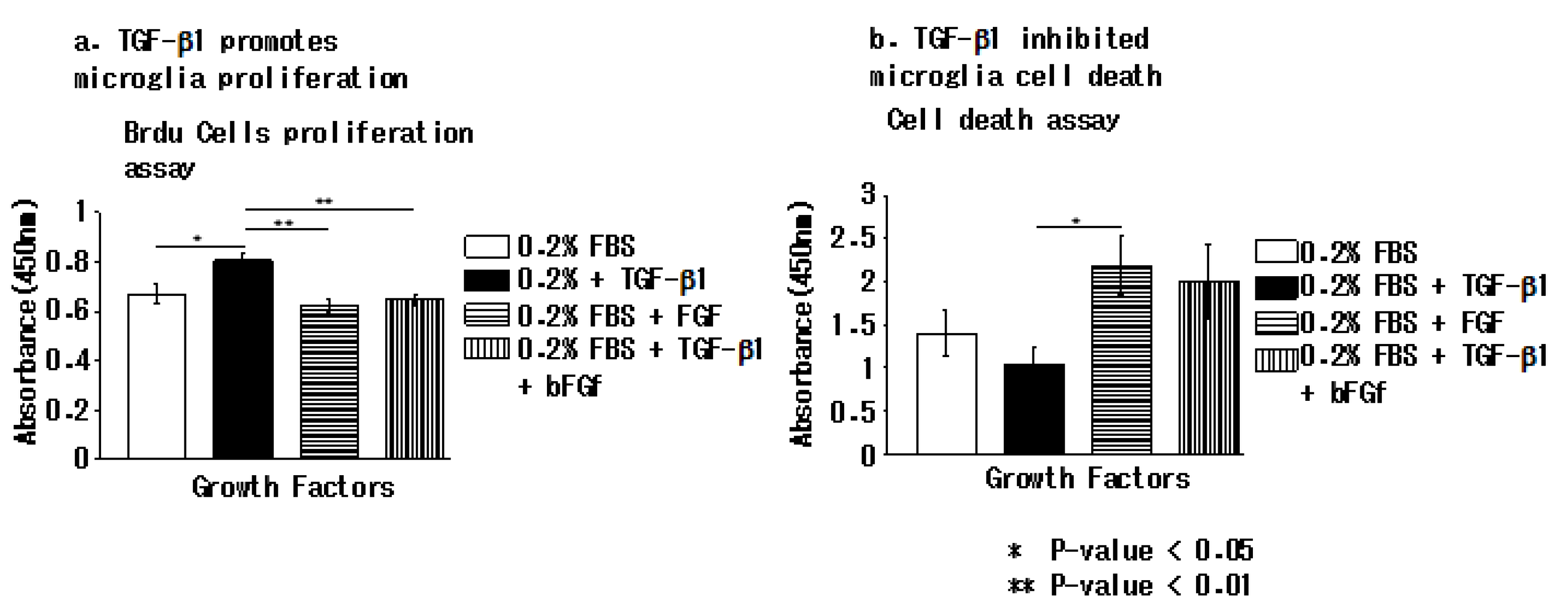
3.4. TGFβ-1 Inhibits Apoptotic Cell Death in EOC 2 Cells In Vitro
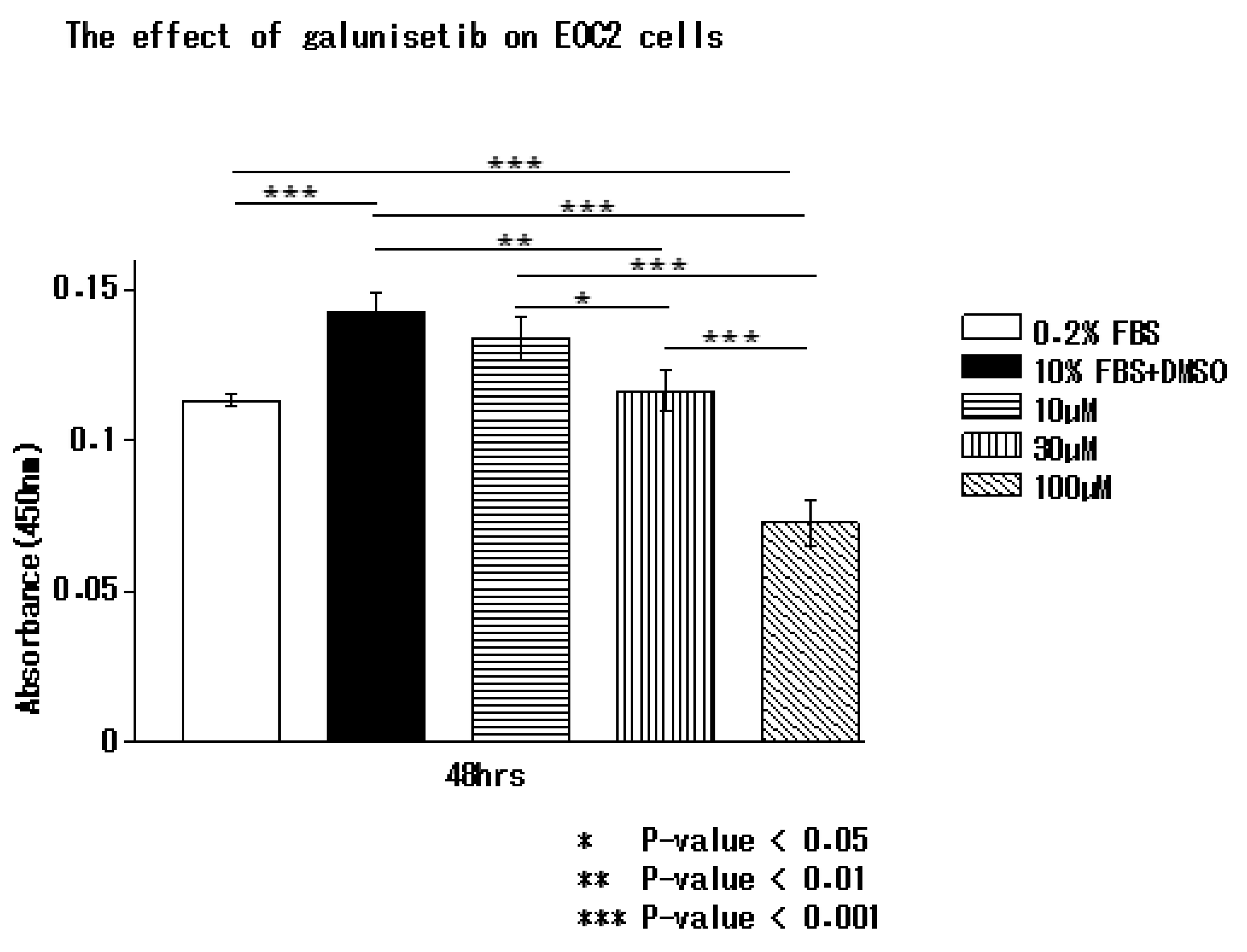
3.5. Inhibition of TGF-β1 by Galunisertib Reduces Microglial Proliferation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| bFGF | basic fibroblast growth factor |
| BrdU | 5-bromo-2′-deoxyuridine |
| CSF 1 | colony stimulating factor 1 |
| CNS | central nervous system |
| CNTF | ciliary neurotrophic factor |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EGF | epidermal growth factor |
| ELISA | enzyme-linked immunosorbent assay |
| FBS | fetal bovine serum |
| PDGF-AA | platelet-derived growth factor-AA |
| PDGF-BB | platelet-derived growth factor-BB |
| TGF-β | transforming growth factor β |
| TGFβRI | transforming growth factor β receptor type I |
References
- Nakajima, K.; Kohsaka, S. Microglia: Activation and Their Significance in the Central Nervous System. J. Biochem. 2001, 130, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.L.; Schafer, D.P. Microglia: Architects of the Developing Nervous System. Trends Cell Biol. 2016, 26, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Hong, J.-S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-M.; Hong, J.-S. Why neurodegenerative diseases are progressive: Uncontrolled inflammation drives disease progression. Trends Immunol. 2008, 29, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Beggs, S.; Salter, M.W. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav. Immun. 2007, 21, 624–633. [Google Scholar] [CrossRef]
- Kim, D.; Kim, M.A.; Cho, I.H.; Kim, M.S.; Lee, S.; Jo, E.K.; Choi, S.Y.; Park, K.; Kim, J.S.; Akira, S.; et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J. Biol. Chem. 2007, 282, 14975–14983. [Google Scholar] [CrossRef]
- Annes, J.P.; Munger, J.S.; Rifkin, D.B. Making sense of latent TGFβ activation. J. Cell Sci. 2003, 116, 217–224. [Google Scholar] [CrossRef]
- Schlapbach, R.; Spanaus, K.S.; Malipiero, U.; Lens, S.; Tasinato, A.; Tschopp, J.; Fontana, A. TGF-β induces the expression of the FLICE-inhibitory protein and inhibits Fas-mediated apoptosis of microglia. Eur. J. Immunol. 2000, 30, 3680–3688. [Google Scholar] [CrossRef]
- Sporn, M.B.; Roberts, A.B. Transforming growth factor-beta: Recent progress and new challenges. J. Cell Biol. 1992, 119, 1017–1021. [Google Scholar] [CrossRef]
- Herbertz, S.; Sawyer, J.S.; Stauber, A.J.; Gueorguieva, I.; Driscoll, K.E.; Estrem, S.T.; Cleverly, A.L.; Desaiah, D.; Guba, S.C.; Benhadji, K.A.; et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des. Dev. Ther. 2015, 9, 4479–4499. [Google Scholar]
- Hirotsu, M.; Setoguchi, T.; Matsunoshita, Y.; Sasaki, H.; Nagao, H.; Gao, H.; Sugimura, K.; Komiya, S. Tumour formation by single fibroblast growth factor receptor 3-positive rhabdomyosarcoma-initiating cells. Br. J. Cancer 2009, 101, 2030–2037. [Google Scholar] [CrossRef]
- Nakamura, S.; Nagano, S.; Nagao, H.; Ishidou, Y.; Yokouchi, M.; Abematsu, M.; Yamamoto, T.; Komiya, S.; Setoguchi, T. Arsenic trioxide prevents osteosarcoma growth by inhibition of GLI transcription via DNA damage accumulation. PLoS ONE 2013, 8, e69466. [Google Scholar] [CrossRef]
- Sawada, M.; Suzumura, A.; Yamamoto, H.; Marunouchi, T. Activation and proliferation of the isolated microglia by colony stimulating factor-1 and possible involvement of protein kinase C. Brain Res. 1990, 509, 119–124. [Google Scholar] [CrossRef]
- Giulian, D.; Ingeman, J. Colony-stimulating factors as promoters of ameboid microglia. J. Neurosci. 1988, 8, 4707–4717. [Google Scholar] [CrossRef]
- Flanders, K.C.; Ren, R.F.; Lippa, C.F. Transforming Growth Factor-βS in Neurodegenerative Disease. Prog. Neurobiol. 1998, 54, 71–85. [Google Scholar] [CrossRef]
- Buckwalter, M.S.; Wyss-Coray, T. Modelling neuroinflammatory phenotypes in vivo. J. Neuroinflamm. 2004, 1, 10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doyle, K.P.; Cekanaviciute, E.; Mamer, L.E.; Buckwalter, M.S. TGFβ signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J. Neuroinflamm. 2010, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, K.; Tada, T. Elevation of transforming growth factor-beta 1 level in cerebrospinal fluid of patients with communicating hydrocephalus after subarachnoid hemorrhage. Stroke 1994, 25, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, T.; Tada, T.; Kitazawa, K.; Tanaka, Y.; Hongo, K.; Kameko, M.; Uemura, K.I. Inflammatory cytokine cascade released by leukocytes in cerebrospinal fluid after subarachnoid hemorrhage. Neurol. Res. 2001, 23, 724–730. [Google Scholar] [CrossRef]
- Suthanthiran, M.; Li, B.; Song, J.O.; Ding, R.; Sharma, V.K.; Schwartz, J.E.; August, P. Transforming growth factor-β(1) hyperexpression in African-American hypertensives: A novel mediator of hypertension and/or target organ damage. Proc. Natl. Acad. Sci. USA 2000, 97, 3479–3484. [Google Scholar]
- Pittet, J.F.; Griffiths, M.J.; Geiser, T.; Kaminski, N.; Dalton, S.L.; Huang, X.; Brown, L.A.; Gotwals, P.J.; Koteliansky, V.E.; Matthay, M.A.; et al. TGF-β is a critical mediator of acute lung injury. J. Clin. Investig. 2001, 107, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.M.; Sigurdson, S.L.; Sheppard, D.; Lwebuga-Mukasa, J.S. Differential Modulation of Integrin Receptors and Extracellular Matrix Laminin by Transforming Growth Factor-β1 in Rat Alveolar Epithelial Cells. Exp. Cell Res. 1995, 221, 385–394. [Google Scholar] [CrossRef]
- Border, W.A.; Noble, N.A. TGF-beta in kidney fibrosis: A target for gene therapy. Kidney Int. 1997, 51, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Chalazonitis, A.; Kalberg, J.; Twardzik, D.R.; Morrison, R.S.; Kessler, J.A. Transforming growth factor beta has neurotrophic actions on sensory neurons in vitro and is synergistic with nerve growth factor. Dev. Biol. 1992, 152, 121–132. [Google Scholar] [CrossRef]
- Manaenko, A.; Lekic, T.; Barnhart, M.; Hartman, R.; Zhang, J.H. Inhibition of transforming growth factor-β attenuates brain injury and neurological deficits in a rat model of germinal matrix hemorrhage. Stroke 2014, 45, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Guardia Clausi, M.; Levison, S.W. Delayed ALK5 inhibition improves functional recovery in neonatal brain injury. J. Cereb. Blood Flow Metab. 2017, 37, 787–800. [Google Scholar] [CrossRef]
- Suzumura, A.; Sawada, M.; Yamamoto, H.; Marunouchi, T. Transforming growth factor-beta suppresses activation and proliferation of microglia in vitro. J. Immunol. 1993, 151, 2150–2158. [Google Scholar]
- Rozovsky, I.; Finch, C.E.; Morgan, T.E. Age-Related Activation of Microglia and Astrocytes: In Vitro Studies Show Persistent Phenotypes of Aging, Increased Proliferation, and Resistance to Down-Regulation. Neurobiol. Aging 1998, 19, 97–103. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bureta, C.; Setoguchi, T.; Saitoh, Y.; Tominaga, H.; Maeda, S.; Nagano, S.; Komiya, S.; Yamamoto, T.; Taniguchi, N. TGF-β Promotes the Proliferation of Microglia In Vitro. Brain Sci. 2020, 10, 20. https://doi.org/10.3390/brainsci10010020
Bureta C, Setoguchi T, Saitoh Y, Tominaga H, Maeda S, Nagano S, Komiya S, Yamamoto T, Taniguchi N. TGF-β Promotes the Proliferation of Microglia In Vitro. Brain Sciences. 2020; 10(1):20. https://doi.org/10.3390/brainsci10010020
Chicago/Turabian StyleBureta, Costansia, Takao Setoguchi, Yoshinobu Saitoh, Hiroyuki Tominaga, Shingo Maeda, Satoshi Nagano, Setsuro Komiya, Takuya Yamamoto, and Noboru Taniguchi. 2020. "TGF-β Promotes the Proliferation of Microglia In Vitro" Brain Sciences 10, no. 1: 20. https://doi.org/10.3390/brainsci10010020
APA StyleBureta, C., Setoguchi, T., Saitoh, Y., Tominaga, H., Maeda, S., Nagano, S., Komiya, S., Yamamoto, T., & Taniguchi, N. (2020). TGF-β Promotes the Proliferation of Microglia In Vitro. Brain Sciences, 10(1), 20. https://doi.org/10.3390/brainsci10010020




