Variation of Zooplankton Mean Volume Backscattering Strength from Moored and Mobile ADCP Instruments for Diel Vertical Migration Observation
Abstract
1. Introduction
2. Materials and Methods
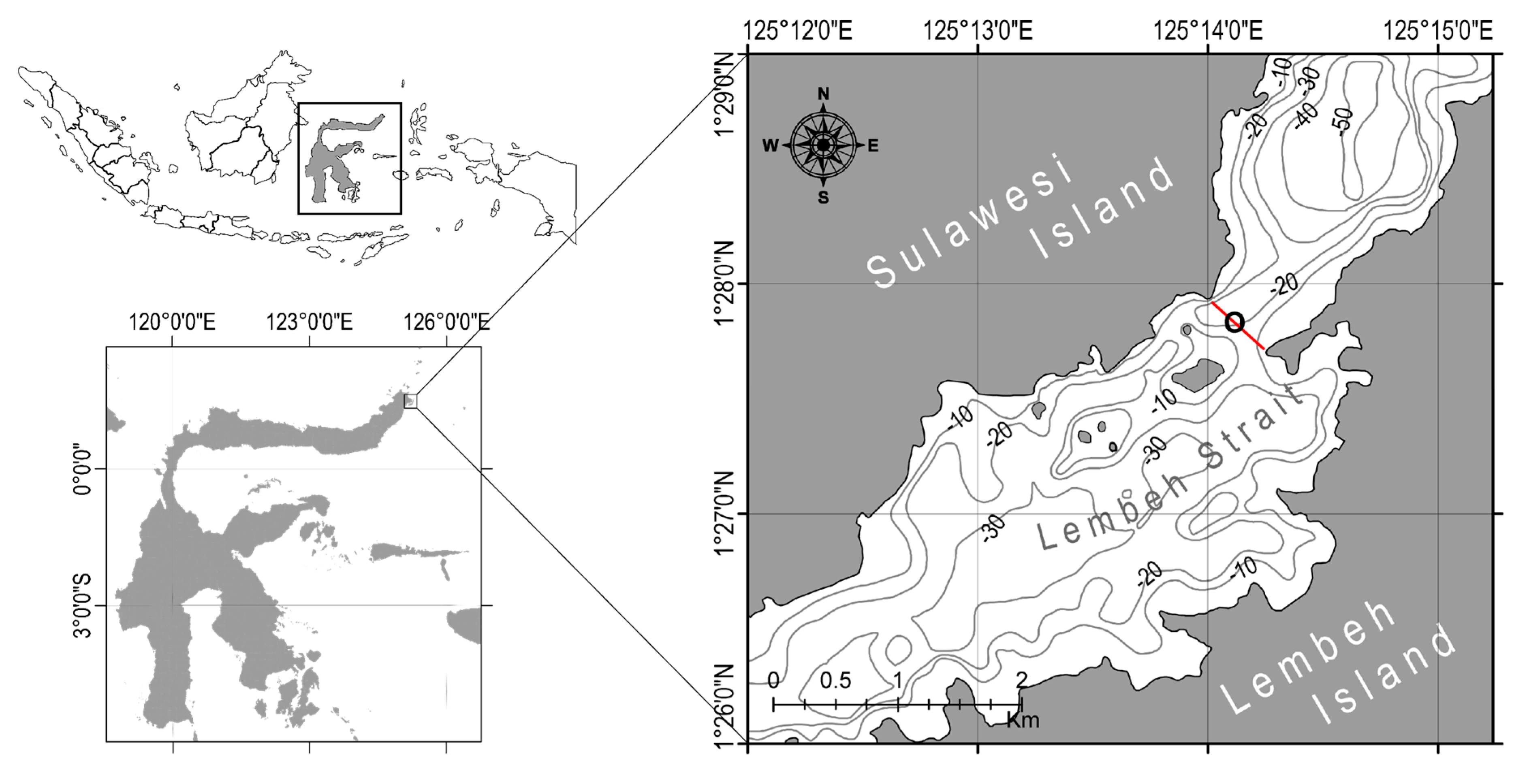
2.1. Time and Location
2.2. Tools and Materials
2.3. Data Collection Procedure
2.3.1. CTD Observation and Net Sampling
2.3.2. Echo Intensity and Vertical Velocity Data Acquisition
2.4. Data Analysis
2.4.1. Mean Volume Backscattering Strength (MVBS) Computation
2.4.2. MVBS Comparison
2.4.3. Vertical Velocity
2.4.4. Center of the Sound Scattering Layer (SSL)
2.5. Distorted wave-Born Approximation (DWBA) Model
3. Results and Discussion
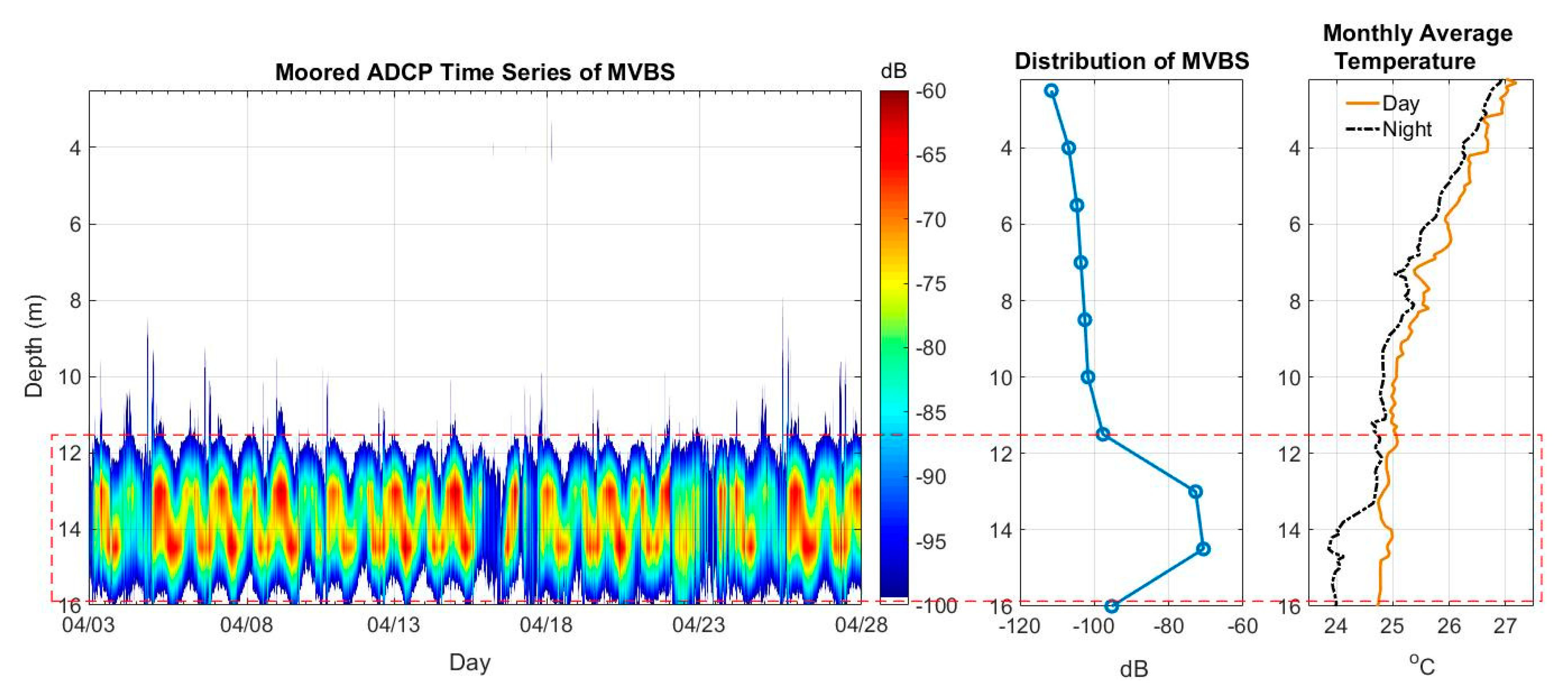
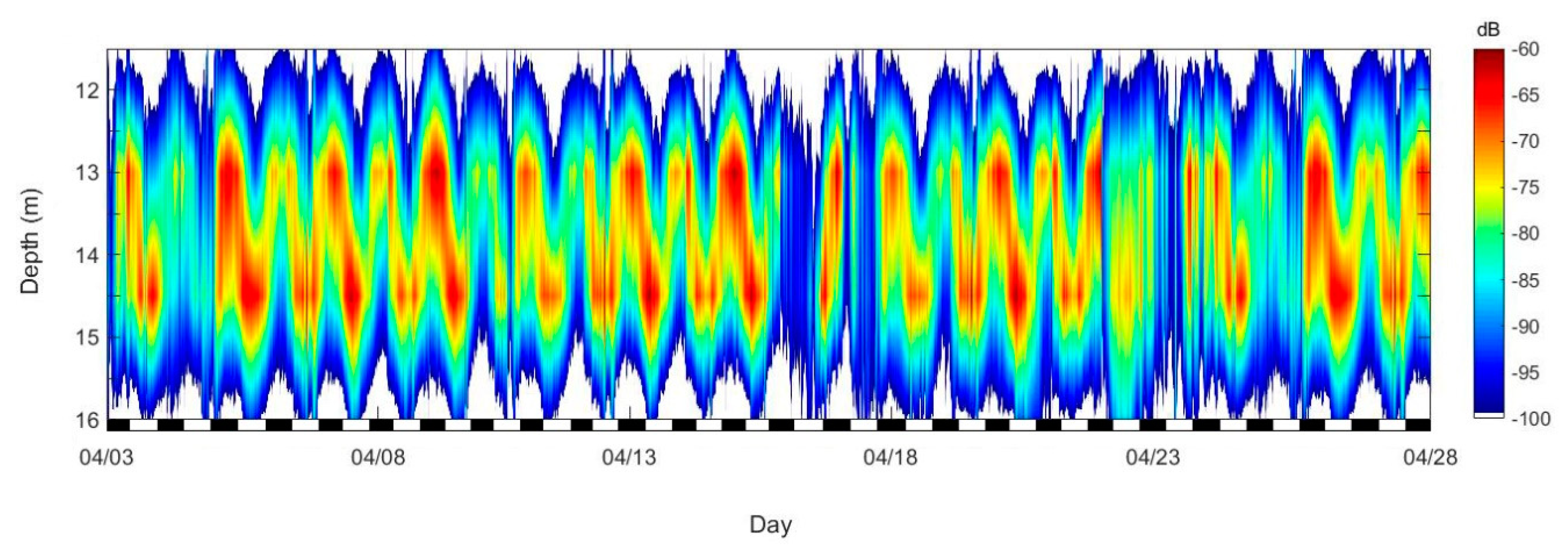
3.1. Moored ADCP
3.2. Mobile ADCP
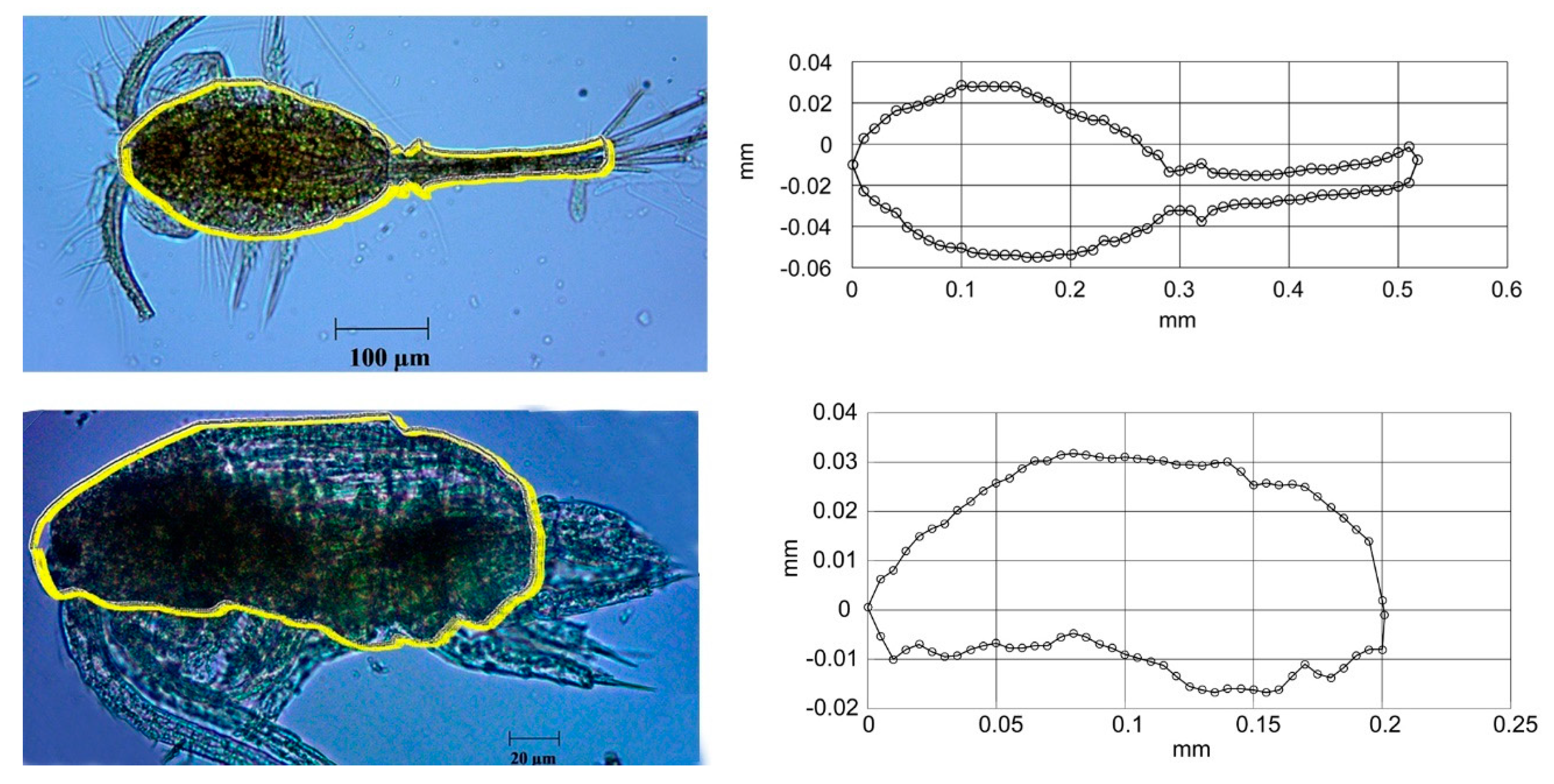
3.3. Biological Sampling
3.4. Relationship Between the MVBS and Zooplankton Abundance
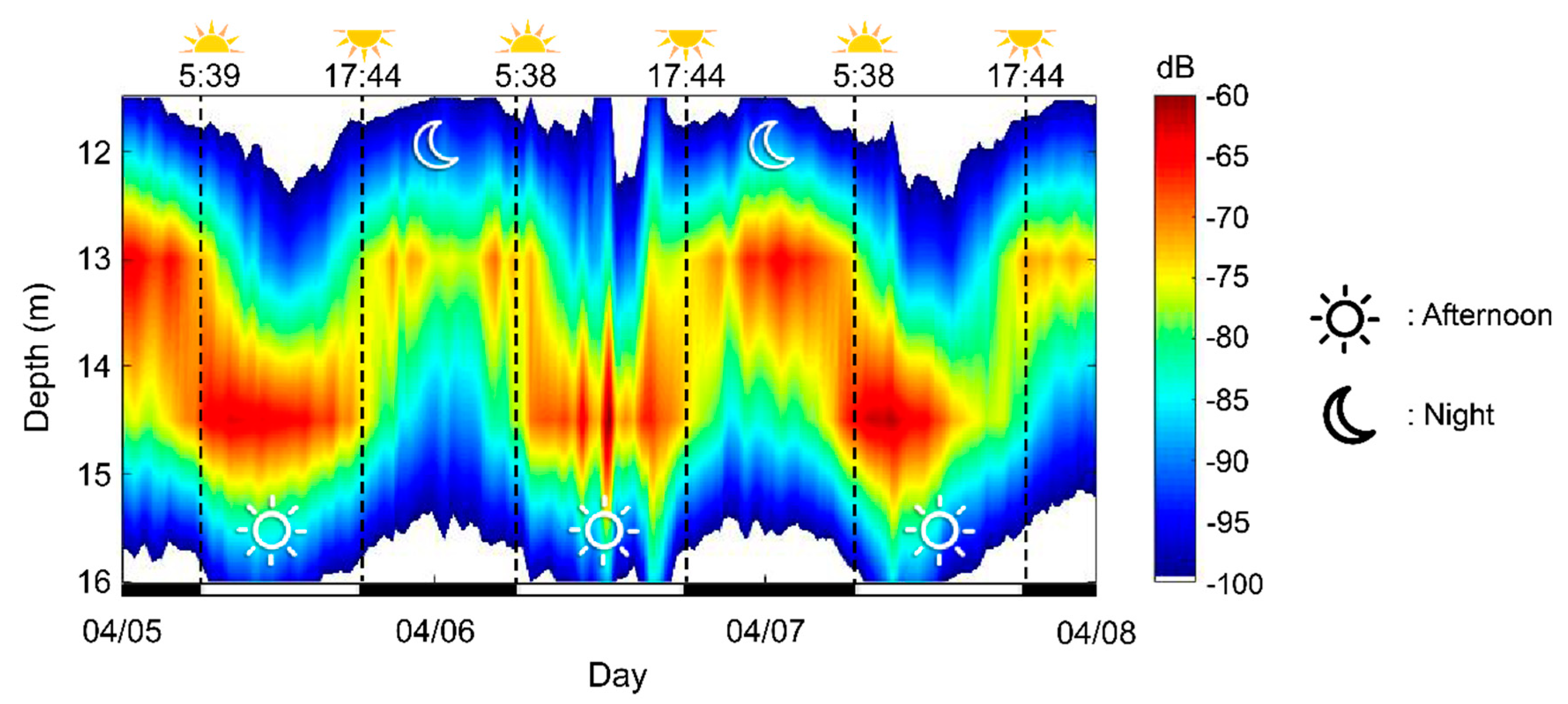
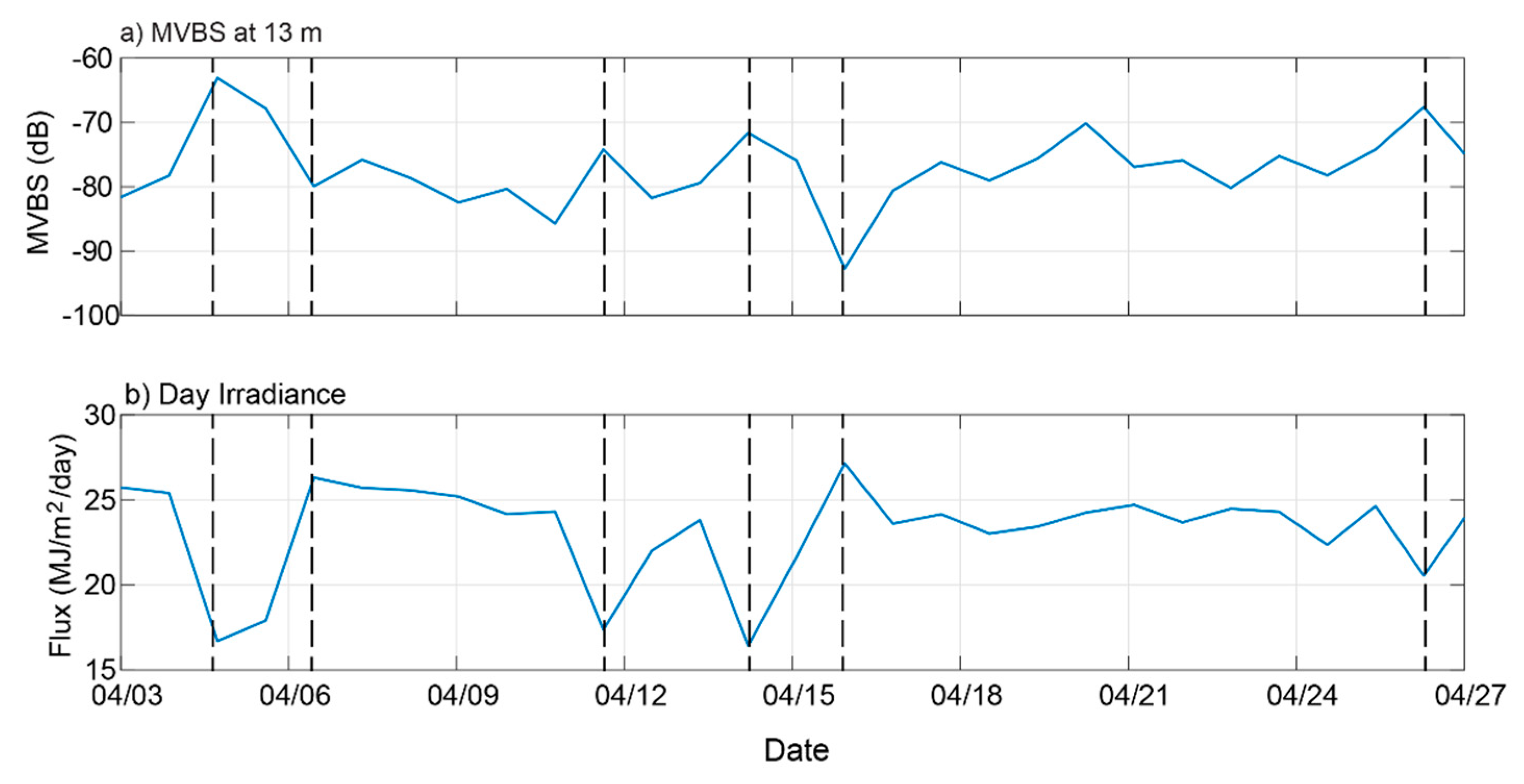
3.5. Diel Vertical Migration
3.6. Theoretical TS using the DWBA Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leonori, I.; De Felice, A.; Canduci, G.; Costantini, I.; Biagiotti, I.; Giuliani, G.; Budillon, G. Krill distribution in relation to environmental parameters in mesoscale structures in the Ross Sea. J. Mar. Syst. 2017, 166, 159–171. [Google Scholar] [CrossRef]
- Brown, J.H. Why are there so many species in the tropics? J. Biogeogr. 2014, 41, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Rumengan, I.F.M.; Akerina, J.; Rampengan, M.M.F.; Masengi, K.W.A. Abundance and diversity of zooplankton in Lembeh Strait, Bitung, Indonesia. Mar. Resour. Indones. 2011, 36, 15–20. [Google Scholar] [CrossRef]
- Cornils, A.; Schulz, J.; Schmitt, P.; Lanuru, M.; Richter, C.; Schnack-Schiel, S.B. Mesozooplankton distribution in the Spermonde Archipelago (Indonesia, Sulawesi) with special reference to the Calanoida (Copepoda). Deep Res. Part II Top. Stud. Oceanogr. 2010, 57, 2076–2088. [Google Scholar] [CrossRef]
- Aboul Ezz, S.M.; Heneash, A.M.M.; Gharib, S.M. Variability of spatial and temporal distribution of zooplankton communities at Matrouh beaches, south-eastern Mediterranean Sea, Egypt. Egypt. J. Aquat. Res. 2014, 40, 283–290. [Google Scholar] [CrossRef]
- Powell, J.R.; Ohman, M.D. Changes in zooplankton habitat, behavior, and acoustic scattering characteristics across glider-resolved fronts in the Southern California Current System. Prog. Oceanogr. 2015, 134, 77–92. [Google Scholar] [CrossRef]
- Simmonds, E.J.; MacLennan, D.N. Fisheries Acoustics: Theory and Practice, 2nd ed.; Fish and Fisheries Series; Blackwell: Oxford, UK, 2005; pp. 266–280. [Google Scholar]
- Saklnan, S.; Gücü, A.C. Spatial distribution of the Black Sea copepod, Calanus euxinus, estimated using multi-frequency acoustic backscatter. ICES J. Mar. Sci. 2017, 74, 832–846. [Google Scholar] [CrossRef]
- Ursella, L.; Cardin, V.; Batistić, M.; Garić, R.; Gačić, M. Evidence of zooplankton vertical migration from continuous Southern Adriatic buoy current-meter records. Prog. Oceanogr. 2018, 167, 78–96. [Google Scholar] [CrossRef]
- La, H.S.; Ha, H.K.; Kang, C.Y.; Wåhlin, A.K.; Shin, H.C. Acoustic backscatter observations with implications for seasonal and vertical migrations of zooplankton and nekton in the Amundsen shelf (Antarctica). Estuar. Coast. Shelf Sci. 2015, 152, 124–133. [Google Scholar] [CrossRef]
- Mukai, T.; Iida, K.; Ando, Y.; Mikami, H.; Maki, Y.; Matsukura, R. Measurements of swimming angles, density, and sound speed of the krill Euphausia pacifica for target strength estimation. In Proceedings of the Oceans ’04 MTTS/IEEE Techno-Ocean ’04, Kobe, Japan, 9–12 November 2004; Volume 4, pp. 383–388. [Google Scholar] [CrossRef]
- Fielding, S.; Griffiths, G.; Roe, H.S.J. The biological validation of ADCP acoustic backscatter through direct comparison with net samples and model predictions based on acoustic-scattering models. ICES J. Mar. Sci. 2004, 61, 184–200. [Google Scholar] [CrossRef]
- Sawada, K.; Mukai, T.; Fukuda, Y.; Matsuura, T. Comparison of zooplankton density estimated by acoustic inversion method and net sampling. J. Acoust. Soc. Am. 2016, 140, 3243. [Google Scholar] [CrossRef]
- Schiano, E.; Pensieri, S.; Bozzano, R.; Picco, P. Analysis of long time series of ADCP backscatter data in the Ligurian Sea to investigate the zooplankton variability. In Proceedings of the Oceans 2013 MTS/IEEE Bergen Challenges Northern Dimension, Bergen, Norway, 10–14 June 2013. [Google Scholar]
- Dwinovantyo, A.; Manik, H.M.; Prartono, T.; Susilohadi, S. Application of Acoustic Doppler Current Profiler (ADCP) to Observe Diel Vertical Migration of Zooplankton. J. Phys. Conf. Ser. 2018, 1075, 012016. [Google Scholar] [CrossRef]
- Yang, C.; Liao, G.; Yuan, Y.; Chen, H.; Zhu, X. The diel vertical migration of sound scatterers observed by an acoustic Doppler current profiler in the Luzon Strait from July 2009 to April 2011. Acta Oceanol. Sin. 2013, 32, 1–9. [Google Scholar] [CrossRef]
- Lee, K.; Mukai, T.; Lee, D.; Iida, K. Verification of mean volume backscattering strength obtained from acoustic Doppler current profiler by using sound scattering layer. Fish. Sci. 2008, 74, 221–229. [Google Scholar] [CrossRef]
- Deines, K.L. Backscatter estimation using Broadband acoustic Doppler current profilers. In Proceedings of the IEEE Sixth Working Conference on Current Measurement (Cat. No.99CH36331), San Diego, CA, USA, 13 March 1999; pp. 249–253. [Google Scholar] [CrossRef]
- Mullison, J. Backscatter Estimation Using Broadband Acoustic Doppler Current Profilers-Updated. In Proceedings of the ASCE Hydraulic Measurements & Experimental Methods Conference, Durham, NH, USA, 9–12 July 2017; pp. 1–5. [Google Scholar]
- Dwinovantyo, A.; Manik, H.M.; Prartono, T.; Susilohadi, S. Quantification and Analysis of Suspended Sediments Concentration Using Mobile and Static Acoustic Doppler Current Profiler Instruments. Adv. Acoust. Vib. 2017, 2017, 4890421. [Google Scholar] [CrossRef]
- Miyashita, K.; Aoki, I. Acoustic measurements of zooplankton using a dual frequency echo sounder. Mar. Ecol. Prog. Ser. 1999, 180, 105–109. [Google Scholar] [CrossRef][Green Version]
- Lee, K.; Mukai, T.; Kang, D.; Iida, K. Application of acoustic Doppler current profiler combined with a scientific echo sounder for krill Euphausia pacifica density estimation. Fish. Sci. 2004, 70, 1051–1060. [Google Scholar] [CrossRef]
- Mackenzie, K.V. Nine-term equation for sound speed in the oceans. J. Acoust. Soc. Am. 1981, 70, 807–812. [Google Scholar] [CrossRef]
- Francois, R.E.; Garrison, G.R. Sound absorption based on ocean measurements. Part II: Boric acid contribution and equation for total absorption. J. Acoust. Soc. Am. 1982, 72, 1879–1890. [Google Scholar] [CrossRef]
- Pratiwi, N.T.M.; Ardhito; Wulandari, D.Y.; Iswantari, A. Horizontal Distribution of Zooplankton in Tangerang Coastal Waters, Indonesia. Procedia Environ. Sci. 2016, 33, 470–477. [Google Scholar] [CrossRef]
- Brierley, A.S.; Saunders, R.A.; Bone, D.G.; Murphy, E.J.; Enderlein, P.; Conti, S.G.; Demer, D.A. Use of moored acoustic instruments to measure short term variability in abundance of Antartic krill. Limnol. Oceanogr. Methods 2006, 4, 18–29. [Google Scholar] [CrossRef]
- Lee, K.; Mukai, T.; Lee, D.-J.; Iida, K. Classification of sound-scattering layers using swimming speed estimated by acoustic Doppler current profiler. Fish. Sci. 2014, 80, 1–11. [Google Scholar] [CrossRef]
- Furusawa, M. Effects of noise and absorption on high frequency measurements of acoustic-backscatter from fish. Int. J. Oceanogr. 2015, 2015, 589463. [Google Scholar] [CrossRef]
- Amakasu, K.; Mukai, T.; Moteki, M. Measurement of the volume-backscattering spectrum from an aggregation of Antarctic krill and inference of their length-frequency distribution. Polar Sci. 2017, 12, 79–87. [Google Scholar] [CrossRef]
- Cisewski, B.; Strass, V.H.; Rhein, M.; Kragefsky, S. Seasonal variation of diel vertical migration of zooplankton from ADCP backscatter time series data in the Lazarev Sea, Antarctica. Deep Res. I 2010, 57, 78–94. [Google Scholar] [CrossRef]
- Cisewski, B.; Strass, V.H. Acoustic insights into the zooplankton dynamics of the eastern Weddell Sea. Prog. Oceanogr. 2016, 144, 62–92. [Google Scholar] [CrossRef]
- Proud, R.; Cox, M.J.; Wotherspoon, S.; Brierley, A.S. A method for identifying Sound Scattering Layers and extracting key characteristics. Methods Ecol. Evol. 2015, 6, 1190–1198. [Google Scholar] [CrossRef]
- Chu, D.; Foote, K.G.; Stanton, T.K. Further analysis of target strength measurements of Antarctic krill at 38 and 120 kHz: Comparison with deformed cylinder model and inference of orientation distribution. J. Acoust. Soc. Am. 1993, 93, 2985–2988. [Google Scholar] [CrossRef]
- Stanton, T.K.; Wiebe, P.H.; Chu, D. Differences between sound scattering by weakly scattering spheres and finite-length cylinders with applications to sound scattering by zooplankton. J. Acoust. Soc. Am. 1998, 103, 254–264. [Google Scholar] [CrossRef]
- Stanton, T.K.; Chu, D.; Wiebe, P.H. Sound scattering by several zooplankton groups. II. Scattering models. J. Acoust. Soc. Am. 1998, 103, 236–253. [Google Scholar] [CrossRef] [PubMed]
- McGehee, D.E.; O’Driscoll, R.L.; Traykovski, L.V.M. Effects of orientation on acoustic scattering from Antarctic krill at 120 kHz. Deep Res. Part II Top. Stud. Oceanogr. 1998, 45, 1273–1294. [Google Scholar] [CrossRef]
- Stanton, T.K.; Chu, D. Review and recommendations for the modelling of acoustic scattering by fluid-like elongated zooplankton: Euphausiids and copepods. ICES J. Mar. Sci. 2000, 57, 793–807. [Google Scholar] [CrossRef]
- Jech, J.M.; Horne, J.K.; Chu, D.; Demer, D.A.; Francis, D.T.I.; Gorska, N.; Jones, B.; Lavery, A.C.; Stanton, T.K.; Macaulay, G.J.; et al. Comparisons among ten models of acoustic backscattering used in aquatic ecosystem research. J. Acoust. Soc. Am. 2015, 138, 3742–3764. [Google Scholar] [CrossRef]
- Thoha, H.; Fitriya, N. The diversity of plankton in Sangihe - Sangir Talaud Islands, Sulawesi, Indonesia. Biosfera 2010, 27, 112–119. [Google Scholar] [CrossRef]
- Hays, G.C. Ocean currents and marine life. Curr. Biol. 2017, 27, R470–R473. [Google Scholar] [CrossRef] [PubMed]
- Lawson, G.L.; Wiebe, P.H.; Ashjian, C.J.; Gallager, S.M.; Davis, C.S.; Warren, J.D. Acoustically-inferred zooplankton distribution in relation to hydrography west of the Antarctic Peninsula. Deep Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 2041–2072. [Google Scholar] [CrossRef]
- Hays, G.C. A review of adaptive significance and ecosystem consequenses of zooplankton diel vertical migrations. Hydrobiologia 2003, 503, 163–170. [Google Scholar] [CrossRef]
- Zhu, X.-H.; Takasugi, Y.; Nagao, M.; Hashimoto, E. Diurnal cycle of soynd scatterers and measurements of turbidity using ADCP in Beppu Bay. J. Oceanogr. 2000, 56, 559–565. [Google Scholar] [CrossRef]
- Frank, T.M.; Widder, E.A. The correlation of downwelling irradiance and staggered vertical igration patterns of zooplankton in Wilkinson Basin, Gulf of Maine. J. Plankton Res. 1997, 19, 1975–1991. [Google Scholar] [CrossRef]
- Ringelberg, J. Changes in light intensity and diel vertical migration: A comparison of marine and freshwater environments. J. Mar. Biol. Ass. U. K. 1995, 75, 15–25. [Google Scholar] [CrossRef]
- Lawson, G.L.; Stanton, T.K.; Ashjian, C.J. Euphausiid distribution along the Western Antarctic Peninsula—Part A: Development of robust multi-frequency acoustic techniques to identify euphausiid aggregations and quantify euphausiid size, abundance, and biomass. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 412–431. [Google Scholar] [CrossRef]
- Amakasu, K.; Furusawa, M. The target strength of Antarctic krill (Euphausia superba) measured by the split-beam method in a small tank at 70 kHz. ICES J. Mar. Sci. 2006, 63, 36–45. [Google Scholar] [CrossRef]
- Amakasu, K.; Ono, A.; Hirano, D.; Ishimaru, T. Distribution and density of Antarctic krill (Euphausia superba) and ice krill (E. crystallorophias) off Adélie Land in austral summer 2008 estimated by acoustical methods. Polar Sci. 2011, 5, 187–194. [Google Scholar] [CrossRef]
- Miyashita, K.; Aoki, I.; Seno, K.; Taki, K. Acoustic identification of isada krill, Euphausia pacifica Hansen, off the Sanriku coast, North-Eastern Japan. Fish. Oceanogr. 1997, 6, 266–271. [Google Scholar] [CrossRef]
- Potiris, E.; Frangoulis, C.; Kalampokis, A.; Ntoumas, M.; Pettas, M.; Petihakis, G.; Zervakis, V. Acoustic Doppler current profiler observations of migration patterns of zooplankton in the Cretan Sea. Ocean Sci. 2018, 14, 783–800. [Google Scholar] [CrossRef]
- Wang, Z.; DiMarco, S.F.; Ingle, S.; Belabbassi, L.; Al-Kharusi, L.H. Seasonal and annual variability of vertically migrating scattering layers in the northern Arabian Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2014, 90, 152–165. [Google Scholar] [CrossRef]
- Thoha, H.; Fitriya, N.; Sianturi, O.R.; Wang, Y. Community structure of zooplankton in the Lembeh Strait, Bitung, and Wori Beach, Manado, North Sulawesi, Indonesia. Acta Oceanol. Sin. 2018, 37, 28–34. [Google Scholar] [CrossRef]
- Gallienne, C.P.; Robins, D.B. Is Oithona the most important copepod in the world’s oceans? J. Plankton Res. 2001, 23, 1421–1432. [Google Scholar] [CrossRef]
- Lo, W.-T.; Shih, C.-T.; Hwang, J.-S. Diel vertical migration of the planktonic copepods at an upwelling station north of Taiwan, western North Pacific. J. Plankton Res. 2004, 26, 89–97. [Google Scholar] [CrossRef]
- Pinot, J.M.; Jansá, J. Time variability of acoustic backscatter from zooplankton in the Ibiza Channel (western Mediterranean). Deep Sea Res. Part I Oceanogr. Res. Pap. 2001, 48, 1651–1670. [Google Scholar] [CrossRef]
- Michalec, F.-G.; Fouxon, I.; Souissi, S.; Holzner, M. Zooplankton can actively adjust their motility to turbulent flow. Proc. Natl. Acad. Sci. USA 2017, 114, E11199–E11207. [Google Scholar] [CrossRef]
- Kang, M.; Furusawa, M.; Miyashita, K. Effective and accurate use of difference in mean volume backscattering strength to identify fish and plankton. ICES J. Mar. Sci. 2002, 59, 794–804. [Google Scholar] [CrossRef]

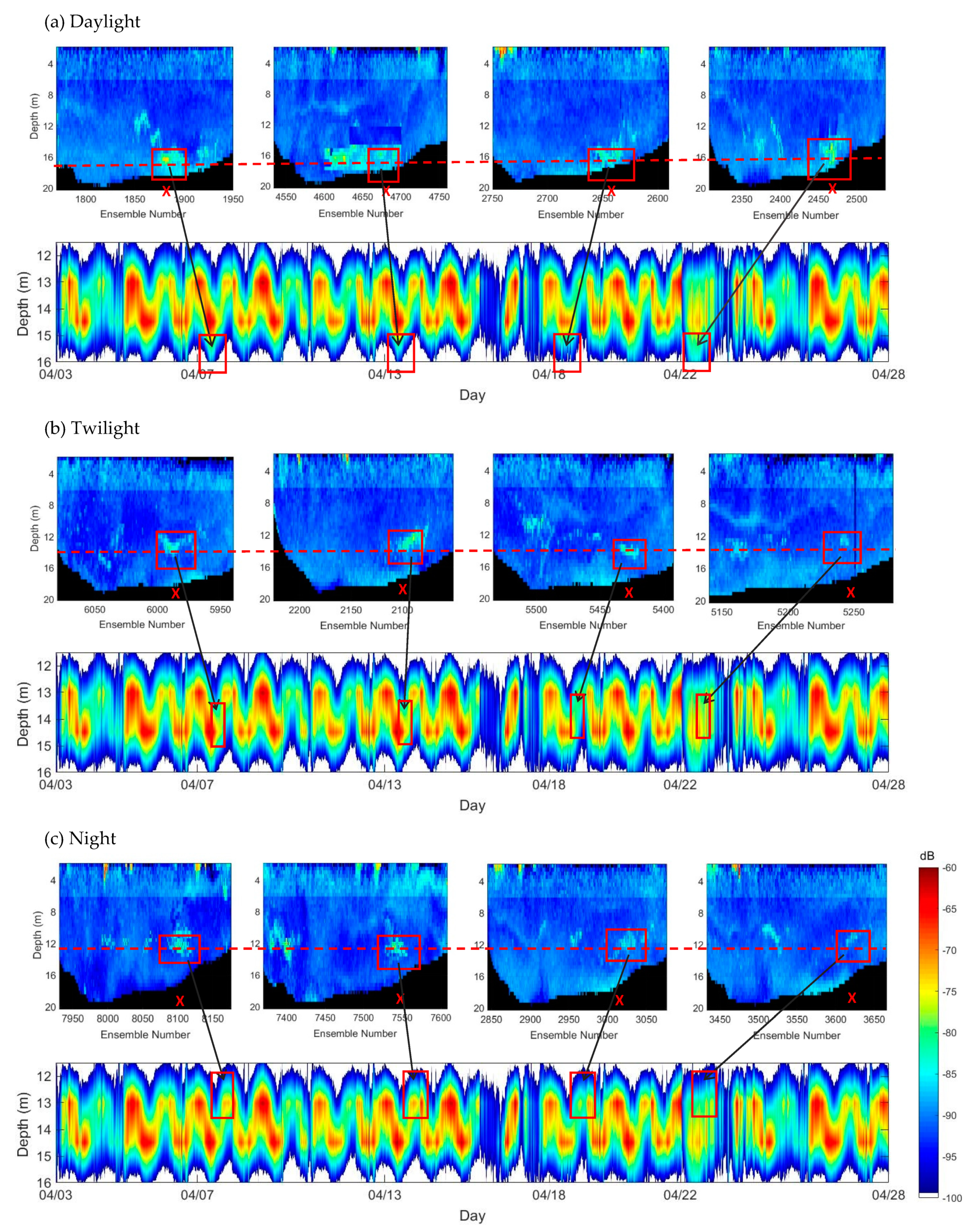
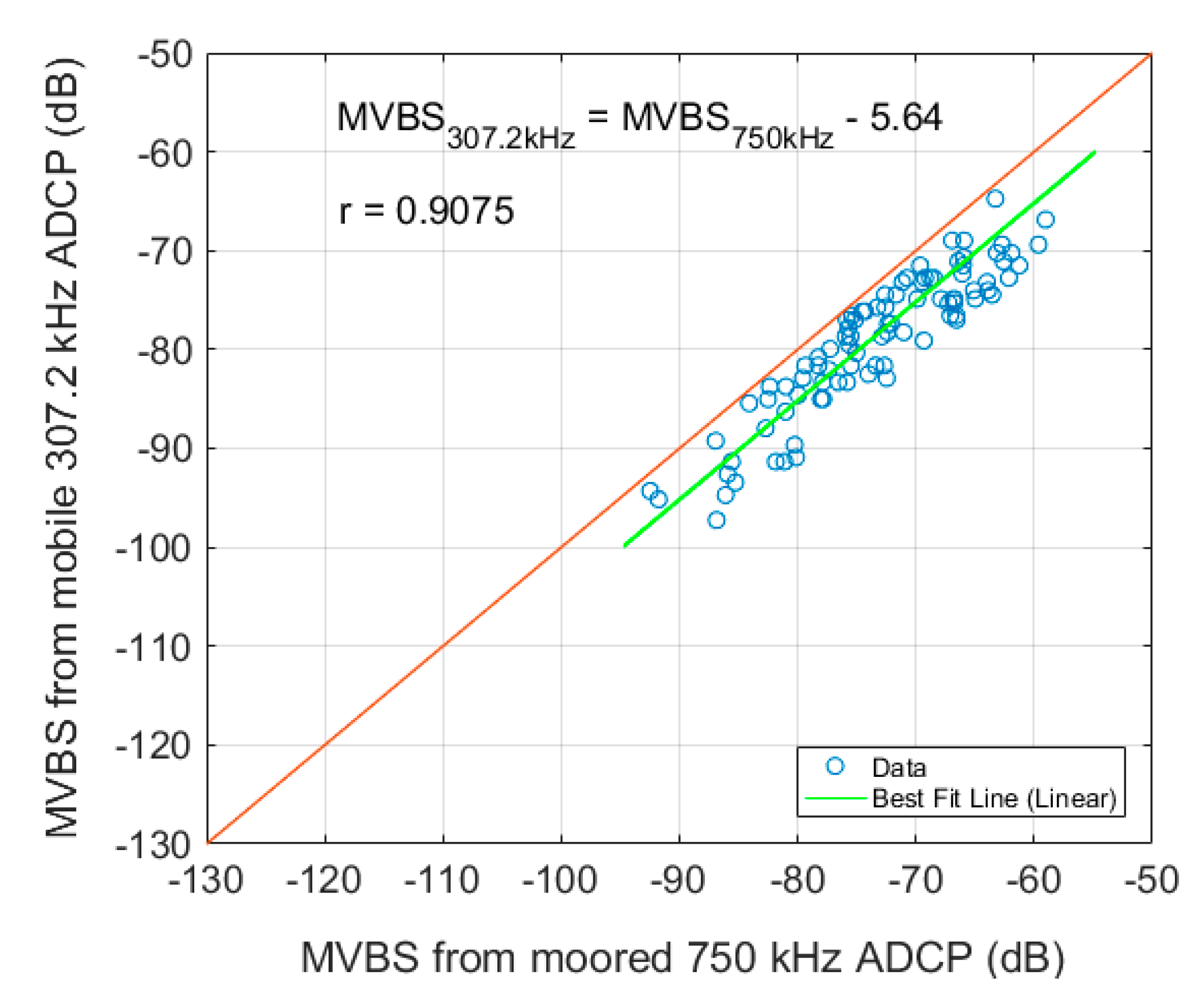
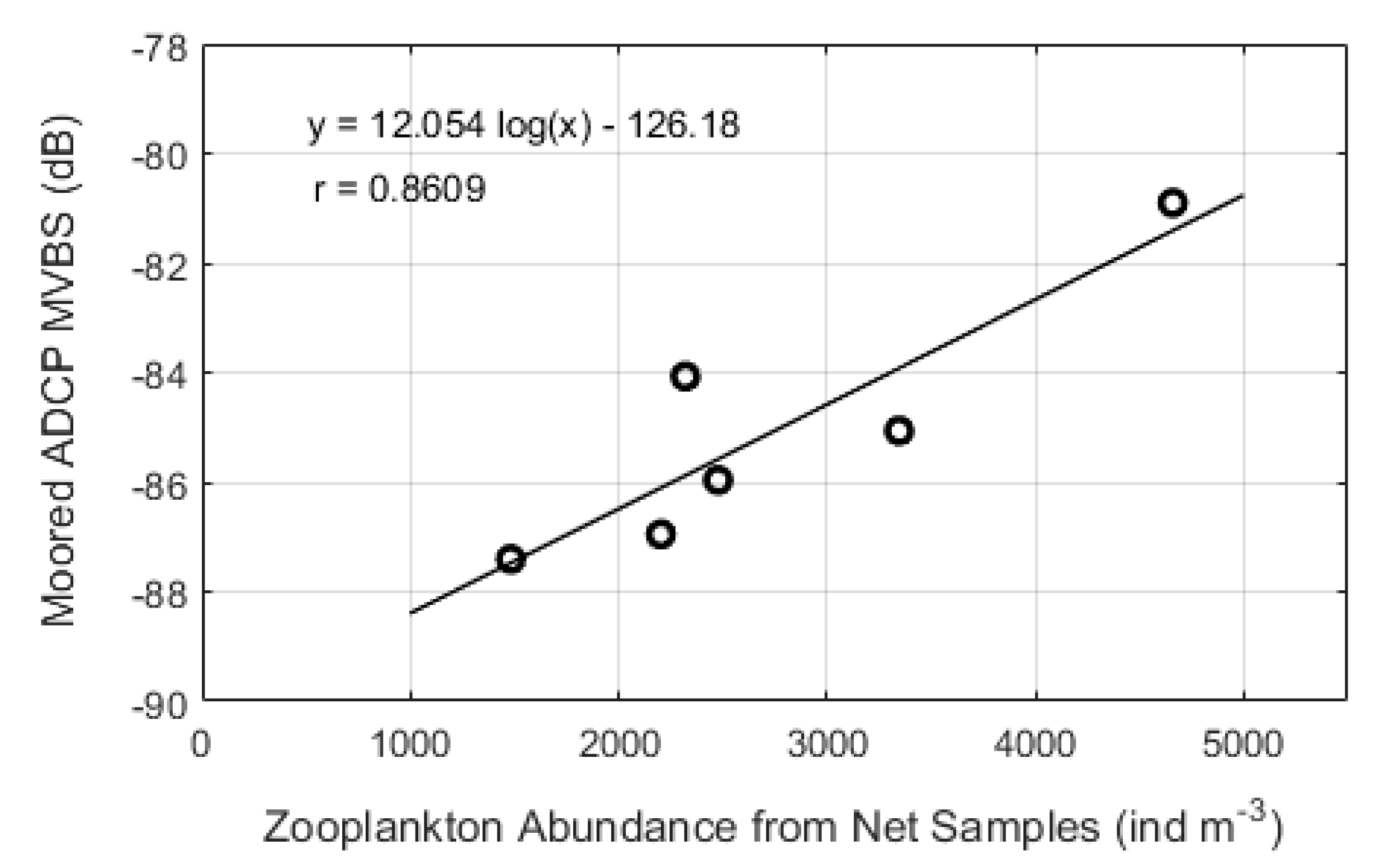

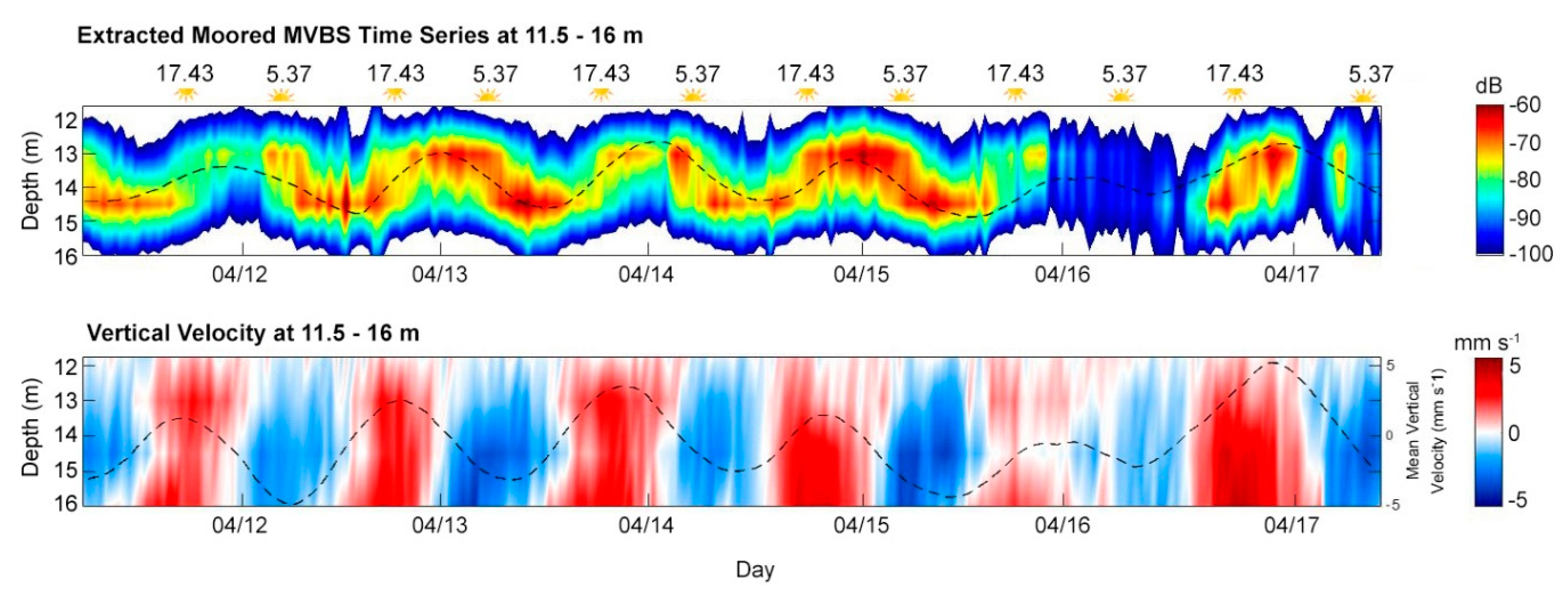

| Parameters | Moored ADCP | Mobile ADCP |
|---|---|---|
| Frequency (kHz) | 750 | 307.2 |
| Sampling interval (s) | 60 | 1 |
| Range (m) | 15 | 50 |
| Bin size/Pulse length (m) | 1.5 | 1 |
| Transducer tilt angle (°) | 20 | 20 |
| Orientation | Upward-looking | Downward-looking |
| Number of transducers | 3 | 4 |
| Transducer depth (m) | 1 1 | 0.65 2 |
| C, system constant (dB) | −160.5 | −151.64 |
| Tx, temperature of transducer (°C) | 28.06 | 28.55 |
| P, emitted wavelength (mm) | 2 | 5 |
| LDBM, logarithmic transmit pulse (dB) | −6.98 | −3.01 |
| PDBW, logarithmic transmit power (dB) | 7.95 | 17.5 |
| Kc, beam specific scaling factor (dB count−1) | 0.72 | 0.43 |
| Er, the minimum value of RSSI | 43 | 40 |
| Percent good threshold (%) | 65 | 65 |
| ORGANISM | Time | |||||
|---|---|---|---|---|---|---|
| 5/4/2016 18:05–18:22 | 7/4/2016 18:13–18:31 | 13/4/2016 17:48–18:13 | 18/4/2016 17:34–18:01 | 22/4/2016 17:20–17:49 | 25/5/2016 16:20–16:43 | |
| PROTOZOA | ||||||
| Favella sp. | 565 | 198 | 734 | 0 | 85 | 85 |
| Tintinnopsis sp. | 0 | 148 | 0 | 44 | 42 | 0 |
| Eutintinnus sp. | 0 | 49 | 0 | 0 | 0 | 85 |
| Leprotintinnus sp. | 0 | 0 | 0 | 44 | 169 | 339 |
| Globigerina sp. | 0 | 0 | 0 | 0 | 127 | 0 |
| CRUSTACEAN | ||||||
| Nauplius (stadia) | 31,572 | 9390 | 15,327 | 5909 | 3558 | 6693 |
| Oithona sp. | 1864 | 890 | 1468 | 1313 | 1567 | 2711 |
| Microsetella sp. | 395 | 148 | 184 | 88 | 0 | 85 |
| Paracalanus sp. | 339 | 593 | 1010 | 1007 | 1779 | 1949 |
| Corycaeus sp. | 0 | 0 | 92 | 44 | 0 | 0 |
| Acartia sp. | 0 | 0 | 0 | 0 | 0 | 169 |
| UROCHORDATE | ||||||
| Oikopleura sp. | 169 | 99 | 0 | 0 | 0 | 1017 |
| CHAETOGNATH | ||||||
| Sagitta sp. | 0 | 0 | 92 | 0 | 0 | 0 |
| NEMATODE | ||||||
| Nematoda Larvae (sp1) | 0 | 0 | 0 | 44 | 42 | 169 |
| GASTROPODS | ||||||
| Gastropoda Larvae (sp1) | 169 | 49 | 642 | 481 | 381 | 508 |
| PELECYPODS | ||||||
| Pelecypoda Larvae (sp1) | 169 | 445 | 367 | 88 | 42 | 85 |
| POLYCHAETE | ||||||
| Polychaeta Larvae (sp1) | 0 | 346 | 734 | 0 | 297 | 0 |
| The number of Taxa | 8 | 11 | 10 | 10 | 11 | 12 |
| Abundance (ind m−3) | 35,242 | 12,355 | 20,650 | 9062 | 8089 | 13,895 |
| Diversity Index | 0.49 | 1.02 | 1.06 | 1.15 | 1.55 | 1.58 |
| Uniformity Index | 0.24 | 0.42 | 0.46 | 0.50 | 0.65 | 0.64 |
| Dominance Index | 0.81 | 0.59 | 0.56 | 0.46 | 0.28 | 0.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dwinovantyo, A.; Manik, H.M.; Prartono, T.; Susilohadi, S.; Mukai, T. Variation of Zooplankton Mean Volume Backscattering Strength from Moored and Mobile ADCP Instruments for Diel Vertical Migration Observation. Appl. Sci. 2019, 9, 1851. https://doi.org/10.3390/app9091851
Dwinovantyo A, Manik HM, Prartono T, Susilohadi S, Mukai T. Variation of Zooplankton Mean Volume Backscattering Strength from Moored and Mobile ADCP Instruments for Diel Vertical Migration Observation. Applied Sciences. 2019; 9(9):1851. https://doi.org/10.3390/app9091851
Chicago/Turabian StyleDwinovantyo, Angga, Henry M. Manik, Tri Prartono, Susilohadi Susilohadi, and Tohru Mukai. 2019. "Variation of Zooplankton Mean Volume Backscattering Strength from Moored and Mobile ADCP Instruments for Diel Vertical Migration Observation" Applied Sciences 9, no. 9: 1851. https://doi.org/10.3390/app9091851
APA StyleDwinovantyo, A., Manik, H. M., Prartono, T., Susilohadi, S., & Mukai, T. (2019). Variation of Zooplankton Mean Volume Backscattering Strength from Moored and Mobile ADCP Instruments for Diel Vertical Migration Observation. Applied Sciences, 9(9), 1851. https://doi.org/10.3390/app9091851







