Effect of Microalgae Incorporation on Quality Characteristics and Functional and Antioxidant Capacities of Ready-to-Eat Fish Burgers Made from Common Carp (Cyprinus carpio)
Abstract
:1. Introduction
2. Material and Methods
2.1. Microalgae
2.2. Catching Fish
2.3. Formulation and Production of Fish Burger
2.4. Physicochemical Properties of Fish Hamburger
weight Residue)/weight Sample)
2.5. Functional Properties of Fish Burger
2.6. Sensory Evaluation
2.7. Color Analysis
2.8. Instrumental Texture Analysis
2.9. Antioxidant Properties Evaluation Using DPPH and Ferric ReducingAantioxidant Power (FRAP) Methods
2.10. Microbiological Analyses
2.11. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Results
3.2. Textural Parameters
3.3. Functional Properties of Carp Burgers
3.4. Microbiological Quality of Carp Burgers
3.5. Color Measurement of Burger Samples
3.6. Antioxidant Properties of Fish Burgers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maiditsch, I.P.; Ladich, F. Effects of Temperature on Auditory Sensitivity in Eurythermal Fishes: Common Carp Cyprinus carpio (Family Cyprinidae) versus Wels Catfish Silurus glanis (Family Siluridae). PLoS ONE 2014, 9, e108583. [Google Scholar] [CrossRef] [PubMed]
- Ljubojevic, D.; Trbovic, D.; Lujic, J.; Bjelic-Cabrilo, O.; Kostic, D.; Novakov, N.; Cirkovic, M. Fatty acid composition of fishes from inland waters. Bulg. J. Agric. Sci. 2013, 19, 62–74. [Google Scholar]
- Cedola, A.; Cardinali, A.; Nobile, M.A.D.; Conte, A. Fish burger enriched by olive oil industrial by-Product. Int. J. Food Sci. Nutr. 2017, 5, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Abdelkafi, S.; Labat, M.; Ben Ali Gam, Z.; Lorquin, J.; Sayadi, S. Optimized conditions for the synthesis of vanillic acid under hypersaline conditions by Halomonas elongata DSM 2581T resting cells. World J. Microbiol. Biotechnol. 2008, 24, 675–680. [Google Scholar] [CrossRef]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT-Food Sci. Technol. 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Alavi, N.; Golmakani, M.T. Improving oxidative stability of virgin olive oil by addition of microalga Chlorella vulgaris biomass. J. Food Sci. Technol. JFST 2017, 54, 2464–2473. [Google Scholar] [CrossRef]
- Ben Amor, F.; Elleuch, F.; Ben Hlima, H.; Garnier, M.; Saint-Jean, B.; Barkallah, M.; Pichon, C.; Abdelkafi, S.; Fendri, I. Proteomic analysis of the Chlorophyta Dunaliella new strain AL-1 revealed global changes of metabolism during high carotenoid production. Mar. Drugs 2017, 15, 293. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Hadrich, B.; Akremi, I.; Dammak, M.; Barkallah, M.; Fendri, I.; Abdelkafi, S. Optimization of lipids’ ultrasonic extraction and production from Chlorella sp. using response-surface methodology. Lipids Health Dis. 2018, 17, 87. [Google Scholar] [CrossRef]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; Oliveira, J.V.; Junior, A.F.; Derner, R.B.; Santanna, E.S. Chemical characterization of six microalgae with potential utility for food application. J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Dammak, M.; Hadrich, B.; Miladi, R.; Barkallah, M.; Hentati, F.; Hachicha, R.; Laroche, C.; Michaud, P.; Fendri, I.; Abdelkafi, S. Effects of nutritional conditions on growth and biochemical composition of Tetraselmis sp. Lipids Health Dis. 2017, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls A and B or leaf in dissolved solvents. Biochem. Soc. Trans. 1985, 11, 591–592. [Google Scholar] [CrossRef]
- Kumar, P.; Ramakritinan, C.M.; Kumaraguru, A.K. Solvent extraction and spectrophotometric determination of pigments of some algal species from the shore of puthumadam, southeast coast of India. Int. J. Oceans Oceanogr. 2010, 4, 29–34. [Google Scholar]
- Kuniak, L.; Marchessault, R.H. Study of the Crosslinking Reaction between Epichlorohydrin and Starch. Starch-Starke 1972, 24, 110–116. [Google Scholar] [CrossRef]
- Okezie, B.O.; Bello, A.B. Physicochemical and functional properties of winged bean flour and isolate compared with soy isolate. J. Food Sci. 1988, 53, 450–454. [Google Scholar] [CrossRef]
- Wong, K.H.; Cheung, P.C.K. Nutritional evaluation of some subtropical red and green seaweeds. Part I—Proximate composition, amino acid profiles and some physicochemical properties. Food Chem. 2000, 71, 475–482. [Google Scholar] [CrossRef]
- Huber, E.; Francio, D.L.; Biasi, V.; Mezzomo, N.; Ferreira, S.R.S. Characterization of vegetable fiber and its use in chicken burger formulation. J. Food Sci. Technol. 2016, 53, 3043–3052. [Google Scholar] [CrossRef] [Green Version]
- Bourne, M.C. Texture profile analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a heated histidine glucose model system I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Yildirim, A.; Mavi, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. Extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef] [PubMed]
- APHA. Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; Downes, F.P., Ito, K., Eds.; American Public Health Association (APHA): Washington, DC, USA, 2001. [Google Scholar]
- Shimamatsu, H. Mass production of Spirulina, an edible microalga. Hydrobiologia 2004, 512, 39–44. [Google Scholar] [CrossRef]
- Selani, M.M.; Shirado, G.A.N.; Margiotta, G.B.; Saldaña, E.; Spada, F.P.; Piedade, S.M.S.; Contreras-Castillo, C.J.; Canniatti-Brazaca, S.G. Effects of pineapple byproduct and canola oil as fat replacers on physicochemical and sensory qualities of low-fat beef burger. Meat Sci. 2016, 112, 69–76. [Google Scholar] [CrossRef]
- Tokuşoglu, O.; üUnal, M.K. Biomass Nutrient Profiles of Three Microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. Food Sci. 2006, 68, 1144–1148. [Google Scholar] [CrossRef]
- Cofrades, S.; López-López, I.; Solas, M.; Bravo, L.; Jiménez-Colmenero, F. Influence of different types and proportions of added edible seaweeds on characteristics of different types and proportions of added edible seaweeds on characteristics of low-salt gel/emulsion meat systems. Meat Sci. 2008, 79, 767–776. [Google Scholar] [CrossRef]
- Fernandez-Martin, F.; Lopez-Lopez, I.; Cofrades, S.; Colmenero, F.J. Influence of adding Sea Spaghetti seaweed and replacing the animal fat with olive oil or a konjac gel on pork meat batter gelation. Potential protein/alginate association. Meat Sci. 2009, 83, 209–217. [Google Scholar] [CrossRef] [Green Version]
- López-Vargas, J.H.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Viuda-Martos, M. Quality characteristics of pork burger added with albedo-fiber powder obtained from yellow passion fruit (Passiflora edulis var. flavicarpa) co-products. Meat Sci. 2014, 97, 270–276. [Google Scholar] [CrossRef]
- Ben Halima, N.; Borchani, M.; Fendri, I.; Khemakhem, B.; Gosset, D.; Baril, P.; Pichon, C.; Ayadi, M.-A.; Abdelkafi, S. Optimised amylases extraction from oat seeds and its impact on bread properties. Int. J. Biol. Macromol. 2015, 72, 1213–1221. [Google Scholar] [CrossRef]
- Kumarathunge, N.C.; Jayasinghe, J.M.P.; Abeyrathne, E.D.N.S. Development of Sea Lettuce (Ulva lactuca) and Catla (Catla catla) Incorporated Protein and Fiber Rich Fish Burger. Int. J. Res. Agric. Sci. 2016, 4, 2348–3997. [Google Scholar]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2016, 99, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Abu-Ghannam, N. Enhancement of the phytochemical and fibre content of beef patties with Himanthalia elongata seaweed. Int. J. Food Sci. Technol. 2013, 48, 2239–2249. [Google Scholar]
- Senthil, M.A.; Mamatha, B.; Mahadevaswamy, M. Effect of using seaweed (Eucheuma) powder on the quality of fish cutlet. Int. J. Food Sci. Nutr. 2005, 56, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Jónsdóttir, R.; Geirsdóttir, M.; Hamaguchi, P.Y.; Jamnik, P.; Kristinsson, H.G.; Undeland, I. The ability of in vitro antioxidant assays to predict the efficiency of a cod protein hydrolysate and brown seaweed extract to prevent oxidation in marine food model systems. J. Sci. Food Agric. 2015, 96, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, H.; Guo, G.; Pu, Y.; Yan, B. The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 26, 22–31. [Google Scholar] [CrossRef]
- Chen, Y.X.; Liu, X.Y.; Xiao, Z.; Huang, Y.F.; Liu, B. Antioxidant activities of polysaccharides obtained from Chlorella pyrenoidosa via different ethanol concentrations. Int. J. Biol. Macromol. 2016, 91, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Arciola, C.R.; Vigani, B.; Crivelli, B.; Moro, P.; Marrubini, G.; Sorrenti, M.; Catenacci, L.; Bruni, G.; Chlapanidas, T.; et al. In Vitro effectiveness of microspheres based on silk sericin and Chlorella vulgaris or Arthrospira platensis for wound healing applications. Materials 2017, 10, 983. [Google Scholar] [CrossRef] [PubMed]
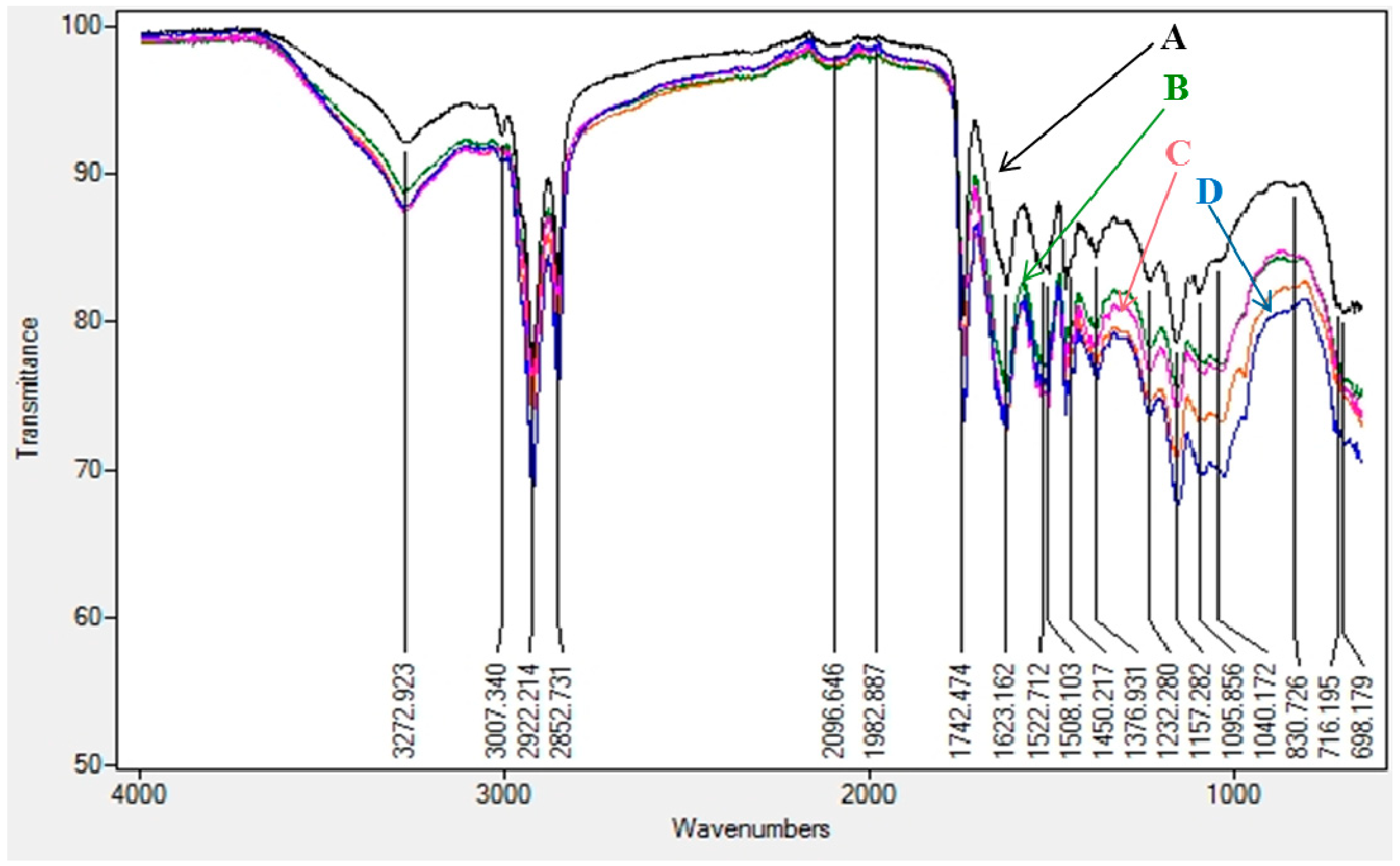
| Color | Odor | Taste | After-taste | Texture | |
|---|---|---|---|---|---|
| Control burger | 4.0 ± 0.3 | 4.0 ± 0.3 | 4.4 ± 0.6 | 4.3 ± 0.2 | 4.0 ± 0.1 |
| 0.5% Isochrysis burger | 4.2 ± 0.3 a | 4.0 ± 0.3 | 4.3 ± 0.2 | 4.4 ± 0.3 | 4.1 ± 0.3 |
| 1% Isochrysis burger | 4.2 ± 0.2 a | 4.1 ± 0.4 | 4.6 ± 0.2 a,e | 4.4 ± 0.2 | 4.2 ± 0.1 a |
| 1.5% Isochrysis burger | 3.8 ± 0.3 a,e,g | 3.7 ± 0.5 b,e,g | 3.7 ± 0.3 c,f,h | 3.8 ± 0.2 c,f,h | 3.8 ± 0.3 a,e,g |
| 0.5% Picochlorum burger | 4.3 ± 0.2 b | 4.0 ± 0.4 | 4.4 ± 0.3 | 4.3 ± 0.2 | 4.2 ± 0.2 a |
| 1% Picochlorum burger | 4.3 ± 0.2 b | 4.0 ± 0.3 | 4.5 ± 0.2 | 4.4 ± 0.2 | 4.2 ± 0.3 a |
| 1.5% Picochlorum burger | 4.0 ± 0.2 e,g | 4.0 ± 0.3 | 3.8 ± 0.3 c,f,h | 4.0 ± 0.3 b,e,g | 4.0 ± 0.2 d,f |
| 0.5% Chlorella burger | 4.3 ± 0.2 b | 4.0 ± 0.2 | 4.7 ± 0.3 b | 4.4 ± 0.4 | 4.2 ± 0.3 a |
| 1% Chlorella burger | 4.4 ± 0.2 c | 4.0 ± 0.5 | 4.6 ± 0.3 a | 4.3 ± 0.3 | 4.2 ± 0.3 a |
| 1.5% Chlorella burger | 3.9 ± 0.1 e,g | 3.5 ± 0.4 c,f,g | 3.9 ± 0.3 c,f,h | 3.9 ± 0.3 b,f,g | 4.0 ± 0.2 d,f |
| Control Burger | 0.5% Isochrysis Burger | 1% Isochrysis Burger | 0.5% Picochlorum Burger | 1% Picochlorum Burger | 0.5% Chlorella Burger | 1% Chlorella Burger | |
|---|---|---|---|---|---|---|---|
| pH | 7.21 ± 0.07 | 7.175 ± 0.005 | 7.17 ± 0.01 | 7.185 ± 0.005 | 7.17 ± 0.01 | 7.17 ± 0.01 | 7.16 ± 0.001 |
| Water activity (aw) | 0.982 ± 0.00 | 0.981 ± 0.001 | 0.88 ± 0.001 a | 0.98 ± 0.001 | 0.971 ± 0.000 a,d | 0.982 ± 0.001 | 0.972 ± 0.001 a,d |
| Moisture (% FW) | 77.03 ± 0.055 | 76.82 ± 0.082 a | 76.68 ± 0.160 b,d | 76.7 ± 0.3 b | 76.65 ± 0.03 b | 76.75 ± 0.19 a | 76.5 ± 0.15 b,d |
| Total solids (% FW) | 22.96 ± 0.06 | 23.18 ± 0.08 | 23.31 ± 0.160 a | 23.3 ± 0.03 a | 23.35 ± 0.03 a | 23.24 ± 0.19 | 23.5 ± 0.145 b,d |
| Proteins (% DW) | 79.66 ± 0.12 | 79.4 ± 0.04 | 79.15 ± 0.05 | 79.36 ± 0.402 | 79.5 ± 0.355 | 79.15 ± 0.650 | 79.02 ± 0.555 |
| Fats (% DW) | 7.8 ± 0.15 | 7.71 ± 0.1 | 7.65 ± 0.120 | 7.65 ± 0.225 | 7.54 ± 0.6 a | 7.70 ± 0.15 | 7.64 ± 0.1 |
| Ash (% DW) | 11.13 ± 0.11 | 11.29 ± 0.06 | 11.77 ± 0.07 c,f | 11.33 ± 0.02 a | 11.9 ± 0.101 c,f | 11.29 ± 0.05 | 11.49 ± 0.03 b,d |
| Carbohydrates (% DW) | 2.8 ± 0.15 | 2.8 ± 0.12 | 2.82 ± 0.13 | 2.8 ± 0.1 | 2.89 ± 0.17 | 2.81 ± 0.2 | 2.84 ± 0.17 |
| Total dietary fibers (g/100g DW) | 0.329 ± 0.025 | 0.375 ± 0.06 | 0.49 ± 0.05 b,d | 0.474 ± 0.07 b | 0.86 ± 0.12 c,f | 0.46 ± 0.06 b | 0.67±0.05 c,e |
| Hardness (N) | Springiness (cm) | Chewiness (N·cm) | Cohesiveness | |
|---|---|---|---|---|
| Control burger | 8.35 | 0.36 | 1.11 | 0.37 |
| 1% Isochrysis burger | 10.03 c | 0.33 | 1.36 b | 0.33 |
| 1% Picochlorum burger | 11.42 c | 0.36 | 1.40 b | 0.34 |
| 1% Chlorella burger | 10.05 c | 0.41 | 1.60 c | 0.39 |
| Control Burger | 1% Isochrysis Burger | 1% Picochlorum Burger | 1% Chlorella Burger | |
|---|---|---|---|---|
| Swelling capacity (SWC) (mL/g DW) * | 3.14 ± 0.115 | 3.59 ± 0.116 c | 3.54 ± 0.1 c | 3.52 ± 0.002 c |
| Water holding capacity (WHC) (g/g DW) * | 2.31 ± 0.135 | 2.79 ±0.02 c | 2.81 ± 0.11 c | 2.84 ± 0.04 c |
| Oil holding capacity (OHC) (g/g DW) * | 0.85 ± 0.07 | 0.96 ± 0.04 a | 0.91 ± 0.002 a | 0.92 ± 0.001a |
| Control Burger | 1% Isochrysis Burger | 1% Picochlorum Burger | 1% Chlorella Burger | |
|---|---|---|---|---|
| L* | 46.75 ± 0.335 | 41.1 ± 0.95 a | 41.26 ± 0.9 a | 43.7 ± 0.7 a |
| a* | 10.65 ± 0.22 | 4.22 ± 0.135 a | 4.93 ± 0.05 a | 4.71 ± 0.03 a |
| b* | 11.4 ± 0.71 | 9.55 ± 0.3 a | 8.48 ± 0.55 a | 7.88 ± 0.48 a |
| Chroma (C*) | 15.6 ± 0.3 | 10.4 ± 0.1 a | 9.8 ± 0.2 a | 9.18 ± 0.2 a |
| Hue (H*) | 47± 0.2 | 66 ± 0.4 a | 60 ± 0.4 a | 60 ± 0.2 a |
| Control Burger | 1% Isochrysis Burger | 1% Picochlorum Burger | 1% Chlorella Burger | |
|---|---|---|---|---|
| Chlorophylls (mg/100g DW) | 0 | 23.28 ± 1.97 a | 26.52 ± 2.7 a | 23.14 ± 1.25 a |
| Phycocyanin (mg/100g DW) | 0 | 0.45 ±0.07 a | 0.47 ± 0.02 a | 0.45 ± 0.08 a |
| Carotenoids (mg/100g DW) | 0 | 12.5 ± 1.47 a | 11.43 ± 0.77 a | 10.31 ±0.8 a |
| Scavenging activity (%) * | 56.28 | 65.61a | 83.81 a | 95.23 a |
| Reducing power * | 0.35 | 0.75 a | 0.46 a | 0.50 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Atitallah, A.; Hentati, F.; Dammak, M.; Hadrich, B.; Fendri, I.; Ayadi, M.-A.; Michaud, P.; Abdelkafi, S.; Barkallah, M. Effect of Microalgae Incorporation on Quality Characteristics and Functional and Antioxidant Capacities of Ready-to-Eat Fish Burgers Made from Common Carp (Cyprinus carpio). Appl. Sci. 2019, 9, 1830. https://doi.org/10.3390/app9091830
Ben Atitallah A, Hentati F, Dammak M, Hadrich B, Fendri I, Ayadi M-A, Michaud P, Abdelkafi S, Barkallah M. Effect of Microalgae Incorporation on Quality Characteristics and Functional and Antioxidant Capacities of Ready-to-Eat Fish Burgers Made from Common Carp (Cyprinus carpio). Applied Sciences. 2019; 9(9):1830. https://doi.org/10.3390/app9091830
Chicago/Turabian StyleBen Atitallah, Ali, Faiez Hentati, Mouna Dammak, Bilel Hadrich, Imen Fendri, Mohamed-Ali Ayadi, Philippe Michaud, Slim Abdelkafi, and Mohamed Barkallah. 2019. "Effect of Microalgae Incorporation on Quality Characteristics and Functional and Antioxidant Capacities of Ready-to-Eat Fish Burgers Made from Common Carp (Cyprinus carpio)" Applied Sciences 9, no. 9: 1830. https://doi.org/10.3390/app9091830
APA StyleBen Atitallah, A., Hentati, F., Dammak, M., Hadrich, B., Fendri, I., Ayadi, M.-A., Michaud, P., Abdelkafi, S., & Barkallah, M. (2019). Effect of Microalgae Incorporation on Quality Characteristics and Functional and Antioxidant Capacities of Ready-to-Eat Fish Burgers Made from Common Carp (Cyprinus carpio). Applied Sciences, 9(9), 1830. https://doi.org/10.3390/app9091830








