Synthesis of Quaternary Ammonium Room-Temperature Ionic Liquids and their Application in the Dissolution of Cellulose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ionic Liquids
2.2. Cellulose Dissolution and Preparation of the Regenerated Cellulose Film
2.3. Analysis Instruments
3. Results and Discussion
3.1. The Physical Properties of the Prepared Ionic Liquids
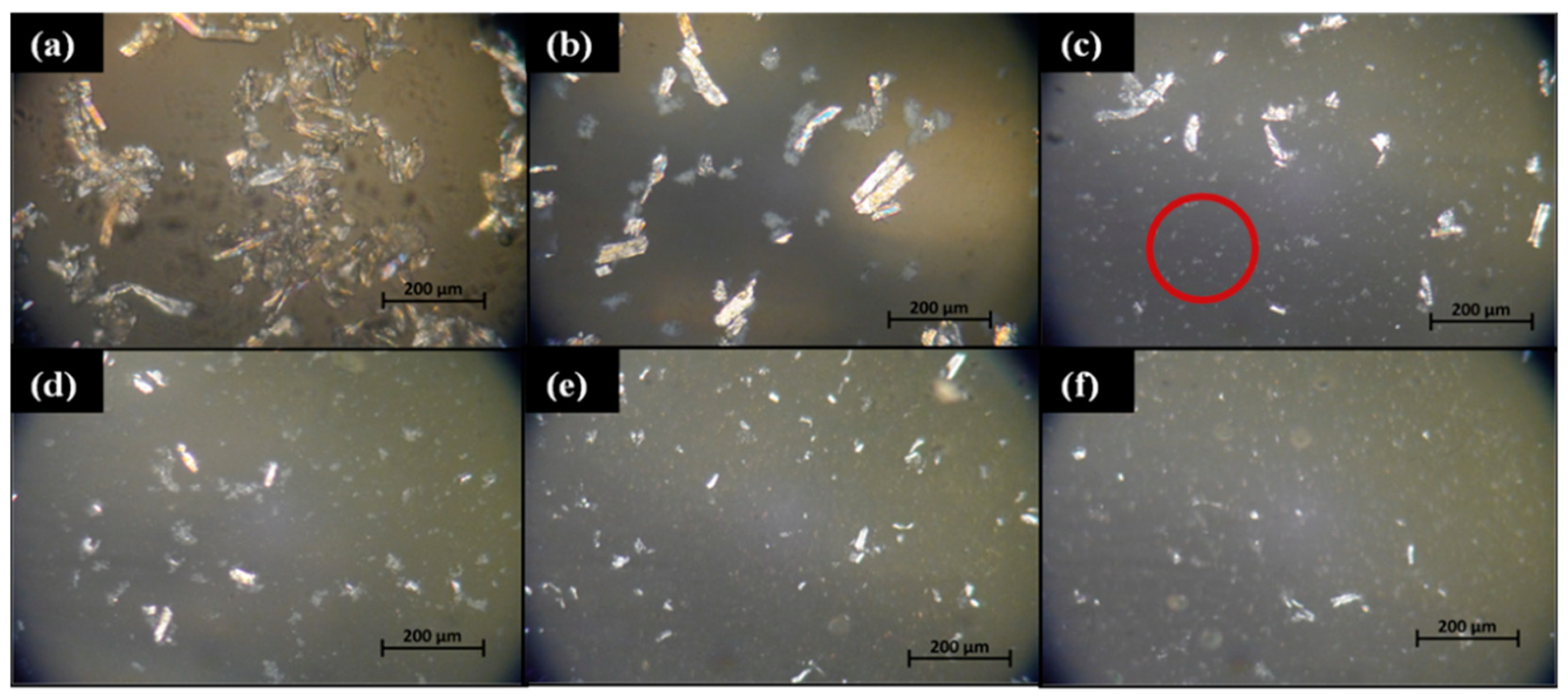
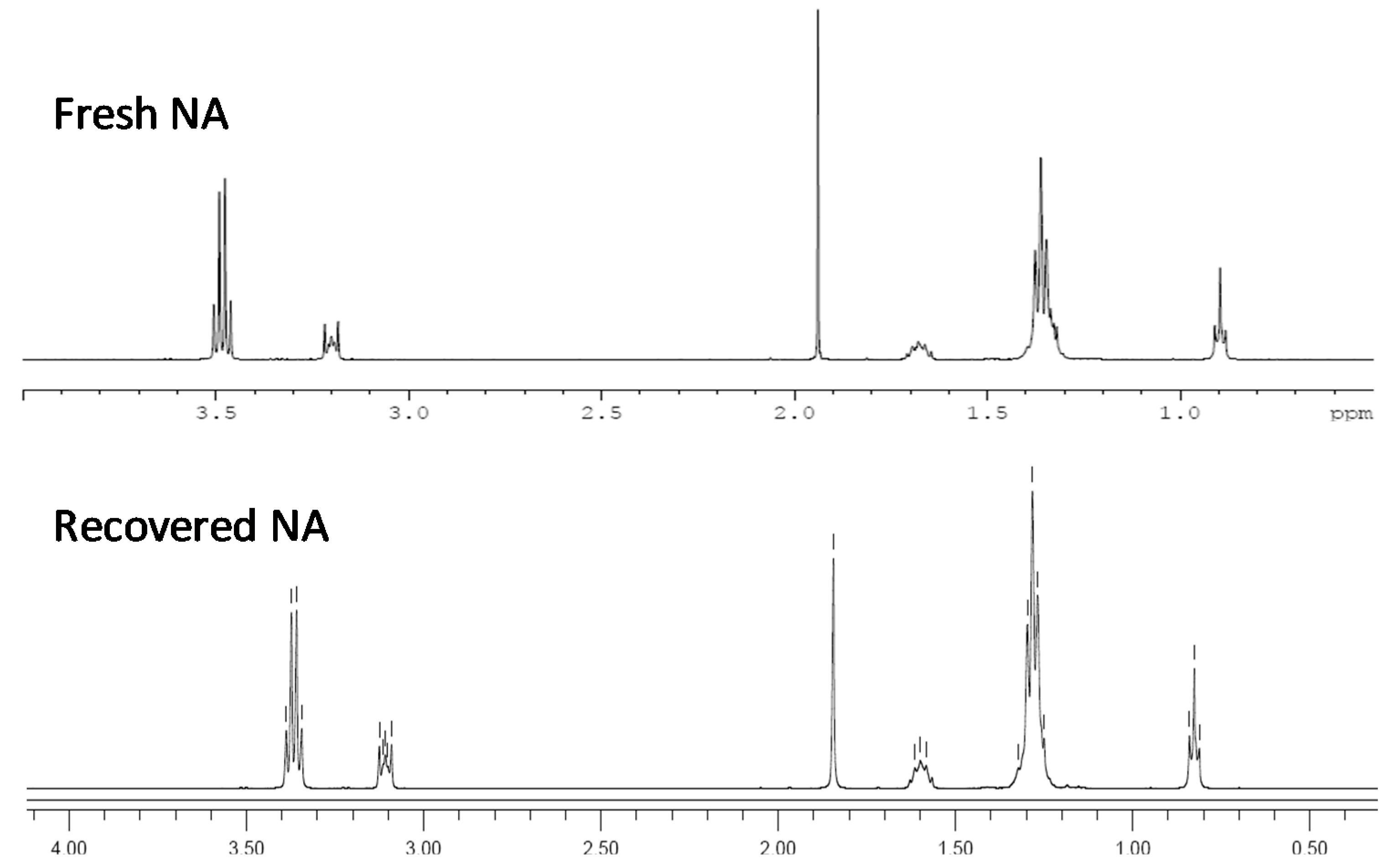
3.2. Cellulose Dissolution
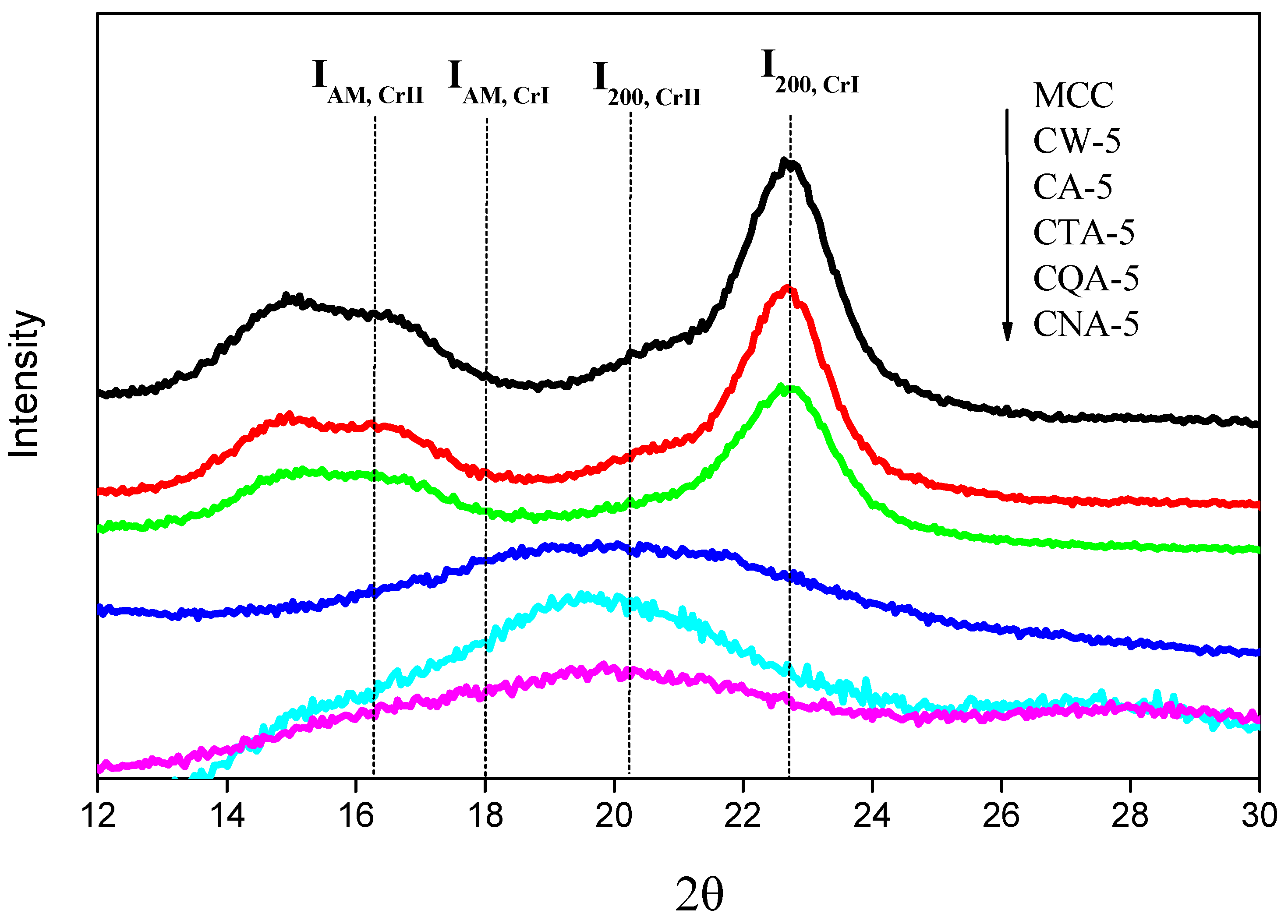
3.3. The Crystallinity of the Regenerated Cellulose Film
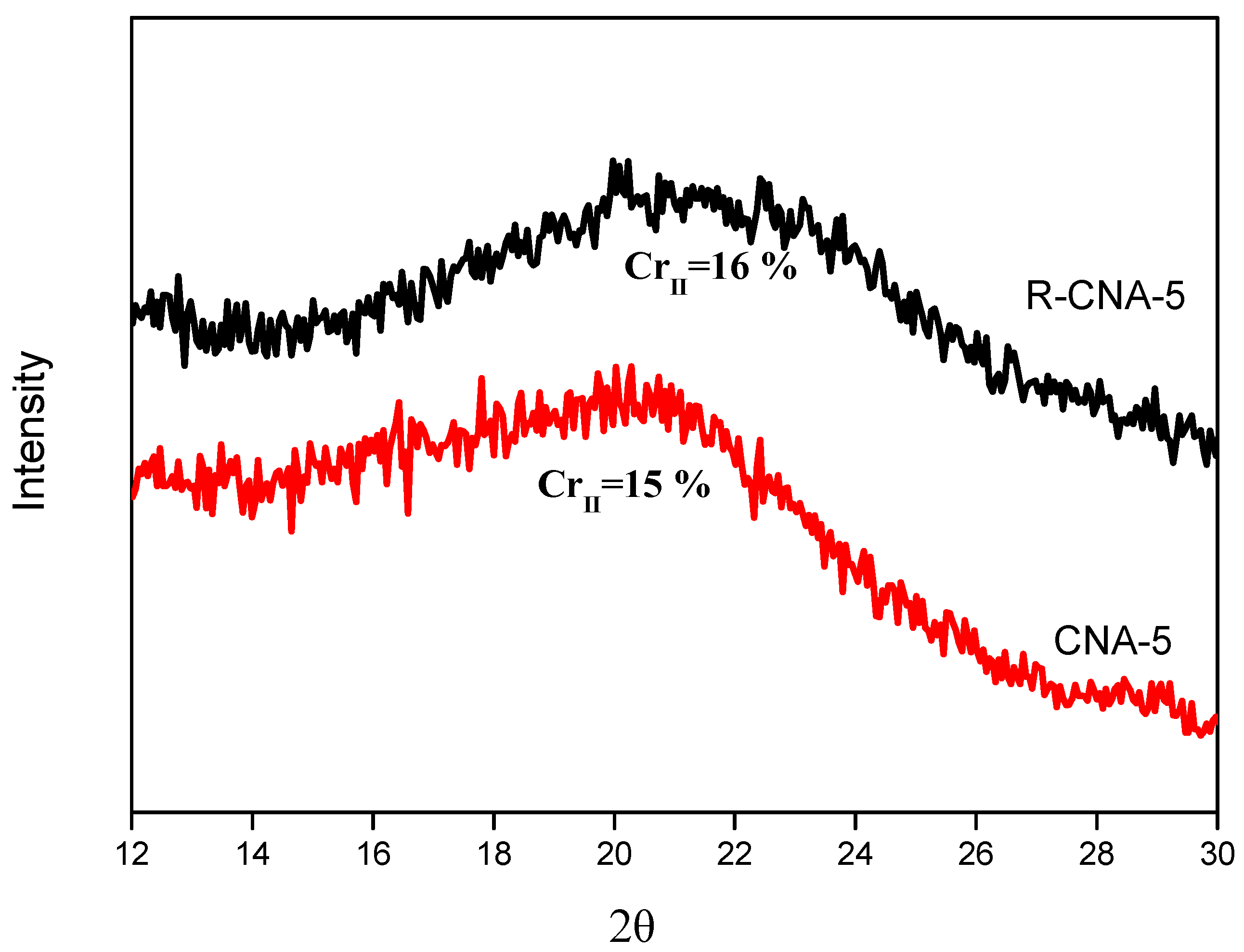
3.4. X-Ray Diffraction Spectrum
3.5. Infrared Spectroscopy
3.6. Degree of Polymerization of the Regenerated Cellulose Film
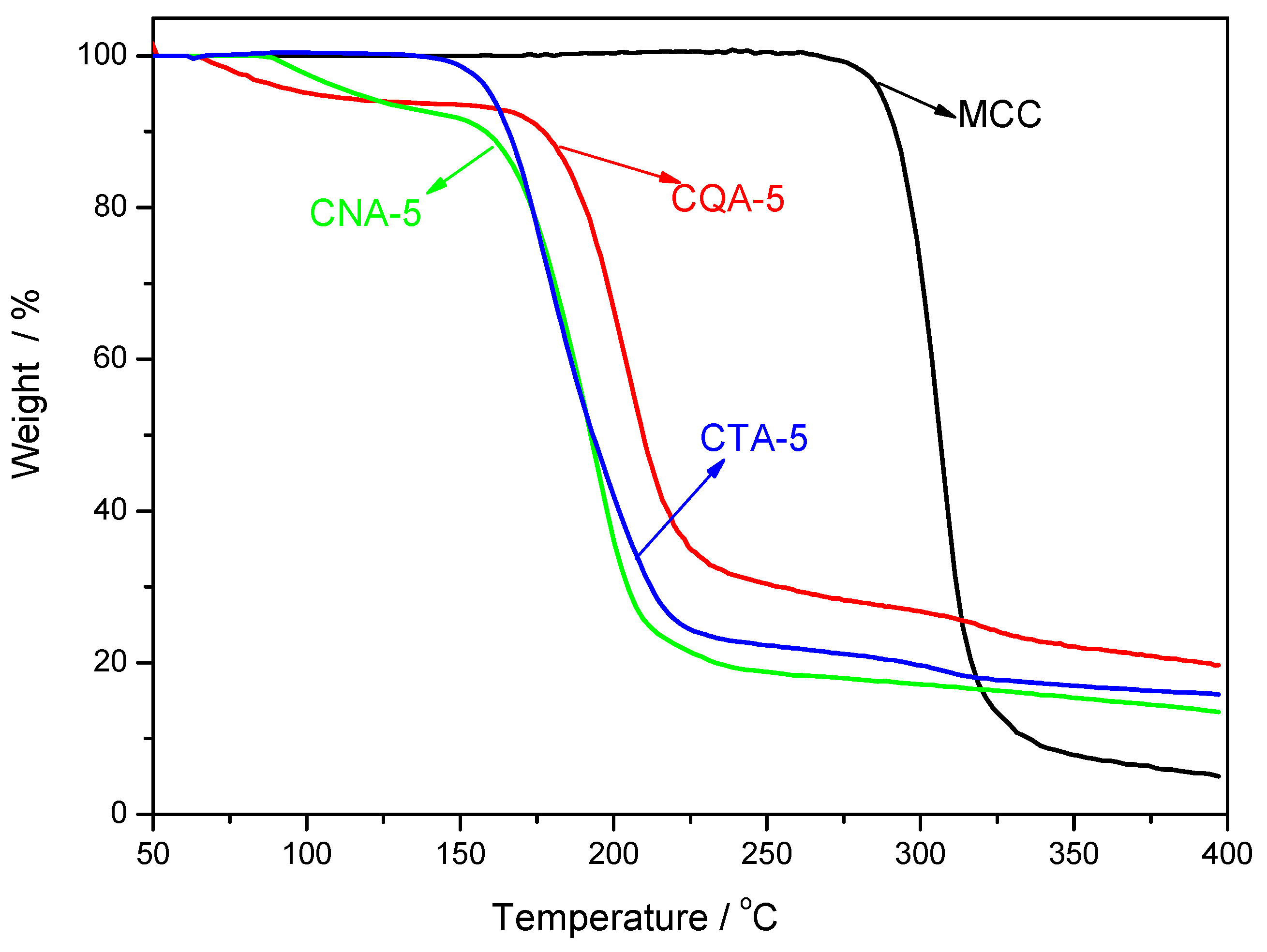
3.7. Thermal Stability of the Regenerated Cellulose Films
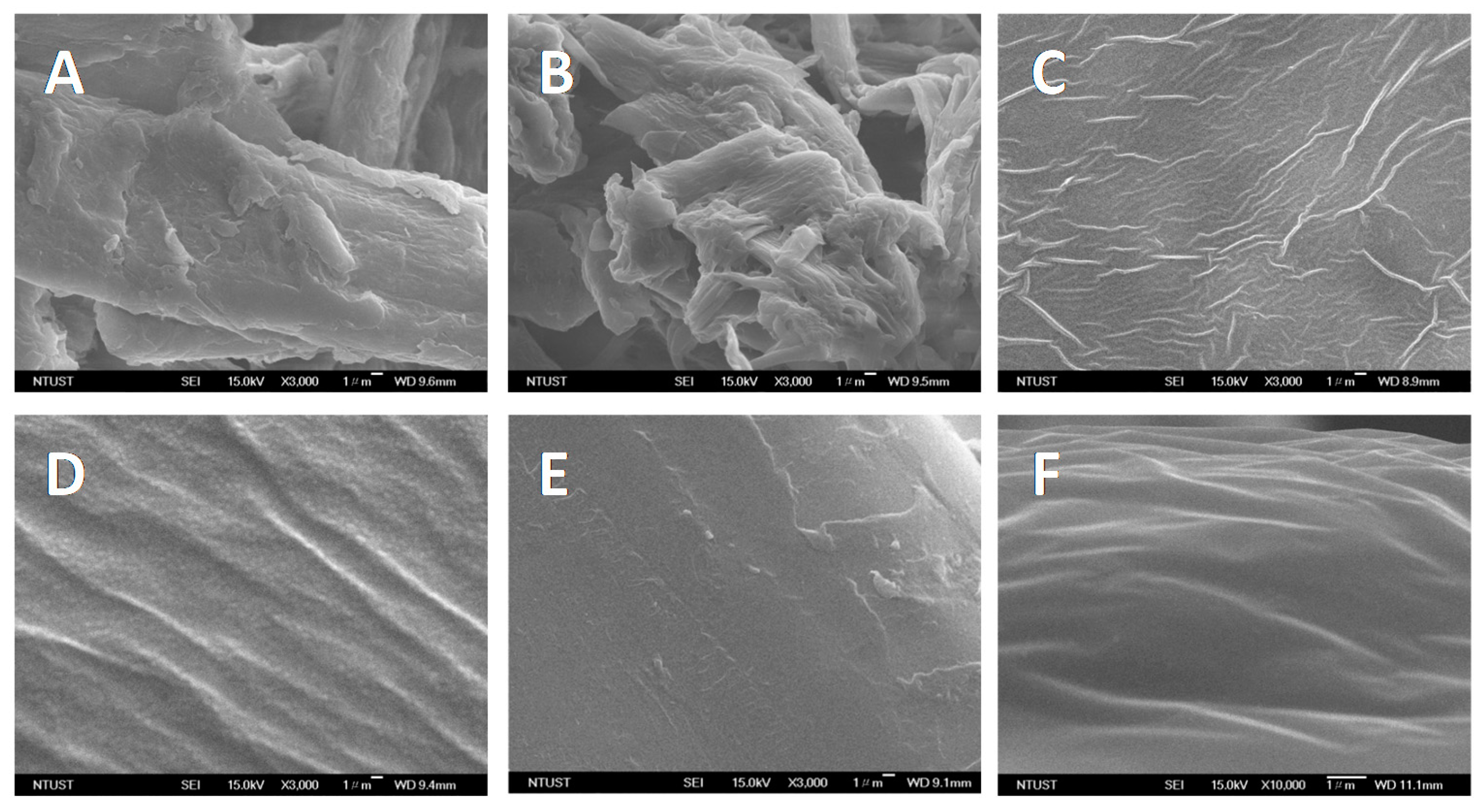
3.8. Morphology of the Regenerated Cellulose Film
3.9. Recycling of the Ionic Liquids
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic Liquids and Their Interaction with Cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Liebert, T. Unconventional Methods in Celulose Functionalization. Progress in Polymer Science. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Sixta, H.; Kosma, P. The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Prog. Polym. Sci. 2001, 26, 1763–1837. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: fascinating biopolymer and sustainable raw material. Angew. Chem. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H.; Li, Z.; Lu, X.; Zhang, X.; Zhang, S.; Zhou, K. Characterization of the regenerated cellulose films in ionic liquids and rheological properties of the solutions. Mater. Chem. Phys. 2011, 128, 220–227. [Google Scholar] [CrossRef]
- Kosan, B.; Michels, C.; Meister, F. Dissolution and forming of cellulose with ionic liquids. Cellulose 2008, 15, 59–66. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Abe, M.; Fukaya, Y.; Ohno, H. Fast and facile dissolution of cellulose with tetrabutylphosphonium hydroxide containing 40 wt% water. Chem. Commun. 2012, 48, 1808–1810. [Google Scholar] [CrossRef]
- Abe, M.; Kuroda, K.; Ohno, H. Maintenance-free cellulose solvents based on onium hydroxides. ACS Sustain. Chem. Eng. 2015, 3, 1771–1776. [Google Scholar] [CrossRef]
- Ema, T.; Komiyama, T.; Sunami, S.; Sakai, T. Synergistic effect of quaternary ammonium hydroxide and crown ether on the rapid and clear dissolution of cellulose at room temperature. RSC Adv. 2014, 4, 2523–2525. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, C.M.; Huang, F.; Jia, H.H.; Wei, P. Wheat straw cellulose dissolution and isolation by tetra-n-butylammonium hydroxide. Carbohydr. Polym. 2013, 94, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Yamada, T.; Ohno, H. Dissolution of wet wood biomass without heating. RSC Adv. 2014, 4, 17136–17140. [Google Scholar] [CrossRef]
- Hyvakko, U.; King, A.W.T.; Kilpelainen, I. Extraction of Wheat Straw with Aqueous Tetra-n-Butylphosphonium Hydroxide. BioResources 2014, 9, 1565–1577. [Google Scholar] [CrossRef]
- Abe, M.; Yamanaka, S.; Yamada, H.; Yamada, T.; Ohno, H. Almost complete dissolution of woody biomass with tetra-n-butylphosphonium hydroxide aqueous solution at 60° C. Green Chem. 2015, 17, 4432–4438. [Google Scholar] [CrossRef]
- Gubitosi, M.; Duarte, H.; Gentile, L.; Olsson, U.; Medronho, B. On cellulose dissolution and aggregation in aqueous tetrabutylammonium hydroxide. Biomacromolecules 2016, 17, 2873–2881. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, W. Synthesis of cationic starch with a high degree of substitution in an ionic liquid. Carbohyd. Polym. 2010, 80, 1172–1177. [Google Scholar] [CrossRef]
- Kroon, M.C.; Buijs, W.; Peters, C.J.; Witkamp, G.J. Quantum chemical aided prediction of the thermal decomposition mechanisms and temperatures of ionic liquids. Thermochim. Acta 2007, 465, 40–47. [Google Scholar] [CrossRef]
- Wang, Y.; Voth, G.A. Unique spatial heterogeneity in ionic liquids. J. Am. Chem. Soc. 2005, 127, 12192–12193. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Yan, T.; Voth, G.A. Understanding Ionic Liquids through Atomistic and Coarse-Grained Molecular Dynamics Simulations. Acc. Chem. Res. 2007, 40, 1193–1199. [Google Scholar] [CrossRef]
- Azubuike, C.P.; Rodrı’guez, H.; Okhamafe, A.O.; Rogers, R.D. Physicochemical properties of maize cob cellulose powders reconstituted from ionic liquid solution. Cellulose 2012, 19, 425–433. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin Jr, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1311. [Google Scholar] [CrossRef]
- O’Connor, R.T.; DuPré, E.F.; Mitcham, D. Applications of Infrared Absorption Spectroscopy to Investigations of Cotton and Modified Cottons: Part I: Physical and Crystalline Modifications and Oxidation. Text. Res. J. 1958, 28, 382–392. [Google Scholar] [CrossRef]
- Kono, H.; Numata, Y. Two-dimensional spin-exchange solid-state NMR study of the crystal structure of cellulose II. Polymer 2004, 45, 4541–4547. [Google Scholar] [CrossRef]
- Guo, L.Y.; Shi, T.J.; Duan, Y.F. Synthesis of [AEIM]Cl and its Solubility for Microcrystalline Cellulose by Microwave Heating Method. Chin. J. Appl. Chem. 2009, 26, 1005–1010. [Google Scholar]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose [correction of cellose] with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]

| ILs | Cation Structure | Molecular Weight | Melting Temperature | η a (cp) | Eflow b (kcal/mol) | Td c |
|---|---|---|---|---|---|---|
| QA | 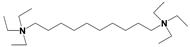 | 461 | <−70 °C | 886 | 10.7 | 180 °C |
| TA | 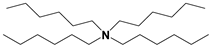 | 414 | <−70 °C | 443 | 9.2 | 173 °C |
| NA |  | 245 | <−70 °C | 59 | 8.1 | 180 °C |
| Sample | Crystal Phase | Crystallinity Index (%) |
|---|---|---|
| MCC | type I | 68 |
| CW-5 | 58 | |
| CA-5 | 44 | |
| CQA-5 | type II | 30 |
| CTA-5 | 26 | |
| CNA-5 | 15 | |
| CNA-2 | 10 | |
| CNA-10 | 19 | |
| CNA-12 | 49 |
| Cellulose Materials | LOI A1432/A898 | TCI A1372/A2904 | Degree of Polymerization |
|---|---|---|---|
| MCC | 2.525 | 0.118 | 309 |
| CW-5 | 2.448 | 0.093 | 299 |
| CA-5 | 1.815 | 0.085 | 208 |
| CQA-5 | 1.124 | 0.054 | 62 |
| CTA-5 | 0.482 | 0.036 | 146 |
| CNA-2 | 0.275 | 0.020 | 79 |
| CNA-5 | 0.382 | 0.023 | 174 |
| CNA-10 | 0.534 | 0.028 | 219 |
| CNA-12 | 0.859 | 0.055 | 269 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, Y.-H.; Lee, Y.-Y.; Chen, S.-H. Synthesis of Quaternary Ammonium Room-Temperature Ionic Liquids and their Application in the Dissolution of Cellulose. Appl. Sci. 2019, 9, 1750. https://doi.org/10.3390/app9091750
Tseng Y-H, Lee Y-Y, Chen S-H. Synthesis of Quaternary Ammonium Room-Temperature Ionic Liquids and their Application in the Dissolution of Cellulose. Applied Sciences. 2019; 9(9):1750. https://doi.org/10.3390/app9091750
Chicago/Turabian StyleTseng, Yao-Hsuan, Yu-Yin Lee, and Shih-Hsun Chen. 2019. "Synthesis of Quaternary Ammonium Room-Temperature Ionic Liquids and their Application in the Dissolution of Cellulose" Applied Sciences 9, no. 9: 1750. https://doi.org/10.3390/app9091750





