Optimization of Salix Carbonation Solid Acid Catalysts for One-Step Synthesis by Response Surface Method
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Walter, B.; Gruson, J.F.; Monnier, G. Motorisation Et Carburants Diesel: Un Large Spectre D’évolutions À Venir Contexte Général Et Thématiques De Recherche. Oil Gas Sci. Technol. 2008, 63, 387–393. [Google Scholar]
- Luque, R.; Lovett, J.C.; Datta, B.; Clancy, J.; Campelo, J.M.; Romero, A.A. Biodiesel as feasible petrol fuel replacement: A multidisciplinary overview. Energy Environ. Sci. 2010, 3, 1706–1721. [Google Scholar] [CrossRef]
- Wilson, K.; Lee, A.F. ChemInform Abstract: Rational Design of Heterogeneous Catalysts for Biodiesel Synthesis. Catal. Sci. Technol. 2012, 2, 884–897. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B.; Korstad, J. Advancements in solid acid catalysts for ecofriendly and economically viable synthesis of biodiesel. Biofuels Bioprod. Biorefin. 2011, 5, 69–92. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of Biodiesel via Acid Catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Koh, M.Y. A review of biodiesel production from Jatropha curcas L. Oil. Renew. Sustain. Energy Rev. 2011, 15, 2240–2251. [Google Scholar] [CrossRef]
- Luque, R.; Clark, J.H. Biodiesel-Like biofuels from simultaneous. transesterification/esterification of waste oils with a biomass-derived solid acid catalyst. ChemCatChem 2015, 3, 594–597. [Google Scholar] [CrossRef]
- Venkateswarulu, T.C.; Raviteja, C.V.; Prabhaker, K.V.; Babu, D.J.; Reddy, A.R.; Indira, M.; Venkatanarayana, A. A Review on Methods of Transesterification of Oils and Fats in Bio-diesel Formation. Int. J. ChemTech Res. 2014, 6, 974–4290. [Google Scholar]
- Liu, H.A.; Bo, Y. Advances in the research of stillingia oil components as feedstock of biodiesel. Genom. Appl. Biol. 2010, 29, 402–408. [Google Scholar]
- Masakuza, T.; Atsushi, T.; Kondo, J.N.; Okamura, M.; Hayashi, S.; Hara, M. Biodiesel made with sugar catalyst. Nature 2005, 438, 178–179. [Google Scholar]
- Lou, W.Y.; Zong, M.H.; Duan, Z.Q. Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour. Technol. 2008, 99, 8752–8758. [Google Scholar] [CrossRef]
- Zong, M.H.; Duan, Z.Q.; Lou, W.Y.; Smith, T.J.; Wu, H. Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green Chem. 2007, 9, 434–437. [Google Scholar] [CrossRef]
- Kastner, J.R.; Miller, J.; Geller, D.P.; Locklin, J.; Keith, L.H.; Johnson, T. Catalytic esterification of fatty acids using solid acid catalysts generated from biochar and activated carbon. Catal. Today 2012, 190, 122–132. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Áron, N. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Geboers, J.; Jacobs, P.A.; Sels, B.F. Recent Advances in the Catalytic Conversion of Cellulose. ChemCatChem 2011, 3, 82–94. [Google Scholar] [CrossRef]
- Dusselier, M.; Mascal, M.; Sels, B.F. Selective Catalysis for Renewable Feedstocks and Chemicals. In Top Chemical Opportunities from Carbohydrate Biomass: A Chemist’s View Biorefinery; Nicholas, K.M., Ed.; Springer International Publishing: Berlin, Germany, 2014; Volume 353, pp. 1–40. [Google Scholar]
- Geboers, J.A.; Van De Vyver, S.; Ooms, R.; Op De Beeck, B.; Jacobs, P.A.; Sels, B.F. Chemocatalytic conversion of cellulose: Opportunities, advances and pitfalls. Catal. Sci. Technol. 2011, 1, 714–726. [Google Scholar] [CrossRef]
- Tuhy, Ł.; Samoraj, M.; Michalak, I.; Chojnacka, K. The Application of Biosorption for Production of Micronutrient Fertilizers Based on Waste Biomass. Appl. Biochem. Biotechnol. 2014, 174, 1376–1392. [Google Scholar] [CrossRef]
- Vamvuka, D. Bio-oil, solid and gaseous biofuels from biomass pyrolysis processes—An overview. Int. J. Energy Res. 2011, 35, 835–862. [Google Scholar] [CrossRef]
- Sims, R.E.H. Bioenergy to mitigate for climate change and meet the needs of society, the economy and the environment. Mitig. Adapt. Strateg. Glob. Chang. 2003, 8, 349–370. [Google Scholar] [CrossRef]
- Shu, Q.; Gao, J.; Nawaz, Z.; Liao, Y.; Wang, D.; Wang, J. Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon-based solid acid catalyst. Appl. Energy 2010, 87, 2589–2596. [Google Scholar] [CrossRef]
- Shu, Q.; Nawaz, Z.; Gao, J.; Liao, Y.; Zhang, Q.; Wang, D.; Wang, J. Synthesis of biodiesel from a model waste oil feedstock using a carbon-based solid acid catalyst: Reaction and separation. Bioresour. Technol. 2010, 101, 5374–5384. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Zhang, X.F.; Wang, K.B.; Yan, X.L.; Zhao, Y.B.; Guo, X.M. Alcoholysis of Salix psammophila liquefaction. Sci. Technol. Rev. 2014, 32, 37–40. [Google Scholar]
- Huang, J.T.; Gao, G.H. Analysis of NMR spectra on liquefied products from Salix psammophila and Caragana intermedia woods. Chem. Ind. For. Prod. 2009, 29, 101–104. [Google Scholar]
- Liu, X.; Wan, Y.; Liu, P.; Zhao, L.; Zou, W. Optimization of process conditions for preparation of activated carbon from waste Salix psammophila and its adsorption behavior on fluoroquinolone antibiotics. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2018, 77, 2555. [Google Scholar] [CrossRef]
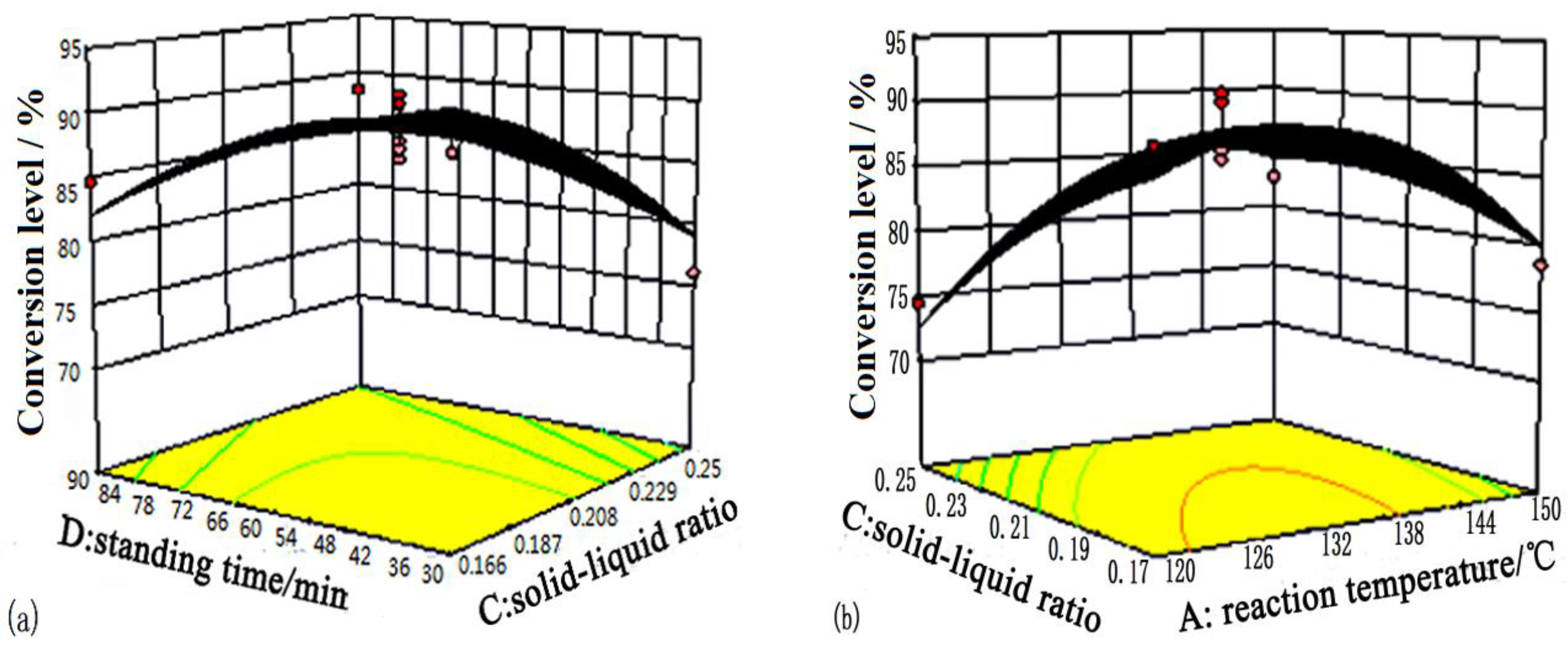
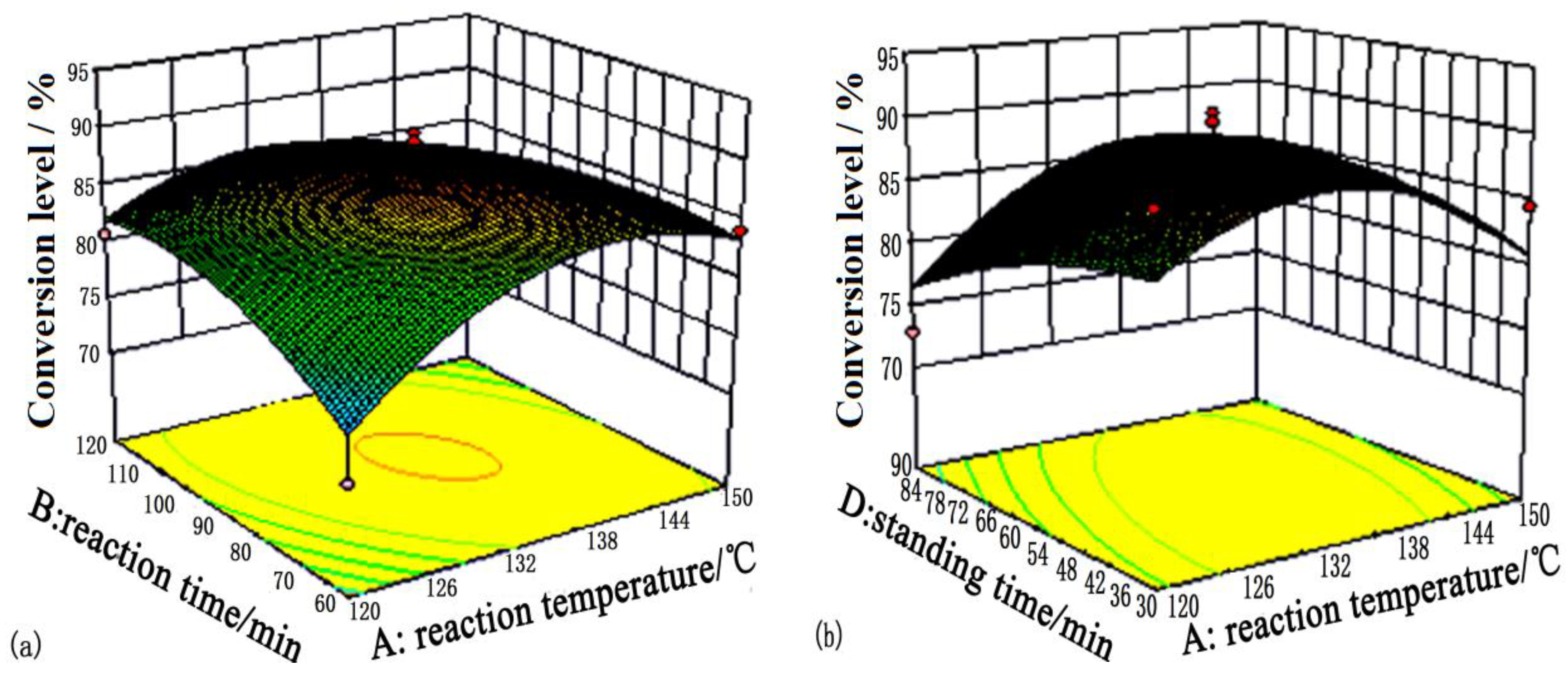
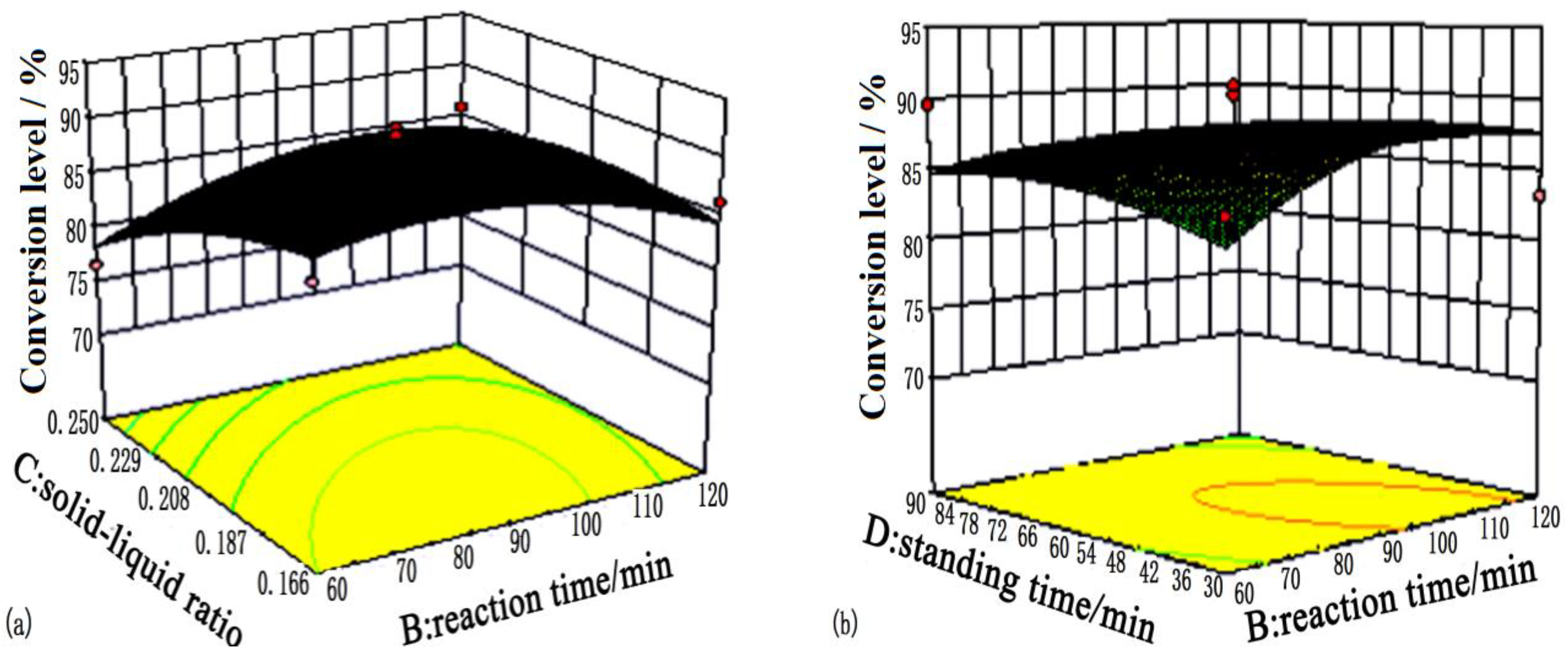
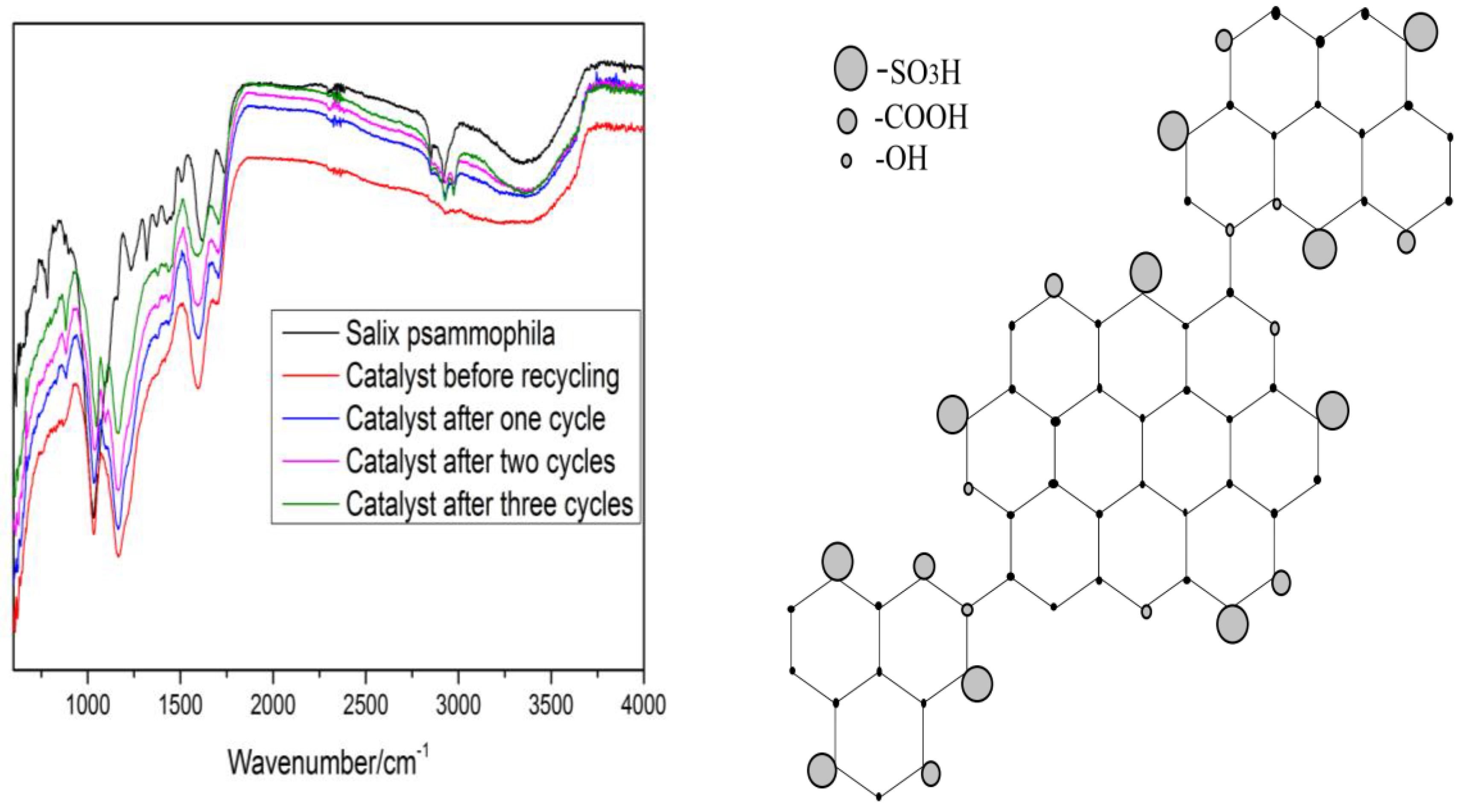
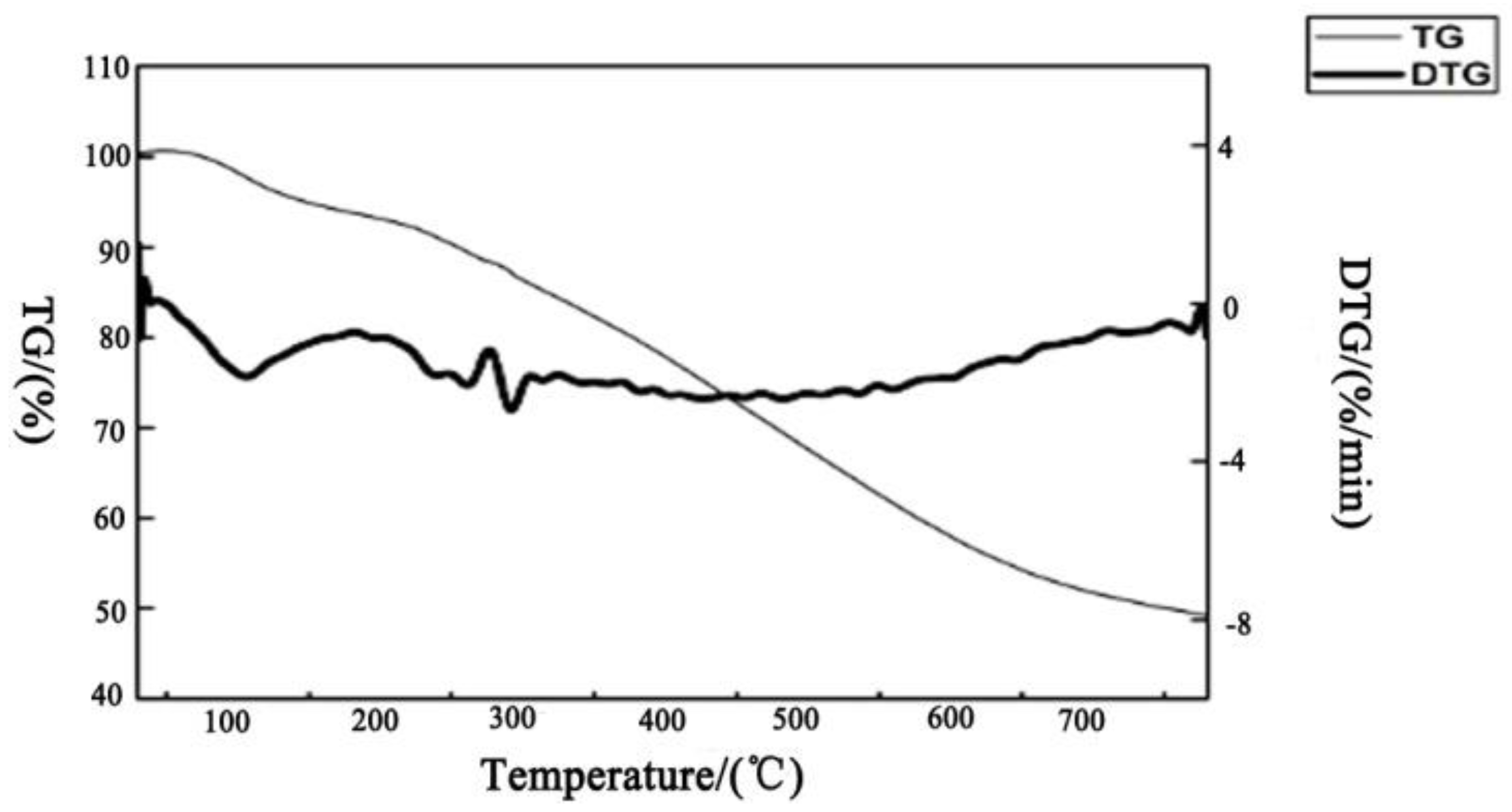
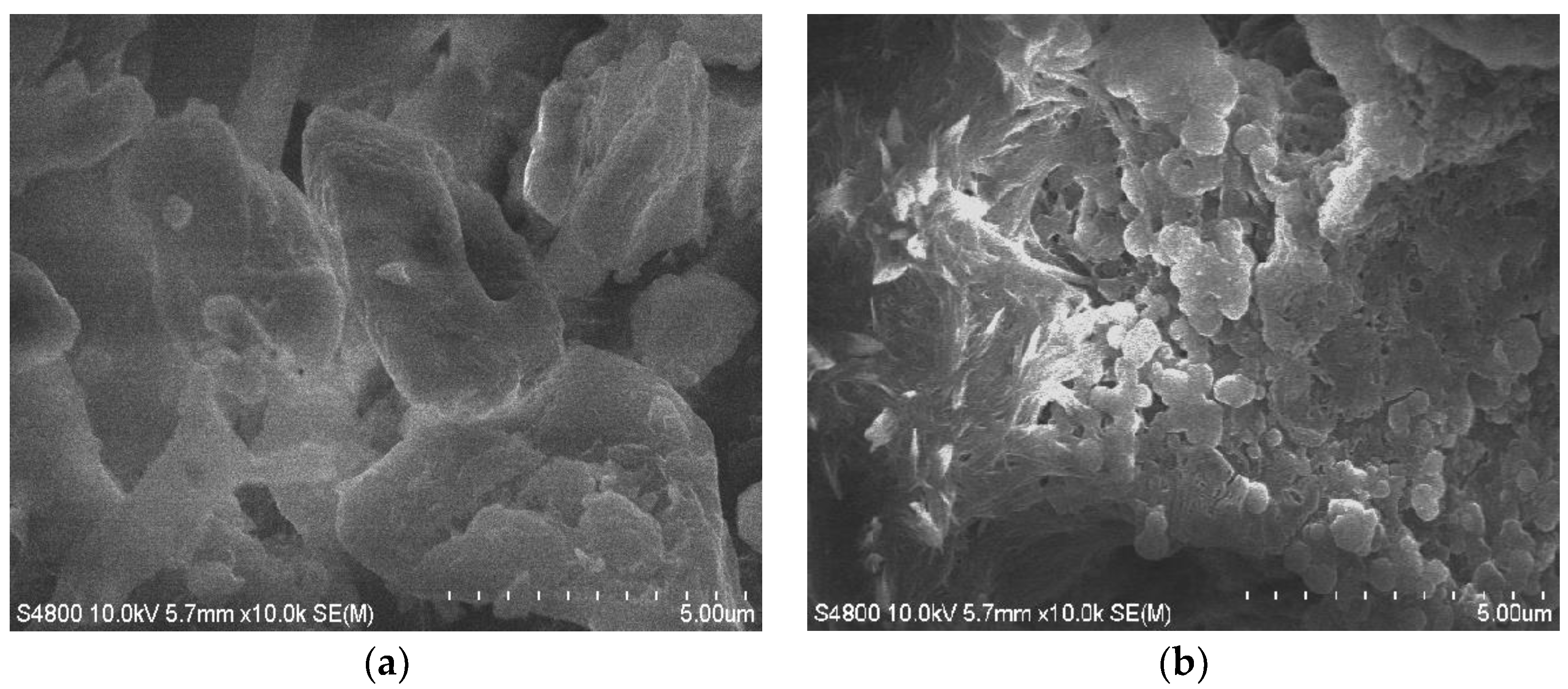
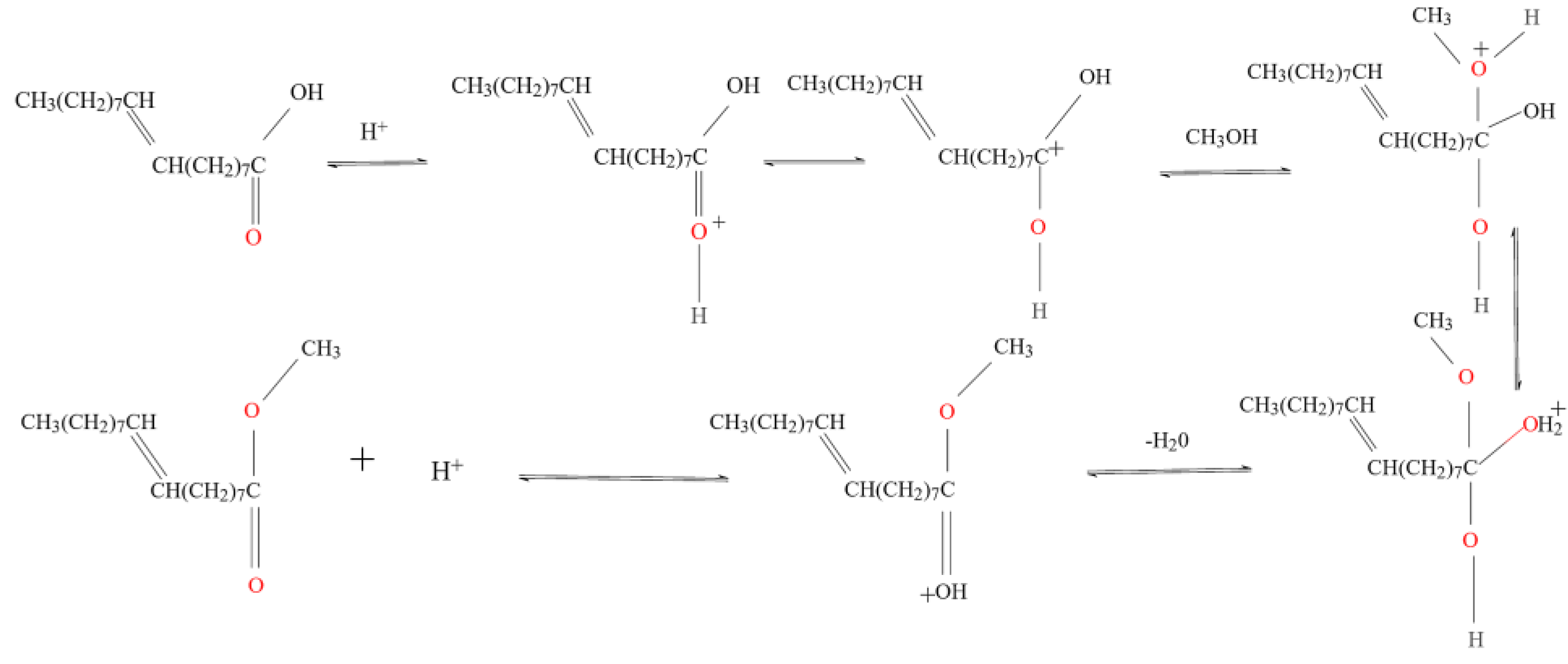
| Levels | A: Reaction Temperature/°C | B: Reaction Time/min | C: Solid-Liquid Ratio | D: Standing Time/min |
|---|---|---|---|---|
| −1 | 120 | 60 | 1:4 | 30 |
| 0 | 135 | 90 | 1:5 | 60 |
| 1 | 150 | 120 | 1:6 | 90 |
| Number | A: Reaction Temperature/°C | B: Reaction Time/min | C: Solid-Liquid Ratio | D: Standing Time/min | Conversion Level/% |
|---|---|---|---|---|---|
| 1 | 135 | 120 | 1:5 | 30 | 83.19 |
| 2 | 135 | 90 | 1:6 | 30 | 89.98 |
| 3 | 135 | 90 | 1:5 | 60 | 86.69 |
| 4 | 135 | 60 | 1:5 | 30 | 84.01 |
| 5 | 135 | 60 | 1:6 | 60 | 86.04 |
| 6 | 150 | 90 | 1:5 | 90 | 78.93 |
| 7 | 150 | 120 | 1:5 | 60 | 81.71 |
| 8 | 120 | 90 | 1:4 | 60 | 74.49 |
| 9 | 135 | 120 | 1:4 | 60 | 85.89 |
| 10 | 135 | 90 | 1:6 | 90 | 84.67 |
| 11 | 135 | 120 | 1:5 | 90 | 79.61 |
| 12 | 150 | 60 | 1:5 | 60 | 84.15 |
| 13 | 150 | 90 | 1:5 | 30 | 84.51 |
| 14 | 120 | 90 | 1:6 | 60 | 88.97 |
| 15 | 135 | 120 | 1:6 | 60 | 86.20 |
| 16 | 120 | 90 | 1:5 | 30 | 88.33 |
| 17 | 135 | 90 | 1:5 | 60 | 87.33 |
| 18 | 150 | 90 | 1:4 | 60 | 82.53 |
| 19 | 120 | 120 | 1:5 | 60 | 80.70 |
| 20 | 150 | 90 | 1:6 | 60 | 78.60 |
| 21 | 135 | 90 | 1:5 | 60 | 85.98 |
| 22 | 135 | 90 | 1:5 | 60 | 90.27 |
| 23 | 135 | 60 | 1:4 | 60 | 76.60 |
| 24 | 120 | 90 | 1:5 | 90 | 72.86 |
| 25 | 120 | 60 | 1:5 | 60 | 71.05 |
| 26 | 135 | 60 | 1:5 | 90 | 89.51 |
| 27 | 135 | 90 | 1:4 | 90 | 88.49 |
| 28 | 135 | 90 | 1:5 | 60 | 90.93 |
| 29 | 135 | 90 | 1:4 | 30 | 76.14 |
| Source Model | Sum of Squares | Degrees Freedom | Mean Square | F-Value | P-Value > F-Value | Significant Level |
|---|---|---|---|---|---|---|
| model | 603.943 | 14 | 43.139 | 2.6279 | 0.041 | significant |
| A | 16.4034 | 1 | 16.403 | 0.9993 | 0.335 | |
| B | 2.9403 | 1 | 2.940 | 0.1791 | 0.679 | |
| C | 76.6085 | 1 | 76.609 | 4.6669 | 0.049 | significant |
| D | 12.1807 | 1 | 12.181 | 0.7420 | 0.404 | |
| AB | 36.5420 | 1 | 36.542 | 2.2261 | 0.158 | |
| AC | 84.7320 | 1 | 84.732 | 5.1617 | 0.039 | significant |
| AD | 24.4530 | 1 | 24.453 | 1.4896 | 0.242 | |
| BC | 20.8392 | 1 | 20.839 | 1.2695 | 0.279 | |
| BD | 20.6116 | 1 | 20.612 | 1.2556 | 0.281 | |
| CD | 77.9689 | 1 | 77.969 | 4.7497 | 0.047 | significant |
| A2 | 206.912 | 1 | 206.91 | 12.605 | 0.003 | significant |
| B2 | 55.2748 | 1 | 55.275 | 3.3672 | 0.088 | |
| C2 | 18.2349 | 1 | 18.235 | 1.1108 | 0.310 | |
| D2 | 14.0723 | 1 | 14.072 | 0.8573 | 0.370 | |
| Residual | 229.816 | 14 | 16.415 | |||
| Lack of Fit | 210.121 | 10 | 21.012 | 4.2675 | 0.087 | not significant |
| Pure Error | 19.6952 | 4 | 4.924 | |||
| Cor Total | 28 |
| C | H | N | O | S | |
|---|---|---|---|---|---|
| Salix psammophila | 51.886% | 6.316% | 0.31% | 41.348% | 0.14% |
| Catalyst | 50.122% | 7.583% | 0.293% | 15.624% | 26.378% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.; Wang, K.; Gong, J. Optimization of Salix Carbonation Solid Acid Catalysts for One-Step Synthesis by Response Surface Method. Appl. Sci. 2019, 9, 1518. https://doi.org/10.3390/app9081518
Lu P, Wang K, Gong J. Optimization of Salix Carbonation Solid Acid Catalysts for One-Step Synthesis by Response Surface Method. Applied Sciences. 2019; 9(8):1518. https://doi.org/10.3390/app9081518
Chicago/Turabian StyleLu, Ping, Kebing Wang, and Juhui Gong. 2019. "Optimization of Salix Carbonation Solid Acid Catalysts for One-Step Synthesis by Response Surface Method" Applied Sciences 9, no. 8: 1518. https://doi.org/10.3390/app9081518
APA StyleLu, P., Wang, K., & Gong, J. (2019). Optimization of Salix Carbonation Solid Acid Catalysts for One-Step Synthesis by Response Surface Method. Applied Sciences, 9(8), 1518. https://doi.org/10.3390/app9081518




