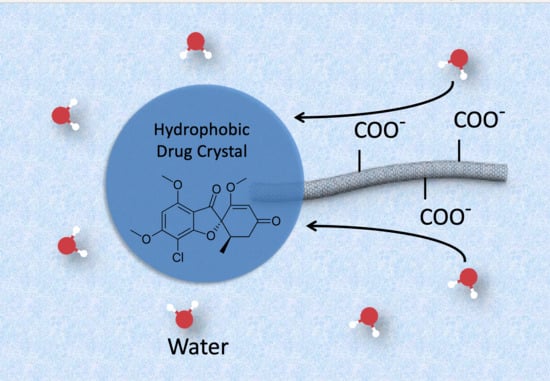
Controlling the Dissolution Rate of Hydrophobic Drugs by Incorporating Carbon Nanotubes with Different Levels of Carboxylation
Abstract
1. Introduction
2. Material and Methods
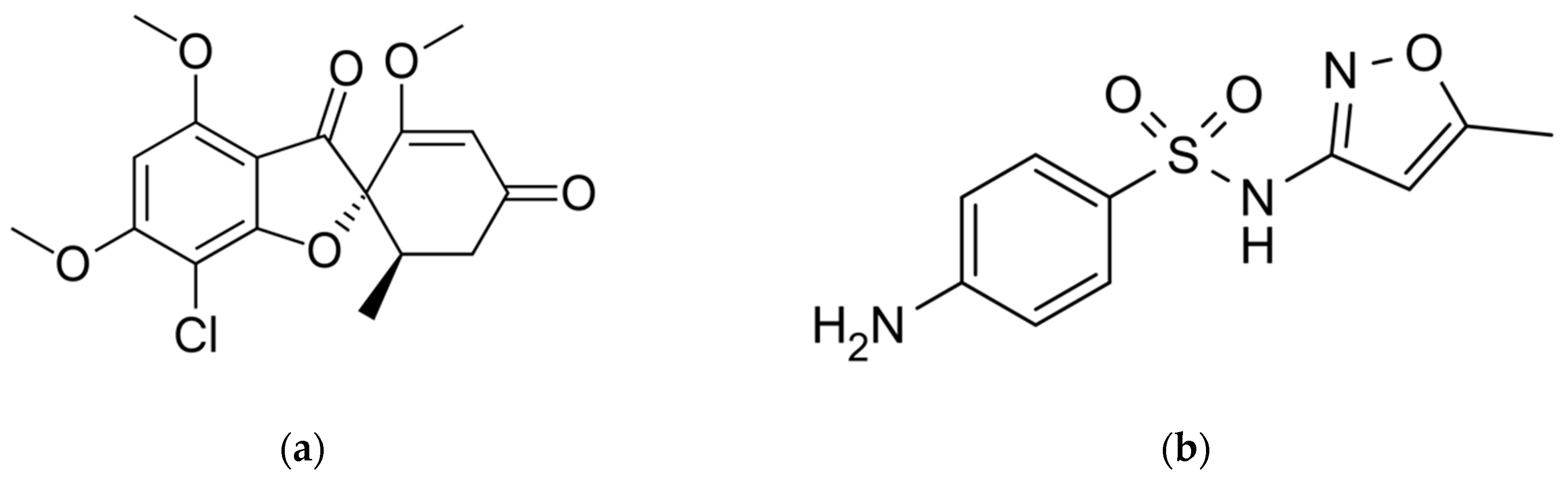
2.1. Materials
2.2. Methods
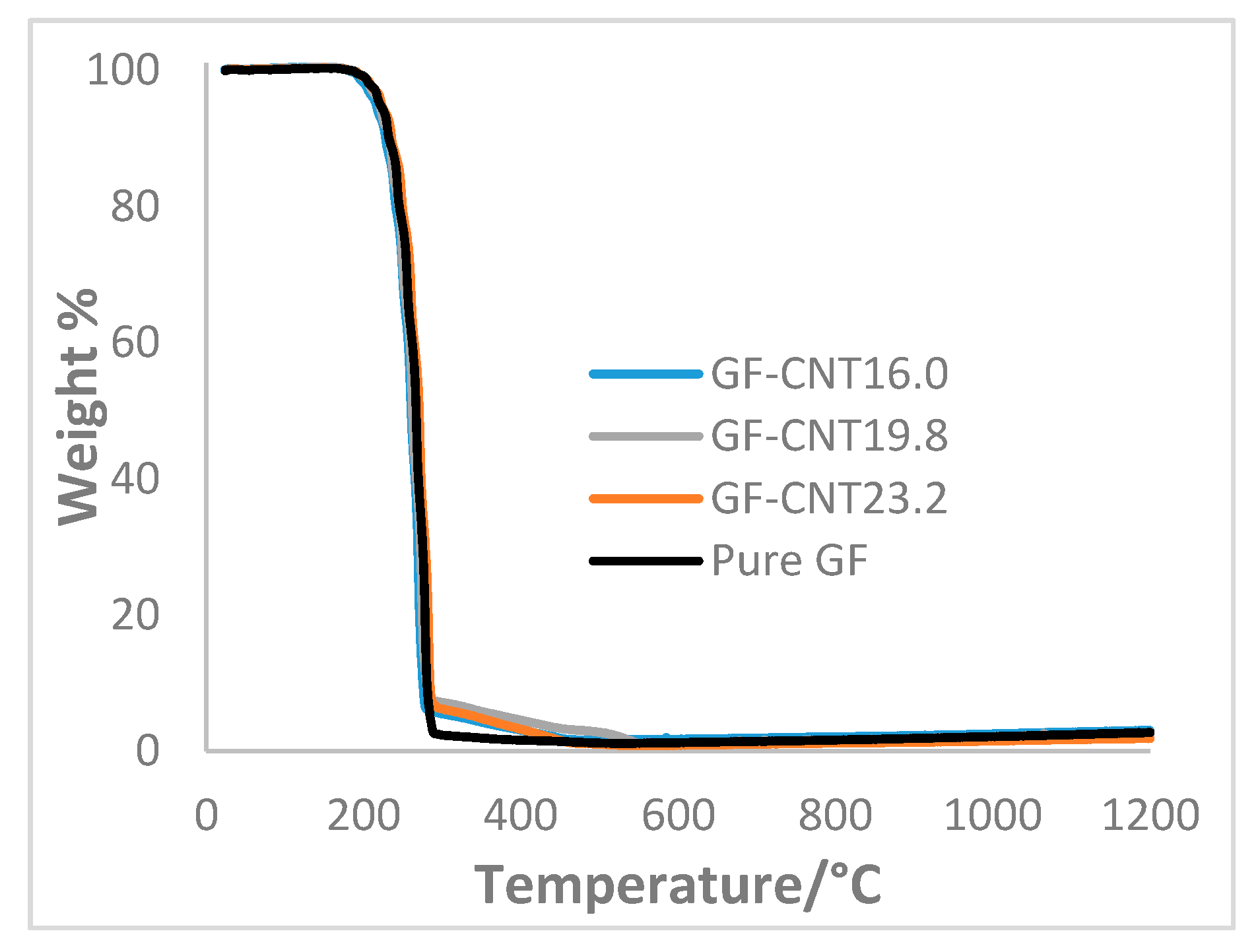
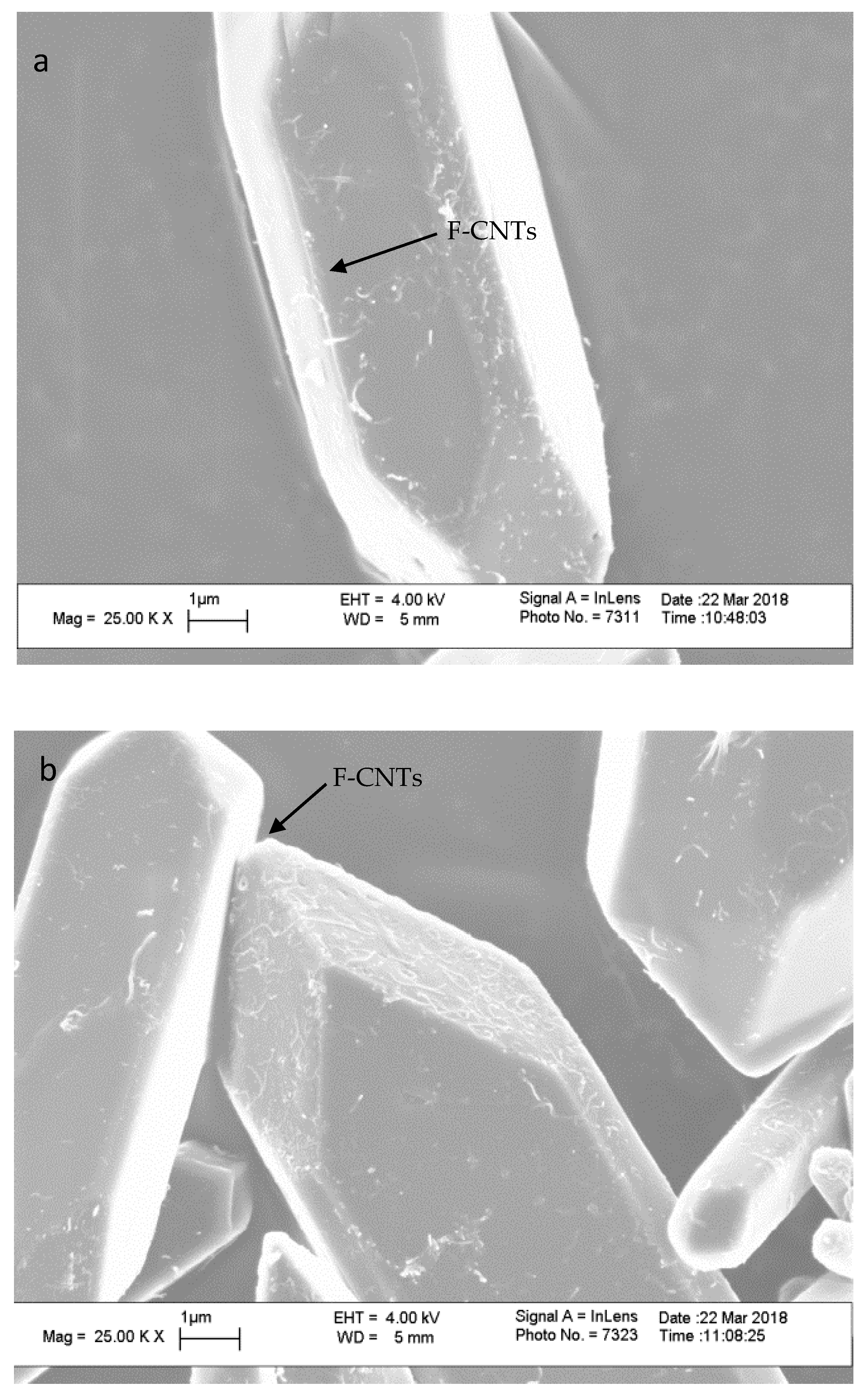
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thorat, A.A.; Dalvi, S.V. Liquid antisolvent precipitation and stabilization of nanoparticles of poorly water soluble drugs in aqueous suspensions: Recent developments and future perspective. Chem. Eng. J. 2012, 181–182, 1–34. [Google Scholar] [CrossRef]
- Xia, D.; Quan, P.; Piao, H.; Piao, H.; Sun, S.; Yin, Y.; Cui, F. Preparation of stable nitrendipine nanosuspensions using the precipitation–ultrasonication method for enhancement of dissolution and oral bioavailability. Eur. J. Pharm. Sci. 2010, 40, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Ryu, T.K.; Kim, S.E.; Kim, J.H.; Moon, S.K.; Choi, S.W. Biodegradable uniform microspheres based on solid-in-oil-in-water emulsion for drug delivery: A comparison of homogenization and fluidic device. J. Bioact. Compat. Polym. 2014, 29, 445–457. [Google Scholar] [CrossRef]
- Kakran, M.; Sahoo, N.G.; Tan, I.L.; Li, L. Preparation of nanoparticles of poorly water-soluble antioxidant curcumin by antisolvent precipitation methods. J. Nanoparticle Res. 2012, 14, 757. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.H.; Chen, A.Z.; Xu, Q.; Hong, Y.J.; Wang, S.B. One-Step Method to Prepare PLLA Porous Microspheres in a High-Voltage Electrostatic Anti-Solvent Process. Materials 2016, 9, 368. [Google Scholar] [CrossRef]
- Imchalee, R.; Charoenchaitrakool, M. Gas anti-solvent processing of a new sulfamethoxazole−l-malic acid cocrystal. J. Ind. Eng. Chem. 2015, 25, 12–15. [Google Scholar] [CrossRef]
- Singh, C.; Tiwari, V.; Mishra, C.P.; Shankar, R.; Sharma, D.; Jaiswal, S. Fabrication of cefpodoxime proxetil nanoparticles by solvent anti-solvent precipitation method for enhanced dissolution. Int. J. Res. Pharm. Nano Sci. 2015, 4, 18. [Google Scholar]
- Park, M.W.; Yeo, S.D. Antisolvent crystallization of carbamazepine from organic solutions. Chem. Eng. Res. Des. 2012, 90, 2202–2208. [Google Scholar] [CrossRef]
- Pachuau, L. Application of Nanocellulose for Controlled Drug Delivery; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef]
- Du, A.W.; Stenzel, M.H. Drug Carriers for the Delivery of Therapeutic Peptides. Biomacromolecules 2014, 15, 1097–1114. [Google Scholar] [CrossRef]
- Sim, T.; Lim, C.; Lee, J.W.; Kim, D.W.; Kim, Y.; Kim, M.; Choi, S.; Choi, H.G.; Lee, E.S.; Kim, K.S.; et al. Characterization and pharmacokinetic study of itraconazole solid dispersions prepared by solvent-controlled precipitation and spray-dry methods. J. Pharm. Pharmacol. 2017, 69, 1707–1715. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Al-Hamidi, H.; Edwards, A.A.; Mohammad, M.A.; Nokhodchi, A. To enhance dissolution rate of poorly water-soluble drugs: Glucosamine hydrochloride as a potential carrier in solid dispersion formulations. Colloids Surf. B Biointerfaces 2010, 76, 170–178. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins. 1. Drug Solubilization and Stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef]
- Cavallaro, G.; Grillo, I.; Gradzielski, M.; Lazzara, G. Structure of Hybrid Materials Based on Halloysite Nanotubes Filled with Anionic Surfactants. J. Phys. Chem. C 2016, 120, 13492–13502. [Google Scholar] [CrossRef]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Stetsyshyn, Y. Stability of Halloysite, Imogolite, and Boron Nitride Nanotubes in Solvent Media. Appl. Sci. 2018, 8, 1068. [Google Scholar] [CrossRef]
- Liu, X.; Gurel, V.; Morris, D.; Murray, D.W.; Zhitkovich, A.; Kane, A.B.; Hurt, R.H. Bioavailability of nickel in single-wall carbon nanotubes. Adv. Mater. 2007, 19, 2790–2796. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423. [Google Scholar] [CrossRef]
- Sato, Y.; Yokoyama, A.; Shibata, K.I.; Akimoto, Y.; Ogino, S.I.; Nodasaka, Y.; Kohgo, T.; Tamura, K.; Akasaka, T.; Uo, M.; et al. Influence of length on cytotoxicity of multi-walled carbon nanotubes against human acute monocytic leukemia cell line THP-1 in vitro and subcutaneous tissue of rats in vivo. Mol. Biosyst. 2005, 1, 176–182. [Google Scholar] [CrossRef]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef]
- Pacurari, M.; Yin, X.J.; Zhao, J.; Ding, M.; Leonard, S.S.; Schwegler-Berry, D.; Ducatman, B.S.; Sbarra, D.; Hoover, M.D.; Castranova, V.; et al. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kappaB, and Akt in normal and malignant human mesothelial cells. Environ. Health Perspect. 2008, 116, 1211–1217. [Google Scholar] [CrossRef]
- Wang, L.; Luanpitpong, S.; Castranova, V.; Tse, W.; Lu, Y.; Pongrakhananon, V.; Rojanasakul, Y. Carbon nanotubes induce malignant transformation and tumorigenesis of human lung epithelial cells. Nano Lett. 2011, 11, 2796–2803. [Google Scholar] [CrossRef]
- Pulskamp, K.; Diabaté, S.; Krug, H.F. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef]
- Kagan, V.E.; Konduru, N.V.; Feng, W.; Allen, B.L.; Conroy, J.; Volkov, Y.; Vlasova, I.I.; Belikova, N.A.; Yanamala, N.; Kapralov, A.; et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat. Nanotechnol. 2010, 5, 354. [Google Scholar] [CrossRef]
- Takagi, D.; Homma, Y.; Hibino, H.; Suzuki, S.; Kobayashi, Y. Single-walled carbon nanotube growth from highly activated metal nanoparticles. Nano Lett. 2006, 6, 2642–2645. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.; Yu, F.; Thakkar, M.; Mitra, S. Variation in chemical, colloidal and electrochemical properties of carbon nanotubes with the degree of carboxylation. J. Nanoparticle Res. 2017, 19, 16. [Google Scholar] [CrossRef]
- Allegri, M.; Perivoliotis, D.K.; Bianchi, M.G.; Chiu, M.; Pagliaro, A.; Koklioti, M.A.; Trompeta, A.-F.A.; Bergamaschi, E.; Bussolati, O.; Charitidis, C.A. Toxicity determinants of multi-walled carbon nanotubes: The relationship between functionalization and agglomeration. Toxicol. Rep. 2016, 3, 230–243. [Google Scholar] [CrossRef]
- Liu, P.; Cottrill, A.L.; Kozawa, D.; Koman, V.B.; Parviz, D.; Liu, A.T.; Yang, J.; Tran, T.Q.; Wong, M.H.; Wang, S.; et al. Emerging trends in 2D nanotechnology that are redefining our understanding of “Nanocomposites”. Nano Today 2018, 21, 18–40. [Google Scholar] [CrossRef]
- Khoshnevis, H.; Mint, S.M.; Yedinak, E.; Tran, T.Q.; Zadhoush, A.; Youssefi, M.; Pasquali, M.; Duong, H.M. Super high-rate fabrication of high-purity carbon nanotube aerogels from floating catalyst method for oil spill cleaning. Chem. Phys. Lett. 2018, 693, 146–151. [Google Scholar] [CrossRef]
- Macchione, M.; Biglione, C.; Strumia, M. Design, Synthesis and Architectures of Hybrid Nanomaterials for Therapy and Diagnosis Applications. Polymers 2018, 10, 527. [Google Scholar] [CrossRef]
- Fabbro, C.; Ali-Boucetta, H.; Ros, T.D.; Kostarelos, K.; Bianco, A.; Prato, M. Targeting carbon nanotubes against cancer. Chem. Commun. 2012, 48, 3911–3926. [Google Scholar] [CrossRef]
- Bhise, K.; Sau, S.; Alsaab, H.; Kashaw, S.K.; Tekade, R.K.; Iyer, A.K. Nanomedicine for cancer diagnosis and therapy: Advancement, success and structure–activity relationship. Ther. Deliv. 2017, 8, 1003–1018. [Google Scholar] [CrossRef]
- Martinelli, V.; Cellot, G.; Toma, F.M.; Long, C.S.; Caldwell, J.H.; Zentilin, L.; Giacca, M.; Turco, A.; Prato, M.; Ballerini, L.; et al. Carbon nanotubes promote growth and spontaneous electrical activity in cultured cardiac myocytes. Nano Lett. 2012, 12, 1831–1838. [Google Scholar] [CrossRef]
- Vashist, A.; Kaushik, A.; Vashist, A.; Sagar, V.; Ghosal, A.; Gupta, Y.K.; Ahmad, S.; Nair, M. Advances in Carbon Nanotubes–Hydrogel Hybrids in Nanomedicine for Therapeutics. Adv. Healthc. Mater. 2018, 7, 1701213. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Niu, X.; Yu, B.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. The use of carbon nanotubes to induce osteogenic differentiation of human adipose-derived MSCs in vitro and ectopic bone formation in vivo. Biomaterials 2012, 33, 4818–4827. [Google Scholar] [CrossRef]
- Zhou, F.; Wu, S.; Yuan, Y.; Chen, W.R.; Xing, D. Mitochondria-targeting photoacoustic therapy using single-walled carbon nanotubes. Small 2012, 8, 1543–1550. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, J.; Yoon, O.J.; Kim, H.W.; Lee, D.Y.; Kim, D.H.; Lee, W.B.; Lee, N.E.; Bonventre, J.V.; Kim, S.S. Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat. Nanotechnol. 2011, 6, 121–125. [Google Scholar] [CrossRef]
- Pantarotto, D.; Partidos, C.D.; Hoebeke, J.; Brown, F.; Kramer, E.; Briand, J.P.; Muller, S.; Prato, M.; Bianco, A. Immunization with peptide-functionalized carbon nanotubes enhances virus-specific neutralizing antibody responses. Chem. Biol. 2003, 10, 961–966. [Google Scholar] [CrossRef]
- Pantarotto, D.; Partidos, C.D.; Graff, R.; Hoebeke, J.; Briand, J.-P.; Prato, M.; Bianco, A. Synthesis, structural characterization, and immunological properties of carbon nanotubes functionalized with peptides. J. Am. Chem. Soc. 2003, 125, 6160–6164. [Google Scholar] [CrossRef]
- McDevitt, M.R.; Chattopadhyay, D.; Kappel, B.J.; Jaggi, J.S.; Schiffman, S.R.; Antczak, C.; Njardarson, J.T.; Brentjens, R.; Scheinberg, D.A. Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J. Nucl. Med. 2007, 48, 1180–1189. [Google Scholar] [CrossRef]
- Podesta, J.E.; Al-Jamal, K.T.; Herrero, M.A.; Tian, B.; Ali-Boucetta, H.; Hegde, V.; Bianco, A.; Prato, M.; Kostarelos, K. Antitumor activity and prolonged survival by carbon-nanotube-mediated therapeutic sirna silencing in a human lung xenograft model. Small 2009, 5, 1176–1185. [Google Scholar] [CrossRef]
- Wulan, P.P.; Wulandari, H.; Ulwan, S.H.; Purwanto, W.W.; Mulia, K. Modification of carbon nanotube’s dispersion using cetyltrimethyl ammonium bromide (CTAB) as cancer drug delivery. Aip Conf. Proc. 2018, 1933, 030008. [Google Scholar]
- Lacerda, L.; Russier, J.; Pastorin, G.; Herrero, M.A.; Venturelli, E.; Dumortier, H.; Al-Jamal, K.T.; Prato, M.; Kostarelos, K.; Bianco, A. Translocation mechanisms of chemically functionalised carbon nanotubes across plasma membranes. Biomaterials 2012, 33, 3334–3343. [Google Scholar] [CrossRef]
- Rezaei, S.J.T.; Hesami, A.; Khorramabadi, H.; Amani, V.; Malekzadeh, A.M.; Ramazani, A.; Niknejad, H. Pt(II) complexes immobilized on polymer-modified magnetic carbon nanotubes as a new platinum drug delivery system. Appl. Organomet. Chem. 2018, 32, e4401. [Google Scholar] [CrossRef]
- Li, R.; Wu, R.; Zhao, L.; Hu, Z.; Guo, S.; Pan, X.; Zou, H. Folate and iron difunctionalized multiwall carbon nanotubes as dual-targeted drug nanocarrier to cancer cells. Carbon 2011, 49, 1797–1805. [Google Scholar] [CrossRef]
- Tan, J.; Saifullah, B.; Kura, A.; Fakurazi, S.; Hussein, M. Incorporation of Levodopa into Biopolymer Coatings Based on Carboxylated Carbon Nanotubes for pH-Dependent Sustained Release Drug Delivery. Nanomaterials 2018, 8, 389. [Google Scholar] [CrossRef]
- Chae, S.; Kim, D.; Lee, K.J.; Lee, D.; Kim, Y.O.; Jung, Y.C.; Rhee, S.D.; Kim, K.R.; Lee, J.O.; Ahn, S.; et al. Encapsulation and Enhanced Delivery of Topoisomerase I Inhibitors in Functionalized Carbon Nanotubes. Acs Omega 2018, 3, 5938–5945. [Google Scholar] [CrossRef]
- Tan, J.M.; Arulselvan, P.; Fakurazi, S.; Ithnin, H.; Hussein, M.Z. A review on characterizations and biocompatibility of functionalized carbon nanotubes in drug delivery design. J. Nanomater. 2014, 2014, 20. [Google Scholar] [CrossRef]
- Chen, K.; Mitra, S. Incorporation of functionalized carbon nanotubes into hydrophobic drug crystals for enhancing aqueous dissolution. Colloids Surf. B Biointerfaces 2019, 173, 386–391. [Google Scholar] [CrossRef]
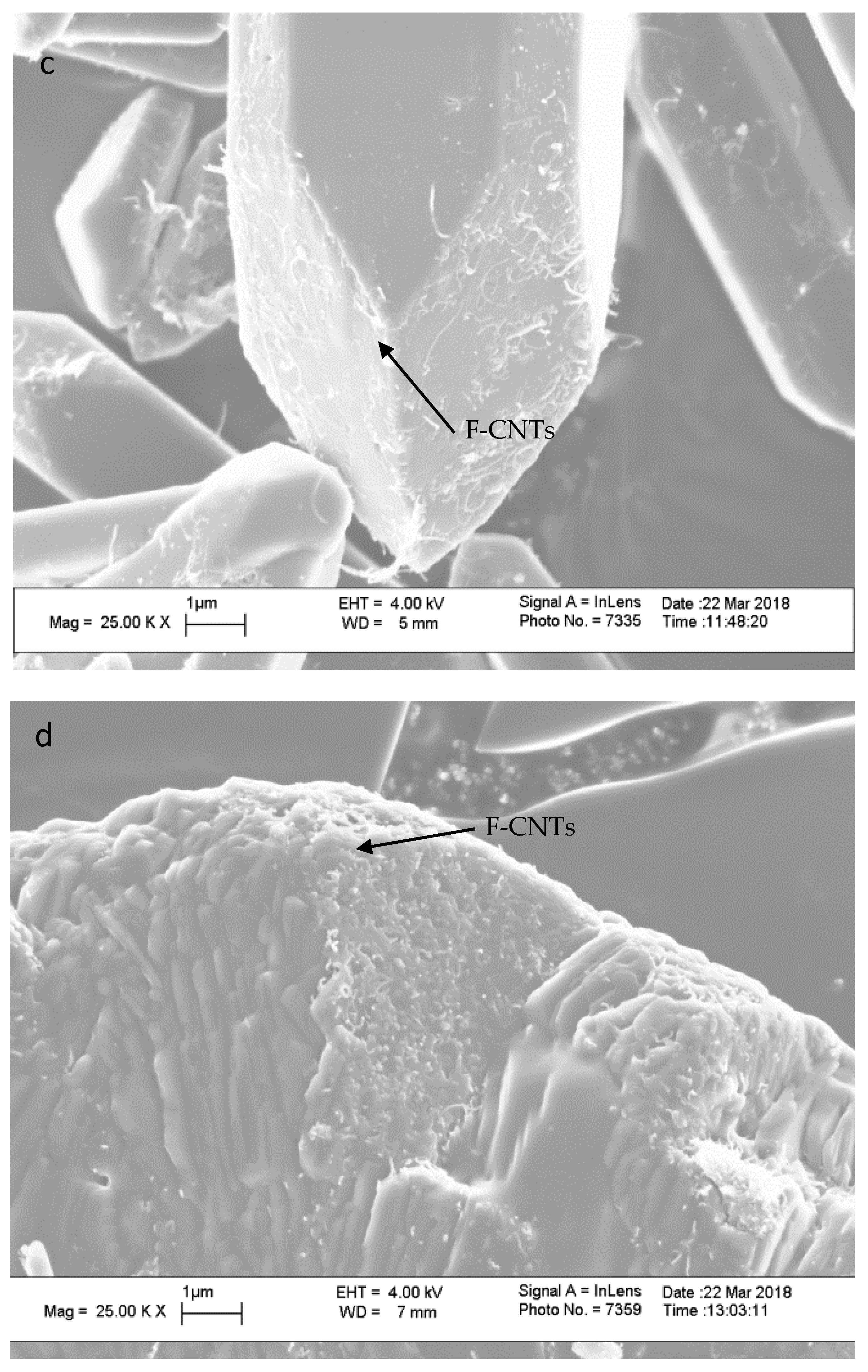

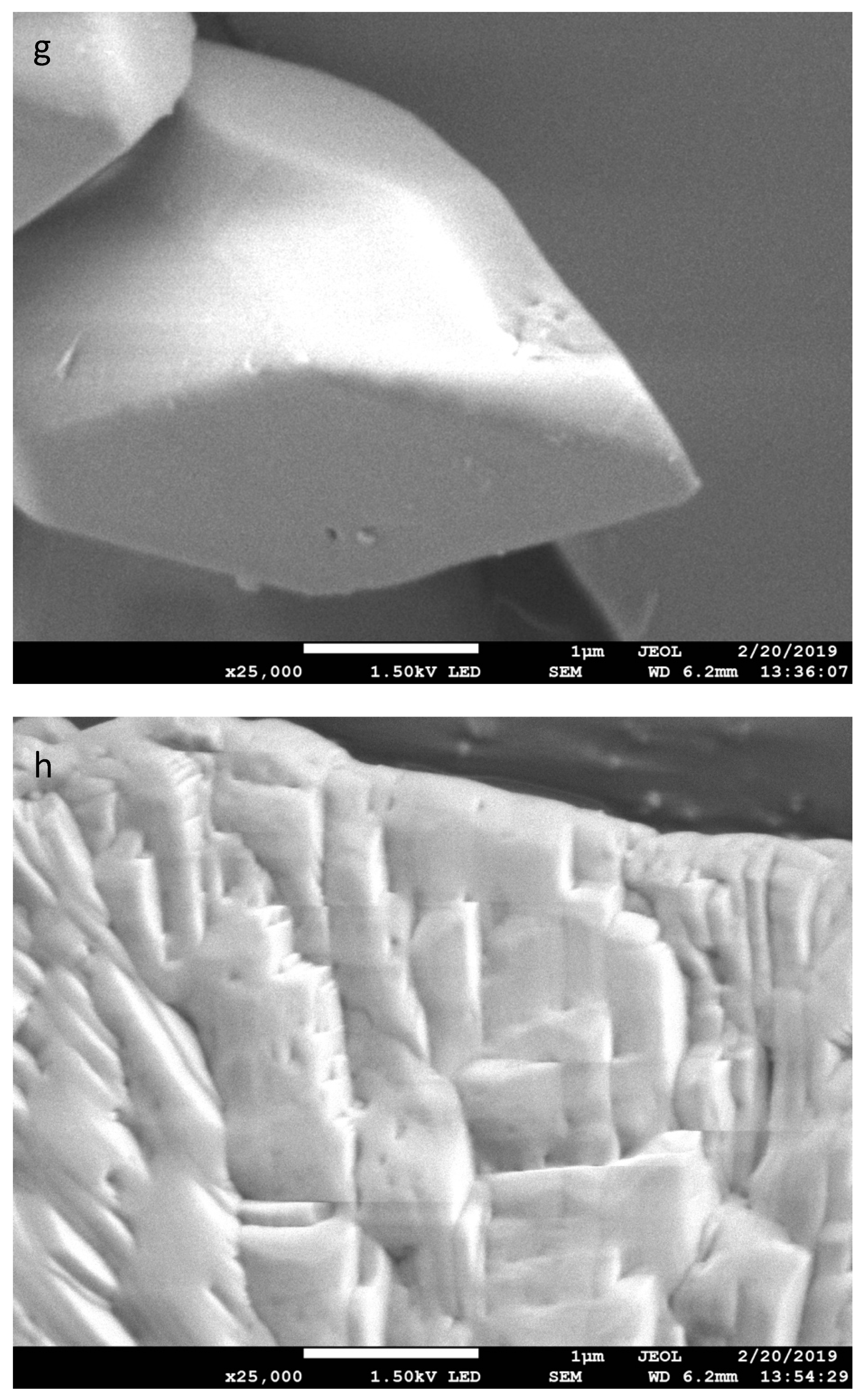


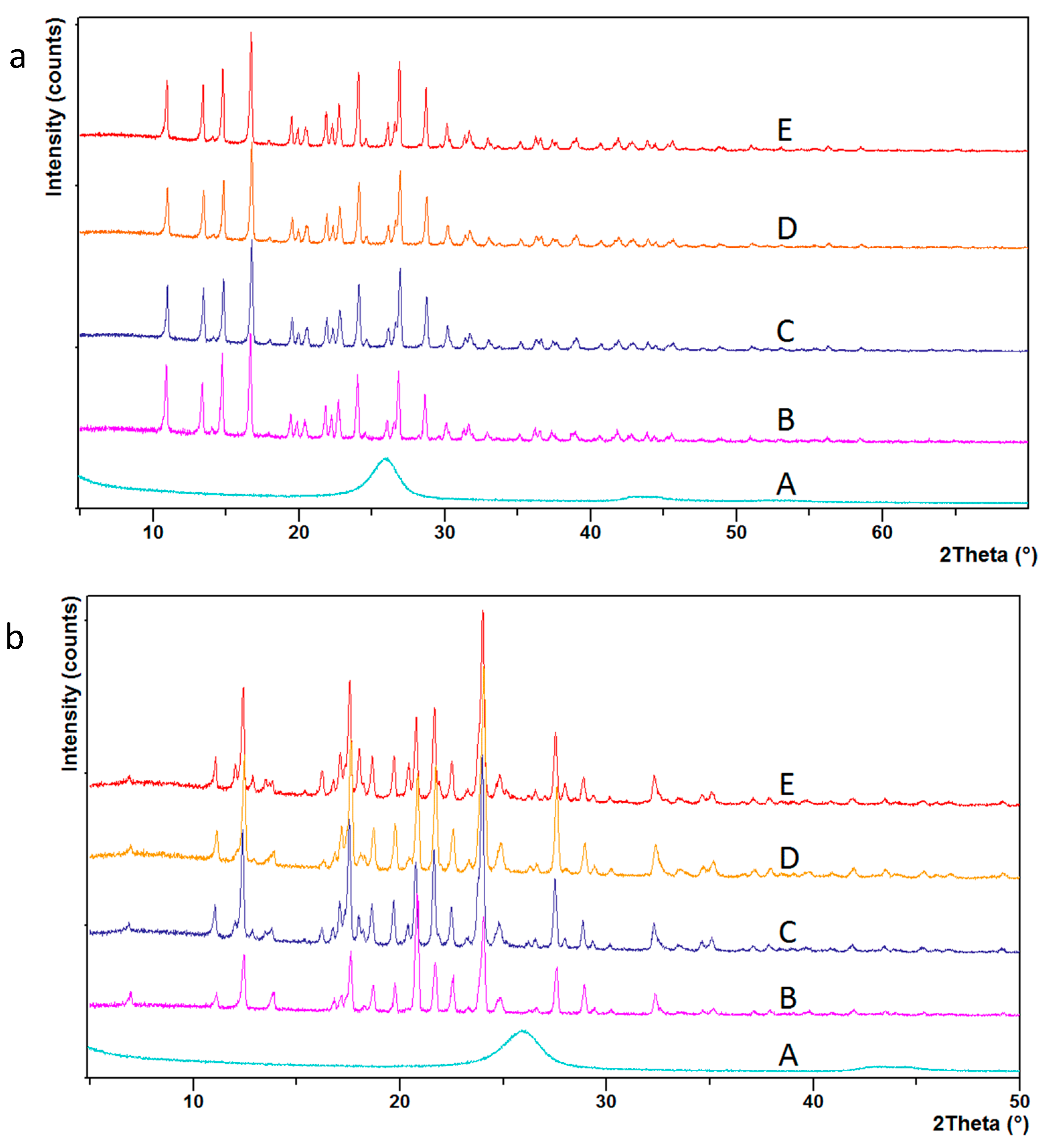

| Treatment Time (min) | % by Weight | C:COOH | |
|---|---|---|---|
| C | O | ||
| 0 | 92.7 | 6.1 | 39.5 |
| 5 | 89.7 | 9.9 | 23.2 |
| 10 | 87.9 | 11.2 | 19.8 |
| 40 | 86.3 | 13.6 | 16.0 |
| Incorporation % | C:COOH | T50 (min) | T80 (min) | Mp (°C) | |
|---|---|---|---|---|---|
| GF-CNTx | 0 | 39.5 | 8.0 | >120.0 | 221.25 |
| 3.9 | 23.2 | 6.0 | 60.0 | 220.75 | |
| 4.2 | 19.8 | 4.5 | 44.0 | 220.92 | |
| 3.8 | 16 | 4.0 | 30.5 | 221.01 | |
| SMZ-CNTx | 0 | 39.5 | 23.5 | 52.5 | 170.37 |
| 2.9 | 23.2 | 8.5 | 16.5 | 170.21 | |
| 1.3 | 19.8 | 7.5 | 15.0 | 170.06 | |
| 1.4 | 16 | 6.0 | 11.5 | 170.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Mitra, S. Controlling the Dissolution Rate of Hydrophobic Drugs by Incorporating Carbon Nanotubes with Different Levels of Carboxylation. Appl. Sci. 2019, 9, 1475. https://doi.org/10.3390/app9071475
Chen K, Mitra S. Controlling the Dissolution Rate of Hydrophobic Drugs by Incorporating Carbon Nanotubes with Different Levels of Carboxylation. Applied Sciences. 2019; 9(7):1475. https://doi.org/10.3390/app9071475
Chicago/Turabian StyleChen, Kun, and Somenath Mitra. 2019. "Controlling the Dissolution Rate of Hydrophobic Drugs by Incorporating Carbon Nanotubes with Different Levels of Carboxylation" Applied Sciences 9, no. 7: 1475. https://doi.org/10.3390/app9071475
APA StyleChen, K., & Mitra, S. (2019). Controlling the Dissolution Rate of Hydrophobic Drugs by Incorporating Carbon Nanotubes with Different Levels of Carboxylation. Applied Sciences, 9(7), 1475. https://doi.org/10.3390/app9071475