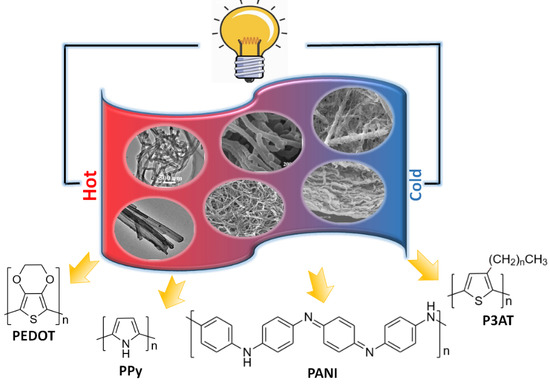
One-Dimensional Nanostructure Engineering of Conducting Polymers for Thermoelectric Applications
Abstract
1. Introduction
2. D-Conducting Polymers and Their Thermoelectric Properties
2.1. PEDOT
2.2. Poly (3-alkylthiophene)
2.3. PPy
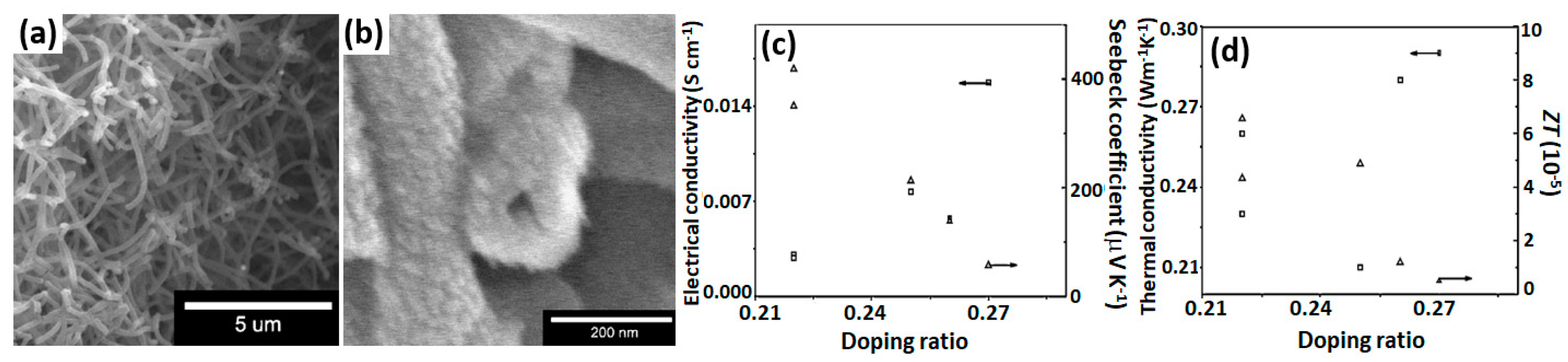
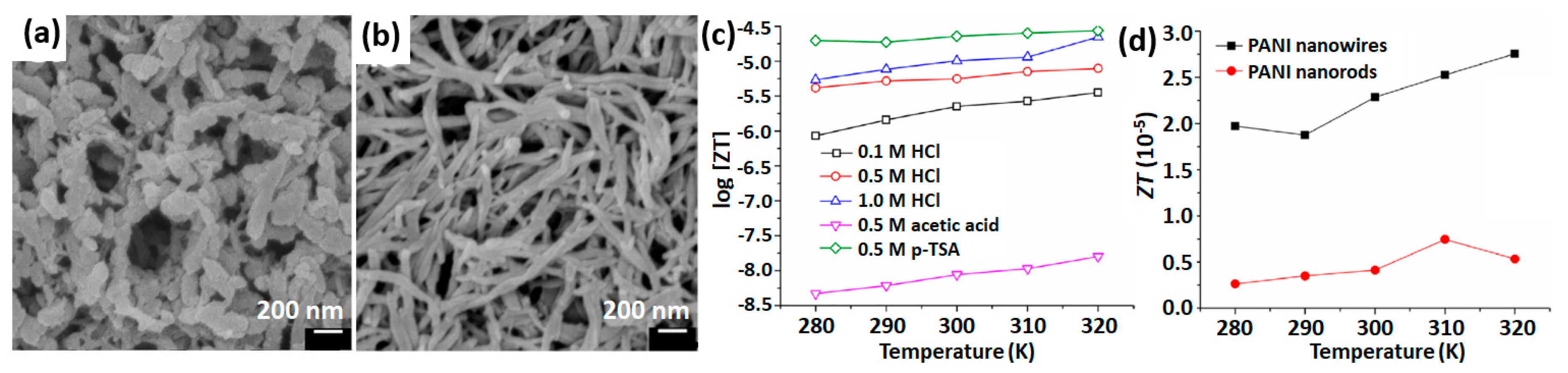
2.4. PANI
3. Strategies to form 1D Conducting Polymer Nanocomposite Thermoelectric Materials
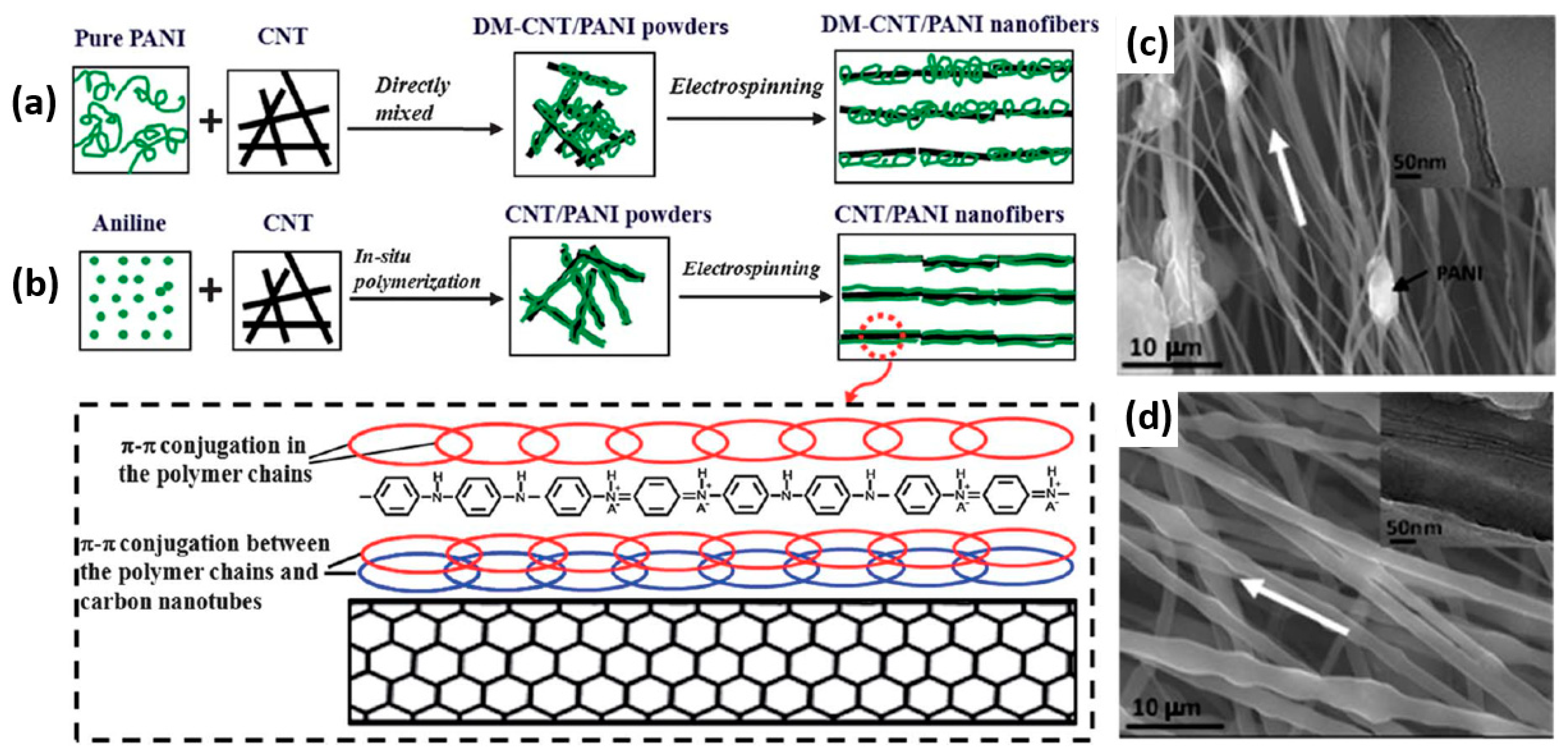
3.1. In Situ Polymerization of Conducting Polymers on 1D Nanofillers
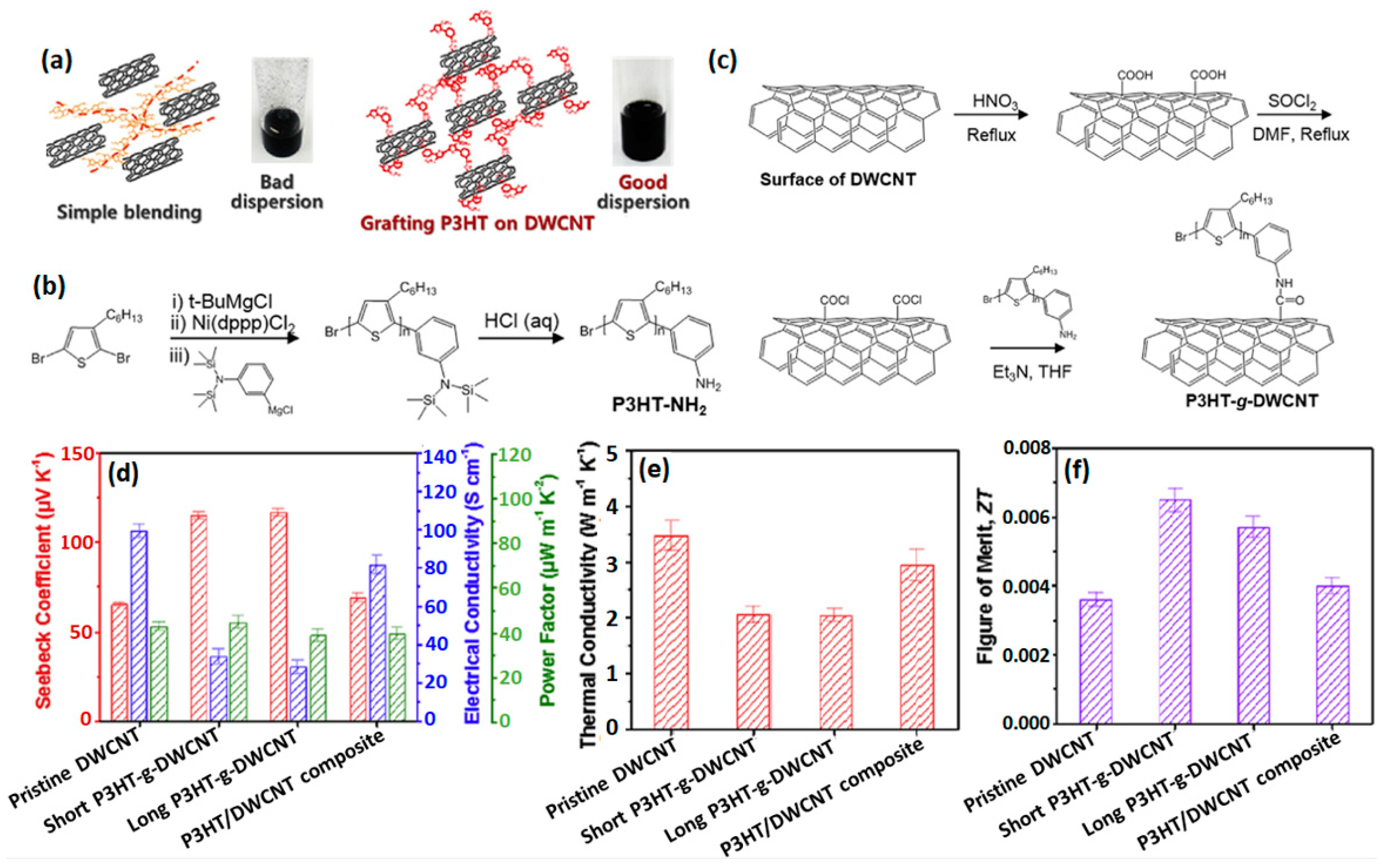
3.2. Covalent Grafting of Conducting Polymers on 1D Nanofillers
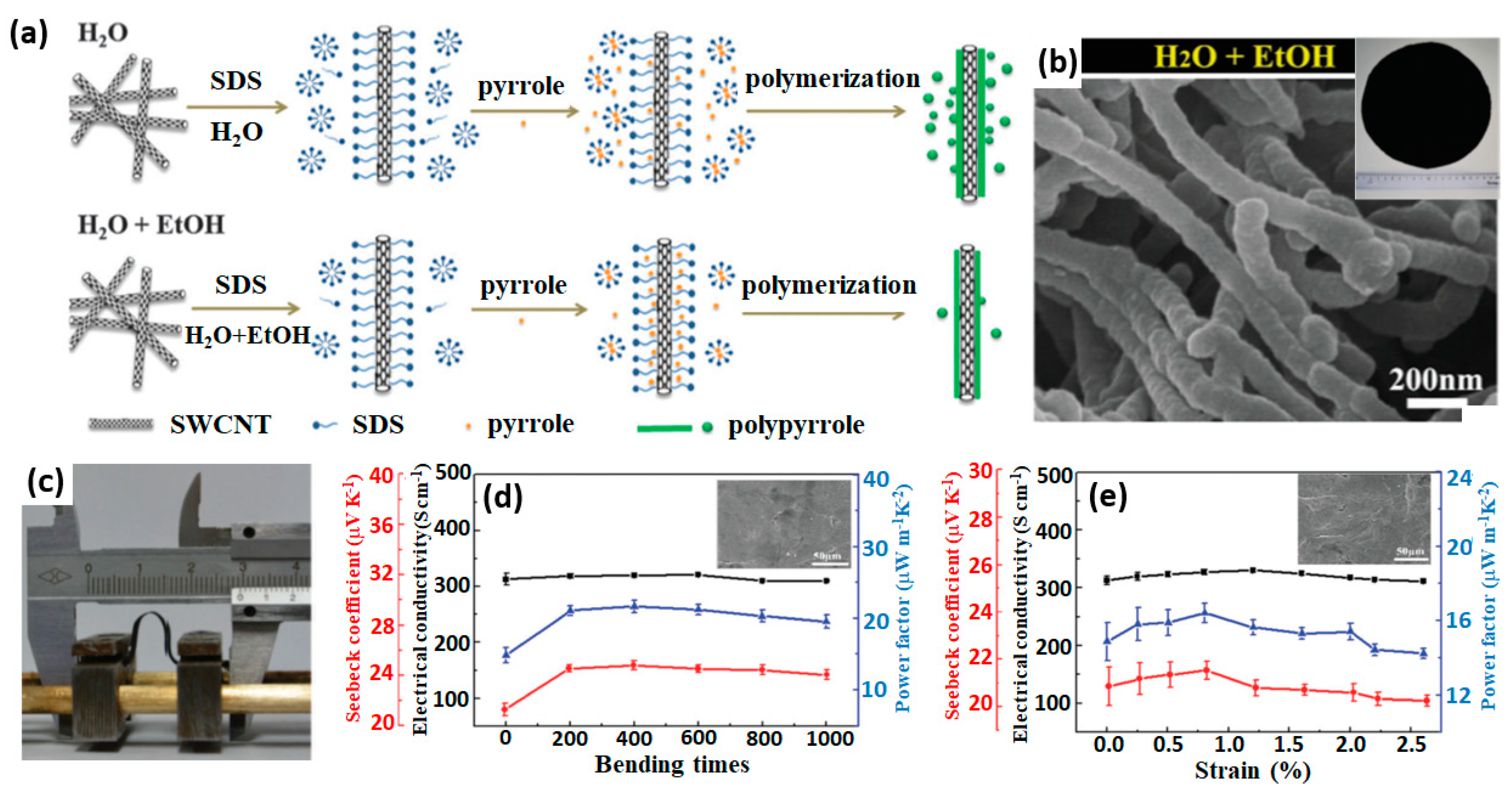
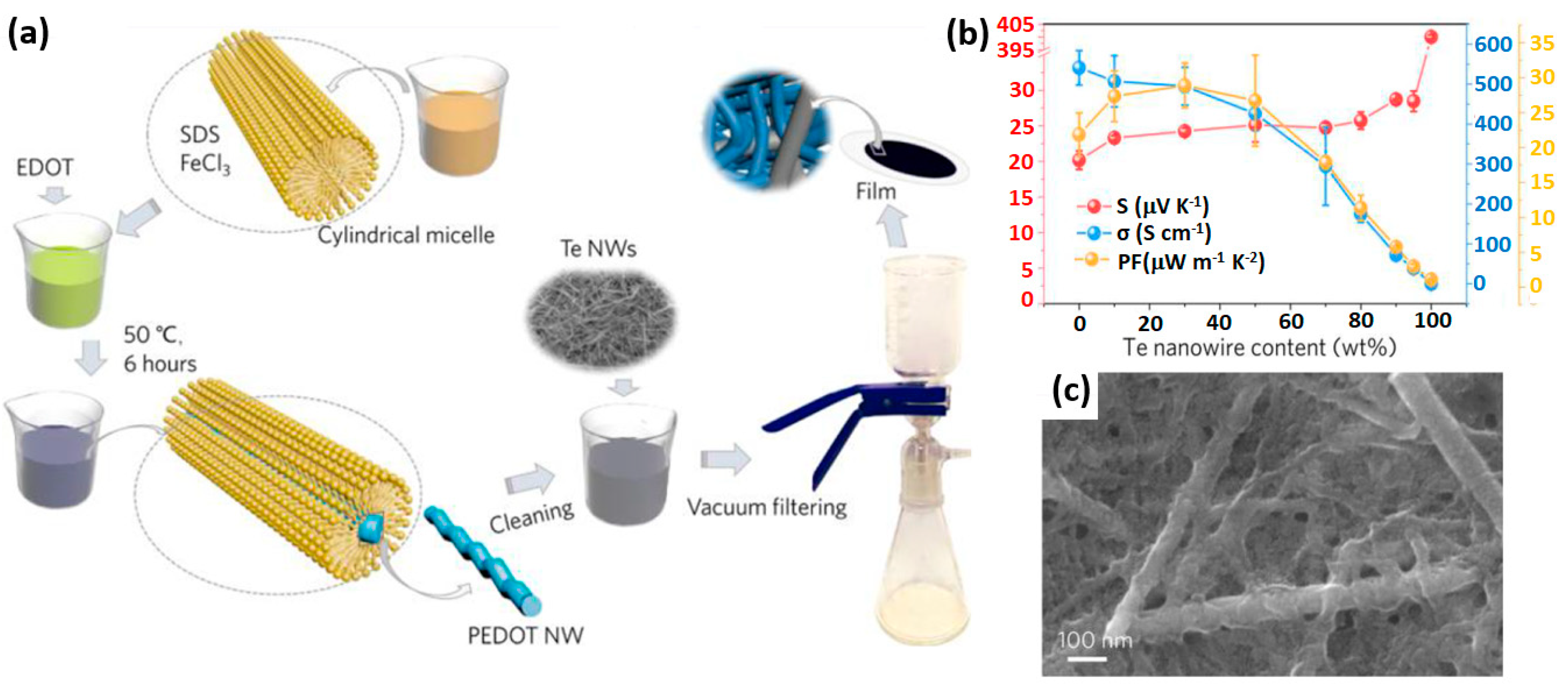
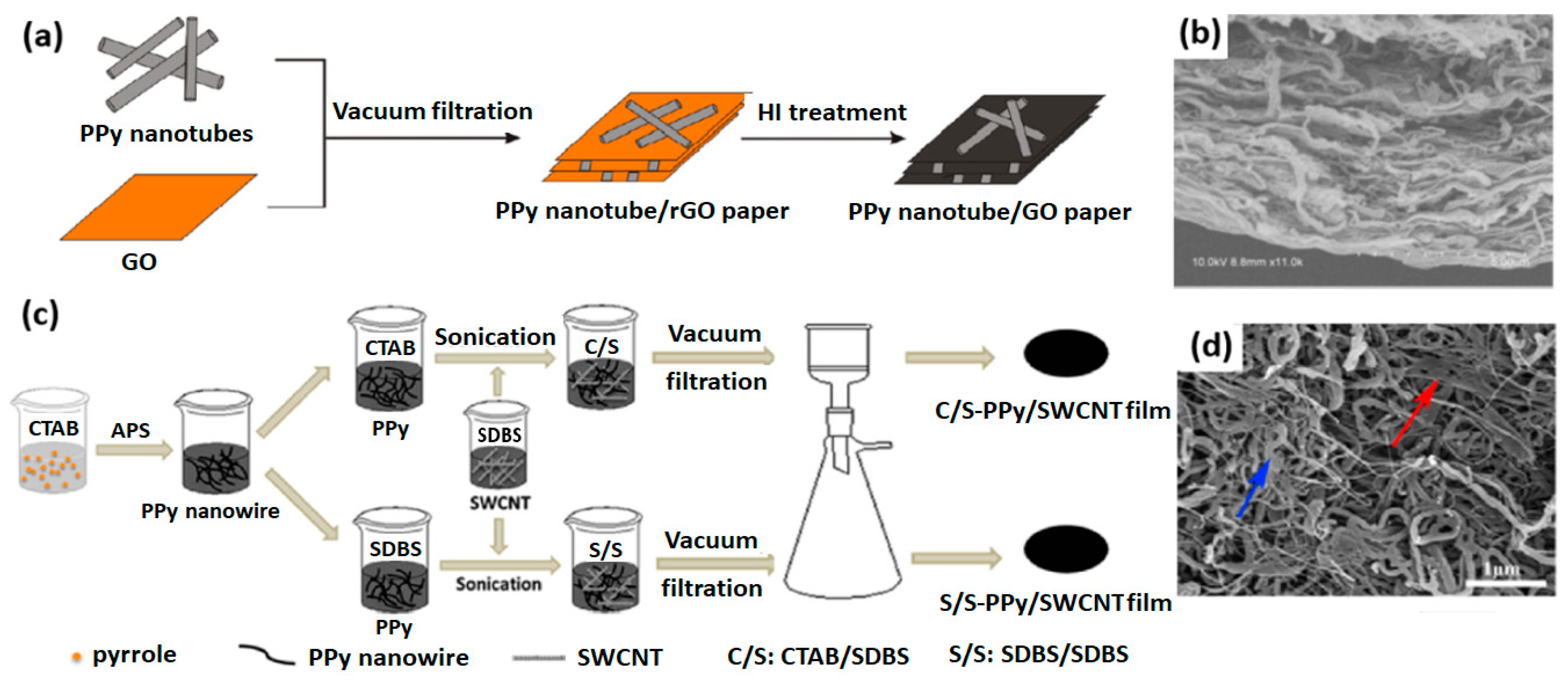
3.3. Self-Assembly of 1D Conducting Polymer with Nanofillers
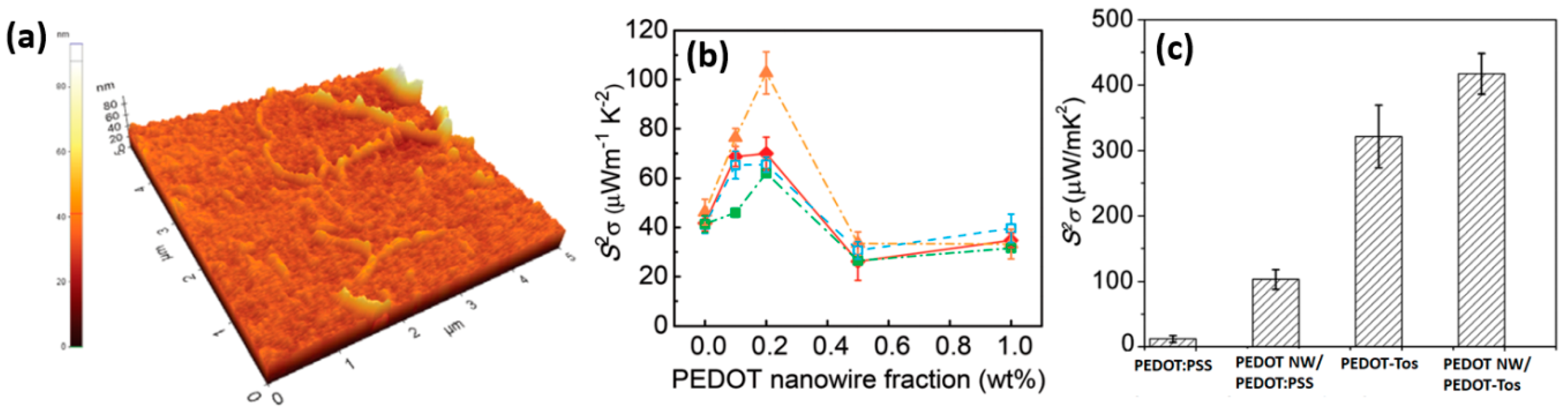
3.4. Incorporation of 1D Conducting Polymer into Polymer Matrix by Physical Mixing
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DiSalvo, F.J. Thermoelectric cooling and power generation. Science 1999, 285, 703–706. [Google Scholar] [CrossRef]
- Bell, L.E. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Tritt, T.M. Thermoelectric phenomena, materials, and applications. In Annual Review of Materials Research; Clarke, D.R., Fratzl, P., Eds.; Annual Reviews: Palo Alto, CA, USA, 2011; Volume 41, pp. 433–448. [Google Scholar]
- Zhang, X.; Zhao, L.-D. Thermoelectric materials: Energy conversion between heat and electricity. J. Materiomics 2015, 1, 92–105. [Google Scholar] [CrossRef]
- Zhao, D.L.; Tan, G. A review of thermoelectric cooling: Materials, modeling and applications. Appl. Therm. Eng. 2014, 66, 15–24. [Google Scholar] [CrossRef]
- Ren, P.; Liu, Y.; He, J.; Lv, T.; Gao, J.; Xu, G. Recent advances in inorganic material thermoelectrics. Inorg. Chem. Front. 2018, 5, 2380–2398. [Google Scholar] [CrossRef]
- Fergus, J.W. Oxide materials for high temperature thermoelectric energy conversion. J. Eur. Ceram. Soc. 2012, 32, 525–540. [Google Scholar] [CrossRef]
- Culebras, M.; Gomez, C.M.; Cantarero, A. Review on polymers for thermoelectric applications. Materials 2014, 7, 6701–6732. [Google Scholar] [CrossRef]
- Du, Y.; Shen, S.Z.; Cai, K.F.; Casey, P.S. Research progress on polymer-inorganic thermoelectric nanocomposite materials. Prog. Polym. Sci. 2012, 37, 820–841. [Google Scholar] [CrossRef]
- Yao, H.; Fan, Z.; Cheng, H.; Guan, X.; Wang, C.; Sun, K.; Ouyang, J. Recent development of thermoelectric polymers and composites. Macromol. Rapid Commun. 2018, 39, 1700727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, Y.M.; Xu, W.; Zhu, D.B. Organic thermoelectric materials: Emerging green energy materials converting heat to electricity directly and efficiently. Adv. Mater. 2014, 26, 6829–6851. [Google Scholar] [CrossRef]
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 14. [Google Scholar] [CrossRef]
- Dubey, N.; Leclerc, M. Conducting polymers: Efficient thermoelectric materials. J. Polym. Sci. B-Polym. Phys. 2011, 49, 467–475. [Google Scholar] [CrossRef]
- Chen, G.M.; Xu, W.; Zhu, D.B. Recent advances in organic polymer thermoelectric composites. J. Mater. Chem. C 2017, 5, 4350–4360. [Google Scholar] [CrossRef]
- He, M.; Qiu, F.; Lin, Z.Q. Towards high-performance polymer-based thermoelectric materials. Energy Environ. Sci. 2013, 6, 1352–1361. [Google Scholar] [CrossRef]
- Ghosh, S.; Maiyalagan, T.; Basu, R.N. Nanostructured conducting polymers for energy applications: Towards a sustainable platform. Nanoscale 2016, 8, 6921–6947. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bai, H.; Shi, G.Q. Conducting polymer nanomaterials: Electrosynthesis and applications. Chem. Soc. Rev. 2009, 38, 2397–2409. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Yoon, H. Recent advances in nanostructured conducting polymers: From synthesis to practical applications. Polymers 2016, 8, 38. [Google Scholar] [CrossRef]
- Yoon, H. Current trends in sensors based on conducting polymer nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef]
- Zhang, L.; Du, W.; Nautiyal, A.; Liu, Z.; Zhang, X. Recent progress on nanostructured conducting polymers and composites: Synthesis, application and future aspects. Sci. China Mater. 2018, 61, 303–352. [Google Scholar] [CrossRef]
- Aleshin, A.N. Polymer nanofibers and nanotubes: Charge transport and device applications. Adv. Mater. 2006, 18, 17–27. [Google Scholar] [CrossRef]
- Chen, C.; Fan, Y.Q.; Gu, J.H.; Wu, L.M.; Passerini, S.; Mai, L.Q. One-dimensional nanomaterials for energy storage. J. Phys. D-Appl. Phys. 2018, 51, 14. [Google Scholar] [CrossRef]
- Tran, H.D.; Li, D.; Kaner, R.B. One-dimensional conducting polymer nanostructures: Bulk synthesis and applications. Adv. Mater. 2009, 21, 1487–1499. [Google Scholar] [CrossRef]
- Yu, H.; Kim, D.Y.; Lee, K.J.; Oh, J.H. Fabrication of one-dimensional organic nanomaterials and their optoelectronic. J. Nanosci. Nanotechnol. 2014, 14, 1282–1302. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.G.; Lee, H.; Wang, D.Z.; Ren, Z.F.; Fleurial, J.P.; Gogna, P. New directions for low-dimensional thermoelectric materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Kanatzidis, M.G. Nanostructured thermoelectrics: The new paradigm? Chem. Mater. 2010, 22, 648–659. [Google Scholar] [CrossRef]
- Vineis, C.J.; Shakouri, A.; Majumdar, A.; Kanatzidis, M.G. Nanostructured thermoelectrics: Big efficiency gains from small features. Adv. Mater. 2010, 22, 3970–3980. [Google Scholar] [CrossRef] [PubMed]
- Pichanusakorn, P.; Bandaru, P. Nanostructured thermoelectrics. Mater. Sci. Eng. R-Rep. 2010, 67, 19–63. [Google Scholar] [CrossRef]
- Li, J.F.; Liu, W.S.; Zhao, L.D.; Zhou, M. High-performance nanostructured thermoelectric materials. NPG Asia Mater. 2010, 2, 152–158. [Google Scholar] [CrossRef]
- Lan, Y.C.; Minnich, A.J.; Chen, G.; Ren, Z.F. Enhancement of thermoelectric figure-of-merit by a bulk nanostructuring approach. Adv. Funct. Mater. 2010, 20, 357–376. [Google Scholar] [CrossRef]
- Minnich, A.J.; Dresselhaus, M.S.; Ren, Z.F.; Chen, G. Bulk nanostructured thermoelectric materials: Current research and future prospects. Energy Environ. Sci. 2009, 2, 466–479. [Google Scholar] [CrossRef]
- Liu, R.; Lee, S.B. MnO2/poly (3, 4-ethylenedioxythiophene) coaxial nanowires by one-step coelectrodeposition for electrochemical energy storage. J. Am. Chem. Soc. 2008, 130, 2942–2943. [Google Scholar] [CrossRef] [PubMed]
- García-Barberá, A.; Culebras, M.; Roig-Sánchez, S.; Gómez, C.M.; Cantarero, A. Three dimensional PEDOT nanowires network. Synth. Met. 2016, 220, 208–212. [Google Scholar] [CrossRef]
- Taggart, D.K.; Yang, Y.; Kung, S.-C.; McIntire, T.M.; Penner, R.M. Enhanced thermoelectric metrics in ultra-long electrodeposited PEDOT nanowires. Nano Lett. 2010, 11, 125–131. [Google Scholar] [CrossRef] [PubMed]
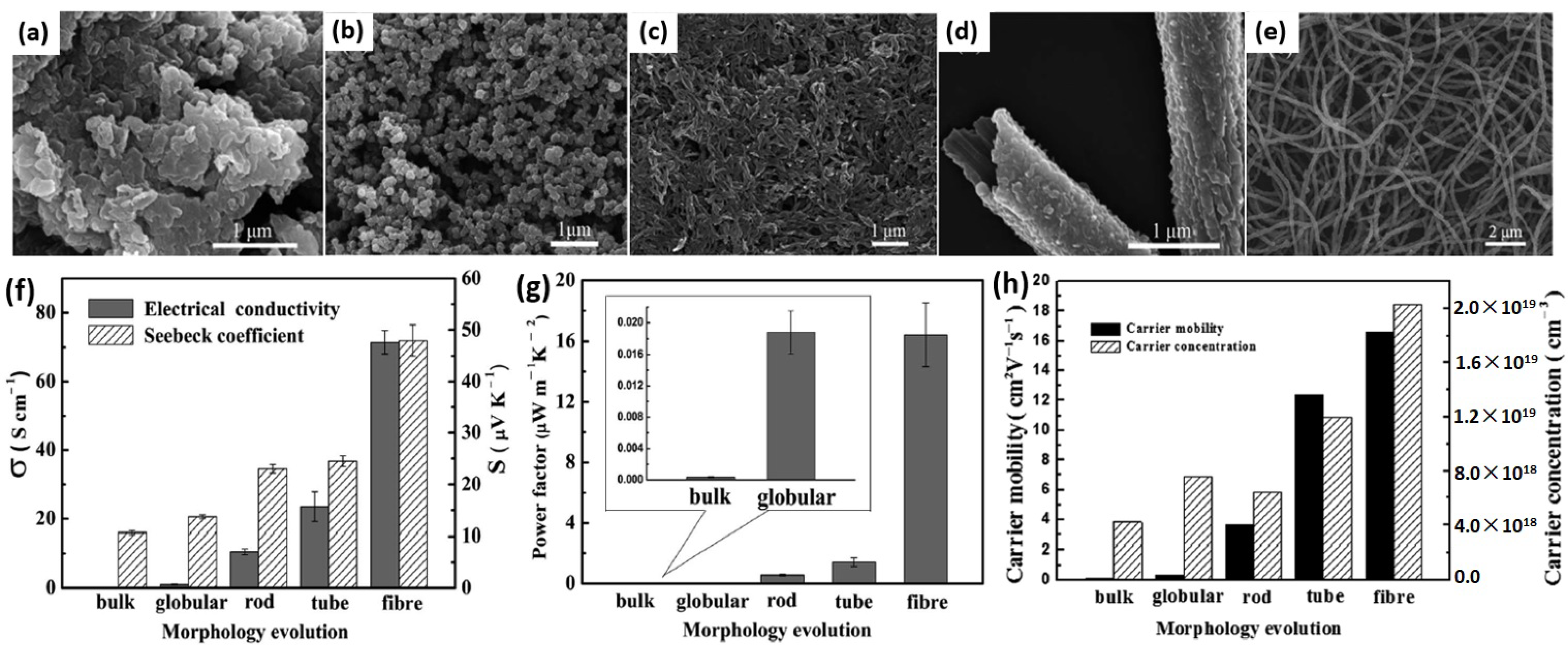
- Hu, X.; Chen, G.; Wang, X.; Wang, H. Tuning thermoelectric performance by nanostructure evolution of a conducting polymer. J. Mater. Chem. A 2015, 3, 20896–20902. [Google Scholar] [CrossRef]
- Liu, R.; Cho, S.I.; Lee, S.B. Poly (3, 4-ethylenedioxythiophene) nanotubes as electrode materials for a high-powered supercapacitor. Nanotechnology 2008, 19, 215710. [Google Scholar] [CrossRef] [PubMed]
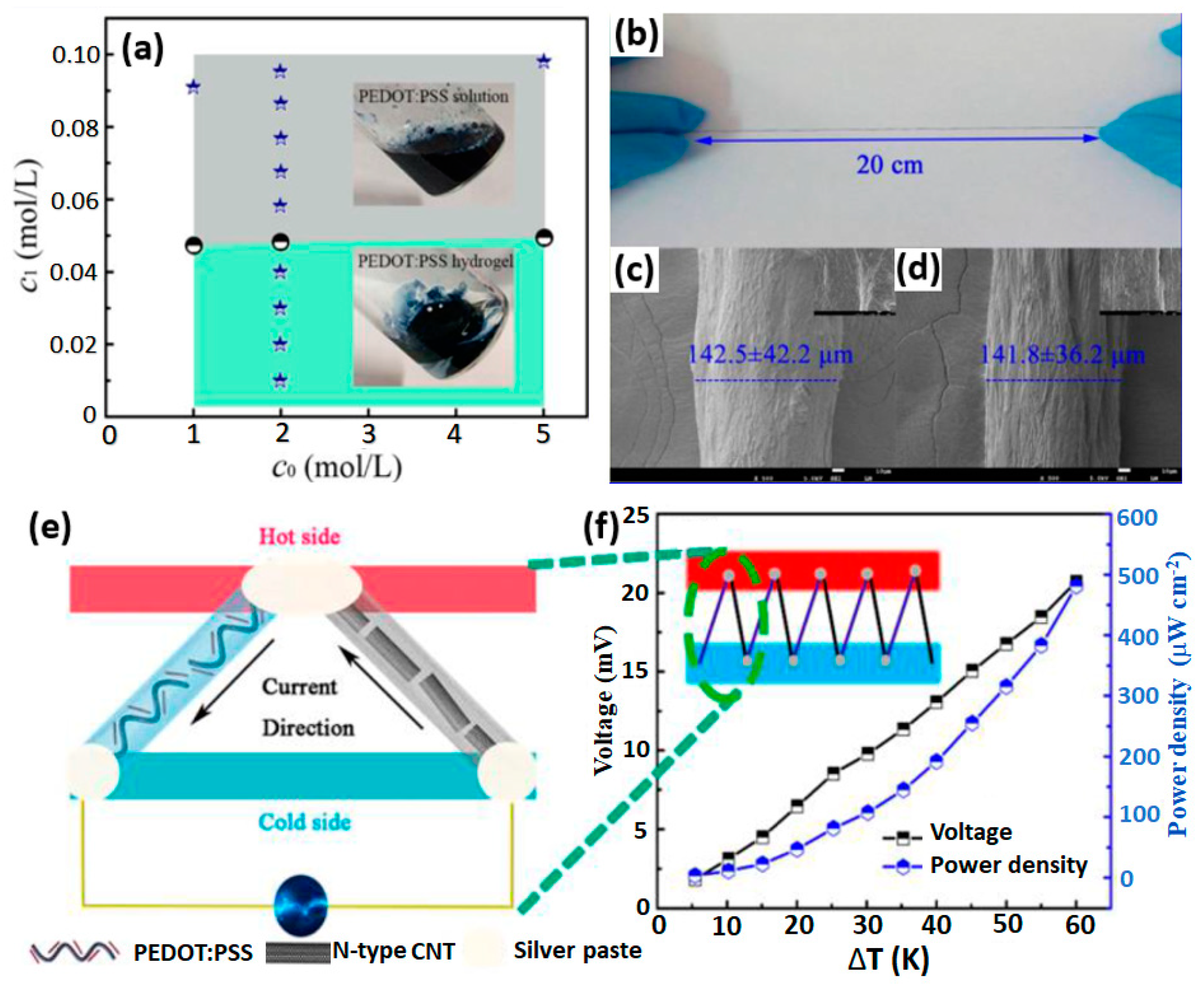
- Yao, B.; Wang, H.; Zhou, Q.; Wu, M.; Zhang, M.; Li, C.; Shi, G. Ultrahigh-conductivity polymer hydrogels with arbitrary structures. Adv. Mater. 2017, 29, 1700974. [Google Scholar] [CrossRef]
- Yoon, H.; Chang, M.; Jang, J. Formation of 1D poly (3, 4-ethylenedioxythiophene) nanomaterials in reverse microemulsions and their application to chemical sensors. Adv. Funct. Mater. 2007, 17, 431–436. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, K.; Xu, F.; Wang, S.; Qiu, Y. Thermoelectric transport in ultrathin poly (3, 4-ethylenedioxythiophene) nanowire assembly. Compos. Part B-Eng. 2018, 136, 234–240. [Google Scholar] [CrossRef]
- Zhao, J.; Tan, D.; Chen, G. A strategy to improve the thermoelectric performance of conducting polymer nanostructures. J. Mater. Chem. C 2017, 5, 47–53. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, S.; Hua, T.; Huang, B.; Liu, S.; Tao, X. Fiber-based thermoelectric generators: Materials, device structures, fabrication, characterization, and applications. Adv. Energy Mater. 2018, 8, 1700524. [Google Scholar] [CrossRef]
- Liu, J.; Jia, Y.; Jiang, Q.; Jiang, F.; Li, C.; Wang, X.; Liu, P.; Liu, P.; Hu, F.; Du, Y. Highly conductive hydrogel polymer fibers toward promising wearable thermoelectric energy harvesting. ACS Appl. Mater. Interfaces 2018, 10, 44033–44040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, G.; Qiu, L.; Feng, Y.; Chen, J.; Yan, J.; Liu, Y.; Huang, X.; Cui, Y.; Sun, Y.; et al. Highly conducting polythiophene thin films with less ordered microstructure displaying excellent thermoelectric performance. Macromol. Rapid Commun. 2018, 39, 1800283. [Google Scholar] [CrossRef] [PubMed]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From fundamental perspectives to applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Imae, I.; Koumoto, T.; Harima, Y. Thermoelectric properties of polythiophenes partially substituted by ethylenedioxy groups. Polymer 2018, 144, 43–50. [Google Scholar] [CrossRef]
- Steyrluthner, R.; Zhang, Y.; Zhang, L.; Kraffert, F.; Cherniawski, B.P.; Bittl, R.; Briseno, A.L.; Bredas, J.-L.; Behrends, J. Impact of morphology on polaron delocalization in a semicrystalline conjugate polymer. Phys. Chem. Chem. Phys. 2017, 19, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. Thermoelectric energy from flexible P3HT films doped with a ferric salt of triflimide anions. Energy Environ. Sci. 2012, 5, 9639–9644. [Google Scholar] [CrossRef]
- Hong, C.T.; Lee, W.; Kang, Y.H.; Yoo, Y.; Ryu, J.; Cho, S.Y.; Jang, K.-S. Effective doping by spin-coating and enhanced thermoelectric power factors in SWCNT/P3HT hybrid films. J. Mater. Chem. A 2015, 3, 12314–12319. [Google Scholar] [CrossRef]
- Xuan, Y.; Liu, X.; Desbief, S.; Leclère, P.; Fahlman, M.; Lazzaroni, R.; Berggren, M.; Cornil, J.; Emin, D.; Crispin, X. Thermoelectric properties of conducting polymers: The case of poly (3-hexylthiophene). Phys. Rev. B 2010, 82, 115454. [Google Scholar] [CrossRef]
- Bhatt, M.P.; Magurudeniya, H.D.; Rainbolt, E.A.; Huang, P.; Dissanayake, D.S.; Biewer, M.C.; Stefan, M.C. Poly(3-hexylthiophene) nanostructured materials for organic electronics applications. J. Nanosci. Nanotechnol. 2014, 14, 1033–1050. [Google Scholar] [CrossRef]
- Laforgue, A.; Robitaille, L. Fabrication of poly-3-hexylthiophene/polyethylene oxide nanofibers using electrospinning. Synth. Met. 2008, 158, 577–584. [Google Scholar] [CrossRef]
- Pierini, F.; Lanzi, M.; Nakielski, P.; Pawłowska, S.; Urbanek, O.; Zembrzycki, K.; Kowalewski, T.A. Single-material organic solar cells based on electrospun fullerene-grafted polythiophene nanofibers. Macromolecules 2017, 50, 4972–4981. [Google Scholar] [CrossRef]
- Samitsu, S.; Shimomura, T.; Heike, S.; Hashizume, T.; Ito, K. Effective production of poly(3-alkylthiophene) nanofibers by means of whisker method using anisole solvent: Structural, optical, and electrical properties. Macromolecules 2008, 41, 8000–8010. [Google Scholar] [CrossRef]
- Sun, S.; Salim, T.; Wong, L.H.; Foo, Y.L.; Boey, F.; Lam, Y.M. A new insight into controlling poly (3-hexylthiophene) nanofiber growth through a mixed-solvent approach for organic photovoltaics applications. J. Mater. Chem. 2011, 21, 377–386. [Google Scholar] [CrossRef]
- Byun, M.; Laskowski, R.L.; He, M.; Qiu, F.; Jeffries-El, M.; Lin, Z. Controlled evaporative self-assembly of hierarchically structured regioregular conjugated polymers. Soft Matter 2009, 5, 1583–1586. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Gao, X.; Xing, R.; Zheng, L.; Wu, S.; Geng, Y.; Han, Y. Oriented poly (3-hexylthiophene) nanofibril with the π−π stacking growth direction by solvent directional evaporation. Langmuir 2011, 27, 4212–4219. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Y.; Zheng, L.; Geng, Y.; Han, Y. Vapor-assisted imprinting to pattern poly (3-hexylthiophene)(P3HT) film with oriented arrangement of nanofibrils and flat-on conformation of P3HT chains. Polymer 2013, 54, 423–430. [Google Scholar] [CrossRef]
- Park, D.; Kim, B.; Jang, M.; Bae, K.; Lee, S.; Joo, J. Synthesis and characterization of polythiophene and poly (3-methylthiophene) nanotubes and nanowires. Synth. Met. 2005, 153, 341–344. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, M.; Kim, M.S.; Kim, D.-C.; Song, H.; Kim, J.; Joo, J. Electrochemical synthesis and nanoscale photoluminescence of poly (3-butylthiophene) nanowire. Electrochem. Solid State Lett. 2008, 11, K69–K72. [Google Scholar] [CrossRef]
- Byun, J.; Kim, Y.; Jeon, G.; Kim, J.K. Ultrahigh density array of free-standing poly (3-hexylthiophene) nanotubes on conducting substrates via solution wetting. Macromolecules 2011, 44, 8558–8562. [Google Scholar] [CrossRef]
- Smith, M.K.; Singh, V.; Kalaitzidou, K.; Cola, B.A. Poly (3-hexylthiophene) nanotube array surfaces with tunable wetting and contact thermal energy transport. ACS Nano 2015, 9, 1080–1088. [Google Scholar] [CrossRef]
- Endrődi, B.; Mellar, J.; Gingl, Z.; Visy, C.; Janáky, C. Reasons behind the improved thermoelectric properties of poly (3-hexylthiophene) nanofiber networks. RSC Adv. 2014, 4, 55328–55333. [Google Scholar] [CrossRef]
- Hiura, S.; Okada, N.; Wakui, J.; Narita, H.; Kanehashi, S.; Shimomura, T. Thermoelectric properties of poly (3-hexylthiophene) nanofiber mat with a large void fraction. Materials 2017, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Li, X.G.; Yang, Y.L. Preparation, properties and applications of polypyrroles. React. Funct. Polym. 2001, 47, 125–139. [Google Scholar] [CrossRef]
- Zhao, C.E.; Wu, J.; Kjelleberg, S.; Loo, J.S.C.; Zhang, Q. Employing a flexible and low-cost polypyrrole nanotube membrane as an anode to enhance current generation in microbial fuel cells. Small 2015, 11, 3440–3443. [Google Scholar] [CrossRef] [PubMed]
- Mateeva, N.; Niculescu, H.; Schlenoff, J.; Testardi, L.R. Correlation of seebeck coefficient and electric conductivity in polyaniline and polypyrrole. J. Appl. Phys. 1998, 83, 3111–3117. [Google Scholar] [CrossRef]
- Maddison, D.S.; Roberts, R.B.; Unsworth, J. Thermoelectric-power of polypyrrole. Synth. Met. 1989, 33, 281–287. [Google Scholar] [CrossRef]
- Kemp, N.T.; Kaiser, A.B.; Liu, C.-J.; Chapman, B.; Mercier, O.; Carr, A.M.; Trodahl, H.J.; Buckley, R.G.; Partridge, A.C.; Lee, J.Y.; et al. Thermoelectric power and conductivity of different types of polypyrrole. J. Polym. Sci. B-Polym. Phys. 1999, 37, 953–960. [Google Scholar] [CrossRef]
- Maddison, D.S.; Unsworth, J.; Roberts, R.B. Electrical-conductivity and thermoelectric-power of polypyrrole with different doping levels. Synth. Met. 1988, 26, 99–108. [Google Scholar] [CrossRef]
- Culebras, M.; Uriol, B.; Gomez, C.M.; Cantarero, A. Controlling the thermoelectric properties of polymers: Application to pedot and polypyrrole. Phys. Chem. Chem. Phys. 2015, 17, 15140–15145. [Google Scholar] [CrossRef]
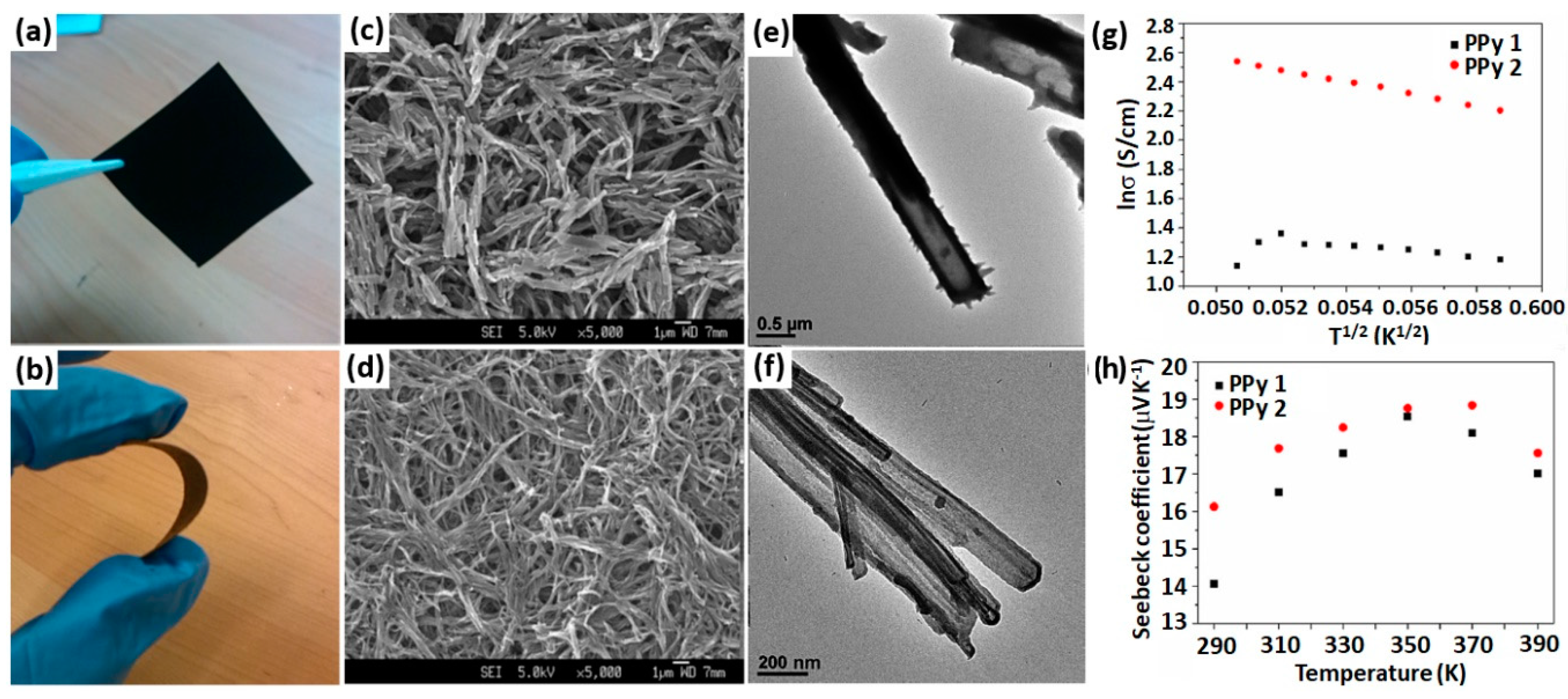
- Wu, J.; Sun, Y.; Pei, W.-B.; Huang, L.; Xu, W.; Zhang, Q. Polypyrrole nanotube film for flexible thermoelectric application. Synth. Met. 2014, 196, 173–177. [Google Scholar] [CrossRef]
- Du, Y.; Niu, H.; Li, J.; Dou, Y.; Shen, S.; Jia, R.; Xu, J. Morphologies tuning of polypyrrole and thermoelectric properties of polypyrrole nanowire/graphene composites. Polymers 2018, 10, 1143. [Google Scholar] [CrossRef]
- Liang, L.; Chen, G.; Guo, C.-Y. Polypyrrole nanostructures and their thermoelectric performance. Mater. Chem. Front. 2017, 1, 380–386. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Dhand, C.; Das, M.; Datta, M.; Malhotra, B. Recent advances in polyaniline based biosensors. Biosens. Bioelectron. 2011, 26, 2811–2821. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.; Kaner, R.B. Polyaniline nanofibers: A unique polymer nanostructure for versatile applications. Acc. Chem. Res. 2008, 42, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.F.; Li, Q.; Xu, Z.S. The chemical modification of polyaniline with enhanced properties: A review. Prog. Org. Coat. 2019, 126, 35–43. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, Z.; Xu, W.; Zhu, D. A three-in-one improvement in thermoelectric properties of polyaniline brought by nanostructures. Synth. Met. 2010, 160, 2371–2376. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.; Xu, W.; Zhang, Q. Investigating thermoelectric properties of doped polyaniline nanowires. Synth. Met. 2014, 189, 177–182. [Google Scholar] [CrossRef]
- Bae, E.J.; Kang, Y.H.; Jang, K.-S.; Cho, S.Y. Enhancement of thermoelectric properties of PEDOT: PSS and tellurium-PESOT: PSS hybrid composites by simple chemical treatment. Sci. Rep. 2016, 6, 18805. [Google Scholar]
- See, K.C.; Feser, J.P.; Chen, C.E.; Majumdar, A.; Urban, J.J.; Segalman, R.A. Water-processable polymer− nanocrystal hybrids for thermoelectrics. Nano Lett. 2010, 10, 4664–4667. [Google Scholar] [CrossRef] [PubMed]
- Coates, N.E.; Yee, S.K.; McCulloch, B.; See, K.C.; Majumdar, A.; Segalman, R.A.; Urban, J.J. Effect of interfacial properties on polymer–nanocrystal thermoelectric transport. Adv. Mater. 2013, 25, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, G.; Wang, X. An unusual coral-like morphology for composites of poly (3, 4-ethylenedioxythiophene)/carbon nanotube and the enhanced thermoelectric performance. Compos. Sci. Technol. 2017, 144, 43–50. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, G.; Wang, H.; Li, X. Template-directed in situ polymerization preparation of nanocomposites of PEDOT: PSS-coated multi-walled carbon nanotubes with enhanced thermoelectric property. Chem. Asian J. 2015, 10, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Mitra, M.; Kargupta, K.; Ganguly, S.; Banerjee, D. Synthesis, characterization and enhanced thermoelectric performance of structurally ordered cable-like novel polyaniline–bismuth telluride nanocomposite. Nanotechnology 2013, 24, 215703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yao, Q.; Chang, J.; Chen, L. Enhanced thermoelectric properties of CNT/PANI composite nanofibers by highly orienting the arrangement of polymer chains. J. Mater. Chem. 2012, 22, 17612–17618. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, L.; Zhang, W.; Liufu, S.; Chen, X. Enhanced thermoelectric performance of single-walled carbon nanotubes/polyaniline hybrid nanocomposites. ACS Nano 2010, 4, 2445–2451. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Ren, D.; Chu, Y.; Wu, Y.; Meng, K.; Miao, J.; Xu, X.; Jiang, Y. Revealing the anisotropy in thermoelectric transport performances in CNT/PANI composites. Synth. Met. 2018, 239, 13–21. [Google Scholar] [CrossRef]
- Chatterjee, M.J.; Banerjee, D.; Chatterjee, K. Composite of single walled carbon nanotube and sulfosalicylic acid doped polyaniline: A thermoelectric material. Mater. Res. Express 2016, 3, 12. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Deng, Y. Semiconductor to metallic behavior transition in multi-wall carbon nanotubes/polyaniline composites with improved thermoelectric properties. Mater. Lett. 2016, 164, 132–135. [Google Scholar] [CrossRef]
- Sobha, A.P.; Narayanankutty, S.K. Electrical and thermoelectric properties of functionalized multiwalled carbon nanotube/polyaniline composites prepared by different methods. IEEE Trans. Nanotechnol. 2014, 13, 835–841. [Google Scholar] [CrossRef]
- Yan, H.Y.; Kou, K.C. Enhanced thermoelectric properties in polyaniline composites with polyaniline-coated carbon nanotubes. J. Mater. Sci. 2014, 49, 1222–1228. [Google Scholar] [CrossRef]
- Zhang, K.; Davis, M.; Qiu, J.J.; Hope-Weeks, L.; Wang, S.R. Thermoelectric properties of porous multi-walled carbon nanotube/polyaniline core/shell nanocomposites. Nanotechnology 2012, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Mitra, M.; Kargupta, K.; Ganguly, S.; Goswami, S.; Banerjee, D. Facile synthesis and thermoelectric properties of aluminum doped zinc oxide/polyaniline (AZO/PANI) hybrid. Synth. Met. 2017, 228, 25–31. [Google Scholar] [CrossRef]
- Liang, L.; Gao, C.; Chen, G.; Guo, C.-Y. Large-area, stretchable, super flexible and mechanically stable thermoelectric films of polymer/carbon nanotube composites. J. Mater. Chem. C 2016, 4, 526–532. [Google Scholar] [CrossRef]
- Song, H.; Cai, K.; Wang, J.; Shen, S. Influence of polymerization method on the thermoelectric properties of multi-walled carbon nanotubes/polypyrrole composites. Synth. Met. 2016, 211, 58–65. [Google Scholar] [CrossRef]
- Liu, J.; Yu, H.Q. Thermoelectric enhancement in polyaniline composites with polypyrrole-functionalized multiwall carbon nanotubes. J. Electron. Mater. 2014, 43, 1181–1187. [Google Scholar] [CrossRef]
- Culebras, M.; Choi, K.; Cho, C. Recent progress in flexible organic thermoelectrics. Micromachines 2018, 9, 638.4. [Google Scholar] [CrossRef]
- An, C.J.; Lee, Y.C.; Kang, Y.H.; Cho, S.Y. Improved interaction between semiconducting polymer and carbon nanotubes in thermoelectric composites through covalent grafting. Carbon 2017, 124, 662–668. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, K.; Zhang, J.; Wang, S.; Qiu, Y. Thermoelectric properties of conducting polymer nanowire–tellurium nanowire composites. ACS Appl. Energy Mater. 2018, 1, 4883–4890. [Google Scholar] [CrossRef]
- Xin, S.; Yang, N.; Gao, F.; Zhao, J.; Li, L.; Teng, C. Free-standing and flexible polypyrrole nanotube/reduced graphene oxide hybrid film with promising thermoelectric performance. Mater. Chem. Phys. 2018, 212, 440–445. [Google Scholar] [CrossRef]
- Liang, L.; Chen, G.; Guo, C.-Y. Enhanced thermoelectric performance by self-assembled layered morphology of polypyrrole nanowire/single-walled carbon nanotube composites. Compos. Sci. Technol. 2016, 129, 130–136. [Google Scholar] [CrossRef]
- Zhang, K.; Qiu, J.; Wang, S. Thermoelectric properties of PEDOT nanowire/pedot hybrids. Nanoscale 2016, 8, 8033–8041. [Google Scholar] [CrossRef] [PubMed]




| 1D Polymer | Preparation Method | σ (S cm−1) | S (µV K−1) | PFmax (μW m−1 K−2) | κ (W m−1 K−1) | ZT | Ref |
|---|---|---|---|---|---|---|---|
| PEDOT nanowires | Hard template | 43 ± 5 | 28 ± 2 | 3.4 | – | – | [33] |
| PEDOT nanowires | Hard template | 12 | −85 | 8.7 | – | – | [34] |
| PEDOT nanofibers | Reverse microemulsion | 71.4 | 48 | 16.4 | – | – | [35] |
| PEDOT nanowires | Hard template + post-treatment | 541 | 20.78 | 23.4 | – | – | [39] |
| PEDOT naonrods | Reverse microemulsion + post-treatment | 16.9 | 23.3 | 0.91 | – | – | [40] |
| PEDOT microfibers | Acid gelation + post-treatment | 172.5 | 14.8 | 4.77 | – | – | [42] |
| P3HT nanofibers | Whisker method + silver doping | 18.3 | 61.1 | 6.84 | 0.80 | 2.6 × 10−3 | [62] |
| P3HT nanofibers | Whisker method | 12.6 | 48.8 | 3.7 | 0.0708 | 1.6 × 10−2 | [63] |
| PPy nanotubes | Soft template | 9.81 | 17.68 | 0.31 | 0.17 | 5.71 × 10−4 | [71] |
| PPy nanowires | Soft template | 22.5 | 11.5 | 0.3 | – | – | [72] |
| PPy nanowires | Soft template | 2.217 | 10.1 | 0.023 | – | – | [73] |
| PANI nanowires | Soft template + acid doping | 0.0077 | 212.4 | 0.035 | 0.21 | 4.86 × 10−5 | [79] |
| PANI nanowires | Soft template + acid doping | 1.24 | 15 | 0.028 | 0.32 | 2.75 × 10−5 | [80] |
| Polymer Composites | Preparation Method | σ (S cm−1) | S (µV K−1) | PFmax (μW m−1 K−2) | κ (W m−1 K−1) | ZT | Ref |
|---|---|---|---|---|---|---|---|
| PEDOT/CNTs | In situ polymerization | 586 | 18 | 19.00 ± 1.43 | – | – | [84] |
| PEDOT/CNTs | In situ polymerization | 15 | 12 | 0.229 ± 0.014 | – | – | [85] |
| PANI/Bi2Te3 nanowires | In situ polymerization | 12 | 35 | 1.57 | 0.1096 | 0.0043 | [86] |
| PANI/CNTs | In situ polymerization | 17 | 10 | 0.18 | – | – | [87] |
| PANI/SWCNTs | In situ polymerization | 125 | 40 | 20 | – | – | [88] |
| PANI/ZnO nanorods | In situ polymerization | 28.15 | 41.55 | 4.86 | 0.87 | 0.0017 | [95] |
| PPy/SWCNTs | In situ polymerization | 399 ± 14 | 22.2 ± 0.1 | 19.7 ± 0.8 | – | – | [96] |
| P3HT/DWCNTs | Covalent grafting | 115 | 35 | 46 | 2.0 | 0.0069 | [100] |
| Te/PEDOT nanowires | Self-assembly | 500 | 24 | 29.05 | – | – | [101] |
| PPy nanotubes/rGO | Self-assembly | 80 | 28 | 7.28 | – | – | [102] |
| PPy nanowires/CNTs | Self-assembly | 300 | 27 | 21.7 | – | [103] | |
| PEDOT nanowires/PEDOT | Physical mixing | 1270 | 59.3 | 446.6 | 0.26 | 0.44 | [104] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, K.W.; Wang, S.-X.; Soo, D.X.Y.; Xu, J. One-Dimensional Nanostructure Engineering of Conducting Polymers for Thermoelectric Applications. Appl. Sci. 2019, 9, 1422. https://doi.org/10.3390/app9071422
Shah KW, Wang S-X, Soo DXY, Xu J. One-Dimensional Nanostructure Engineering of Conducting Polymers for Thermoelectric Applications. Applied Sciences. 2019; 9(7):1422. https://doi.org/10.3390/app9071422
Chicago/Turabian StyleShah, Kwok Wei, Su-Xi Wang, Debbie Xiang Yun Soo, and Jianwei Xu. 2019. "One-Dimensional Nanostructure Engineering of Conducting Polymers for Thermoelectric Applications" Applied Sciences 9, no. 7: 1422. https://doi.org/10.3390/app9071422
APA StyleShah, K. W., Wang, S.-X., Soo, D. X. Y., & Xu, J. (2019). One-Dimensional Nanostructure Engineering of Conducting Polymers for Thermoelectric Applications. Applied Sciences, 9(7), 1422. https://doi.org/10.3390/app9071422