A Novel Method to Characterise Levels of Pharmaceutical Pollution in Large-Scale Aquatic Monitoring Campaigns
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Substances
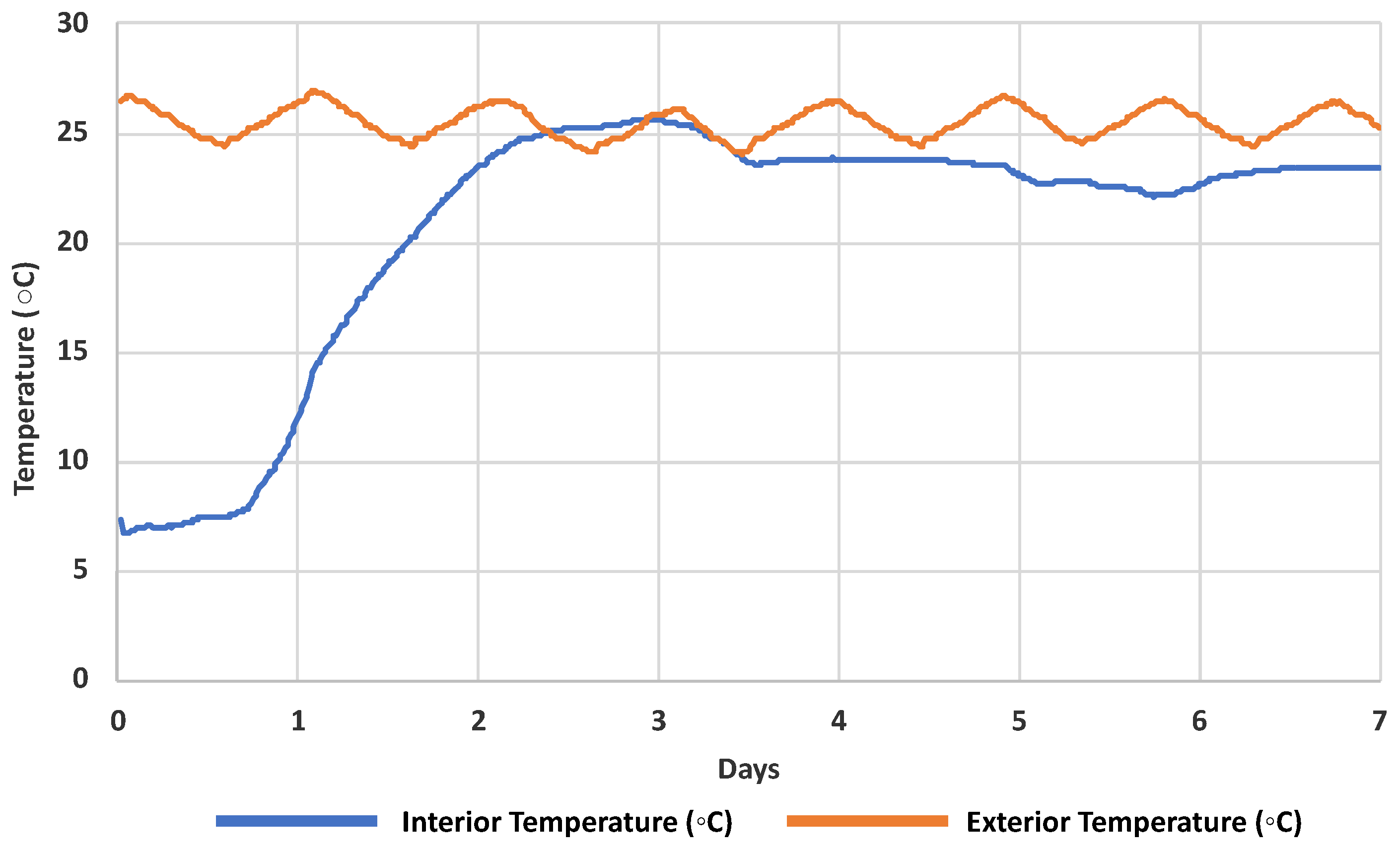
2.2. Sampling Kits and Water Collection Protocol
2.3. HPLC-MS/MS Protocol
2.4. Quality Control
2.5. Method Validation
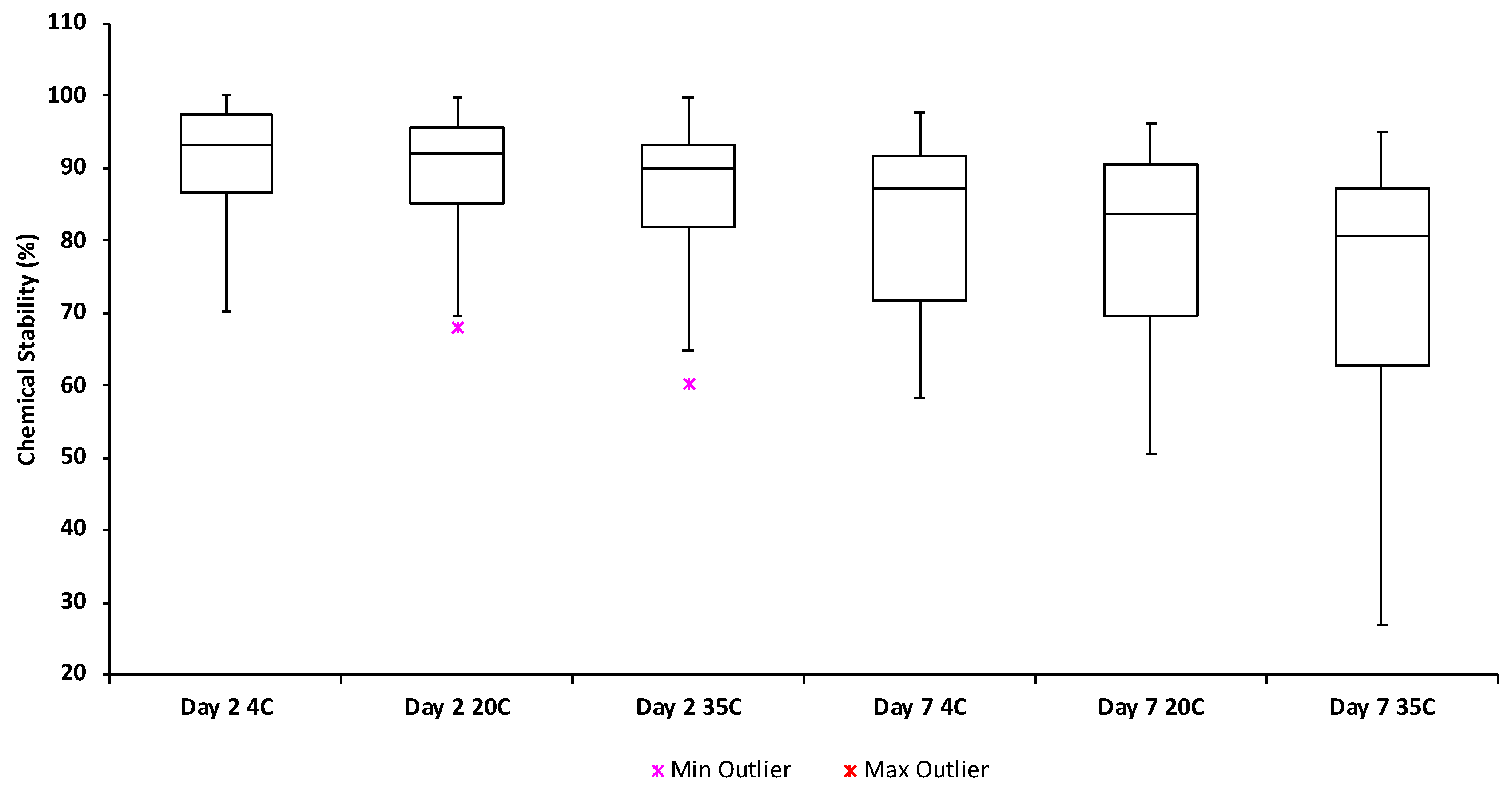
2.5.1. Evaluation of Chemical Stability
2.5.2. Interlaboratory Assessment
3. Results and Discussion
3.1. Method Validation
3.2. Evaluation of Chemical Stability during Shipment
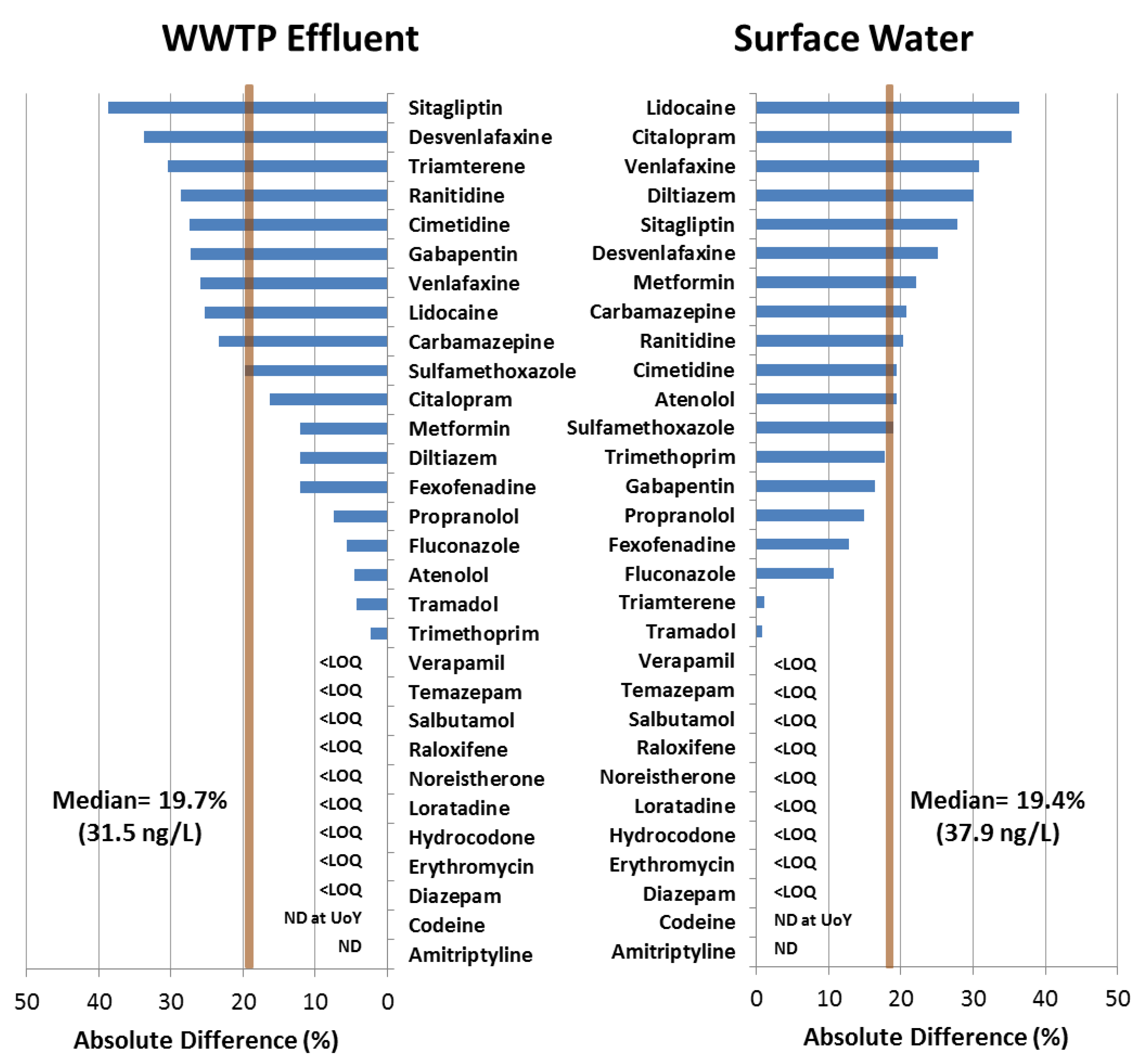
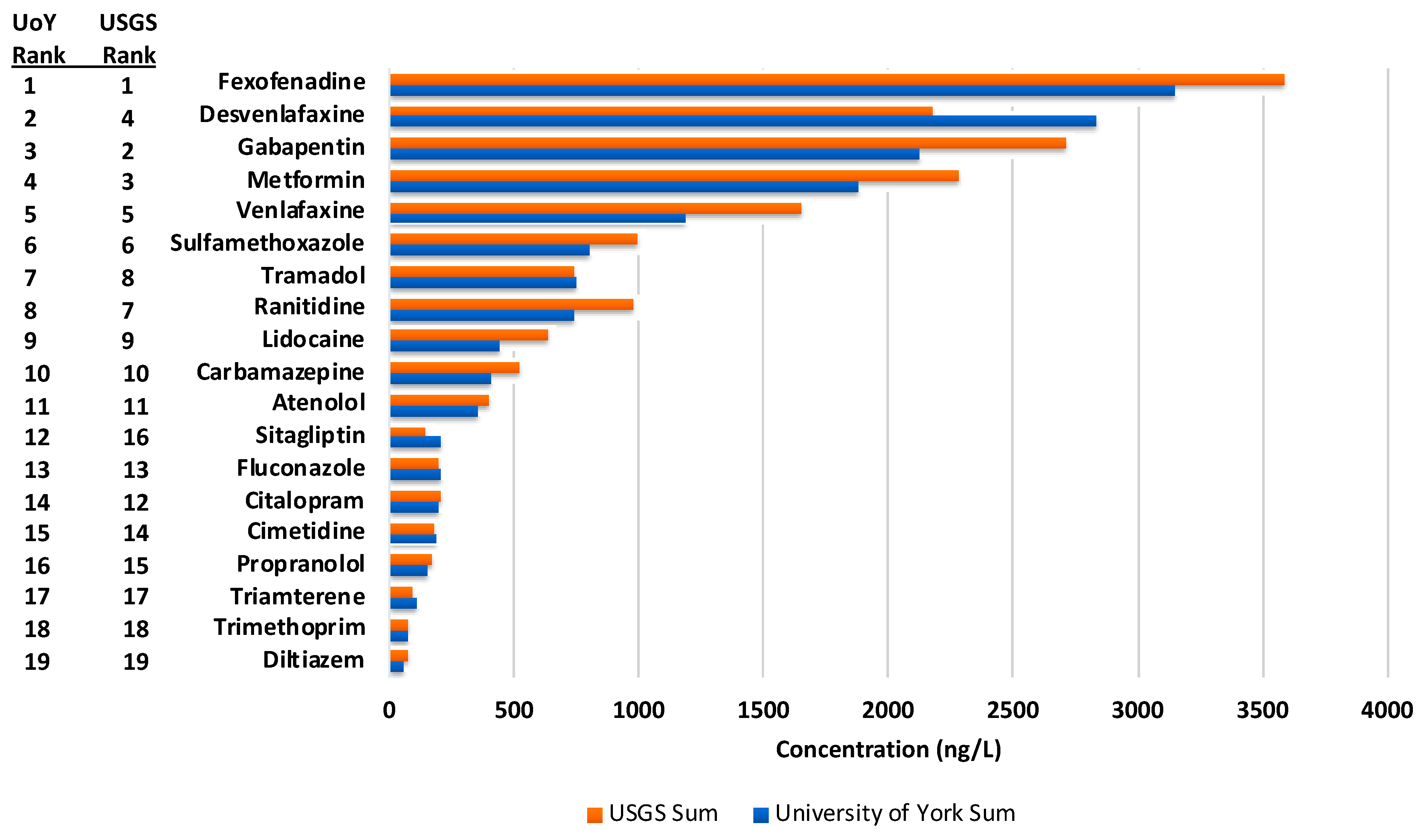
3.3. Interlaboratory Assessment
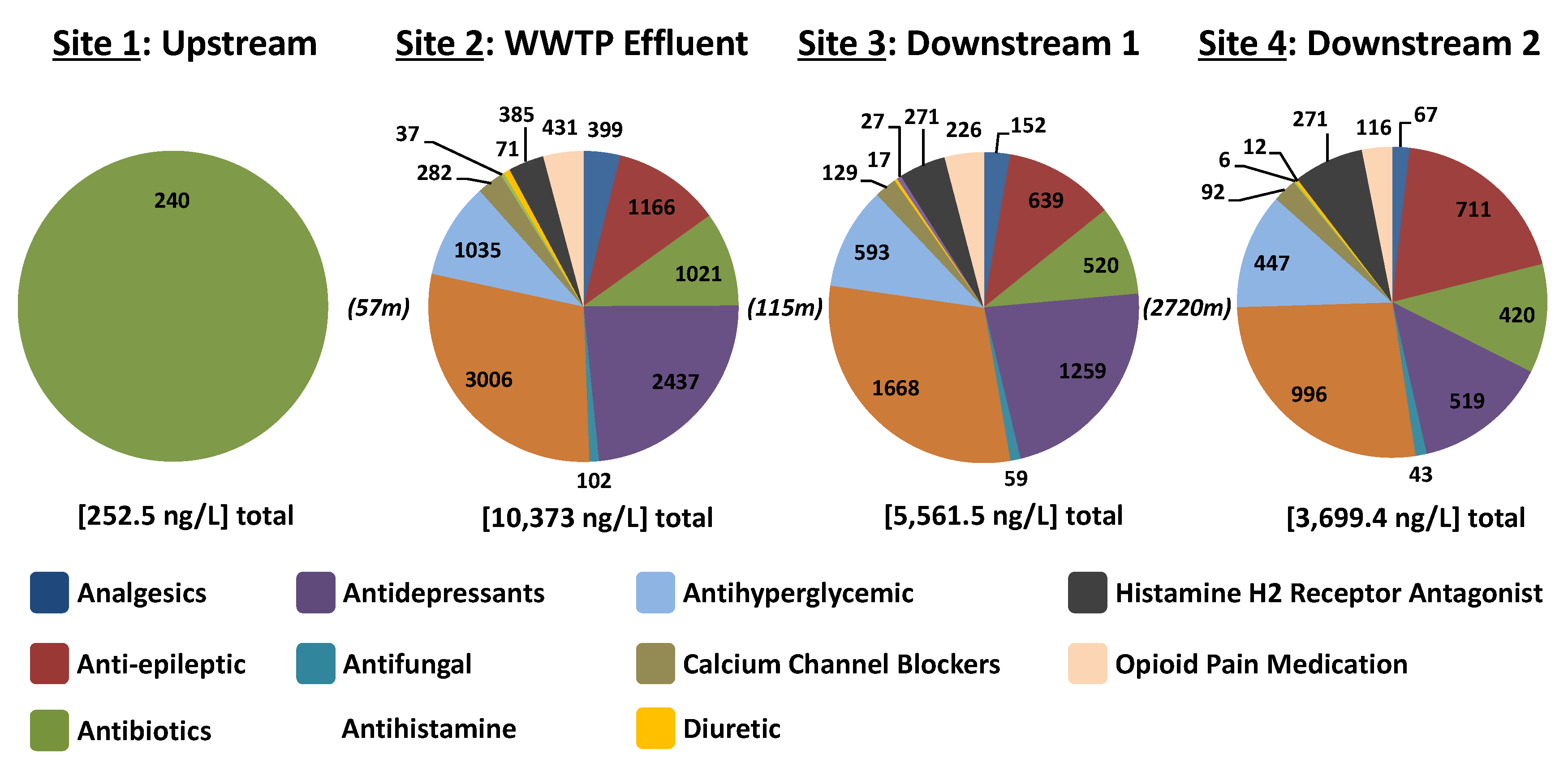
3.4. Concentrations of Studied APIs in Muddy Creek
3.5. Implication for Future Research
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Monette, C.E.; Bracero, S.; Saha, M.S. Methods for field measurement of antibiotic concentrations: Limitations and outlook. FEMS Microbiol. Ecol. 2018, 94, 105. [Google Scholar] [CrossRef] [PubMed]
- Tisler, S.; Zwiener, C. Formation and occurrence of transformation products of metformin in wastewater and surface water. Sci. Total Environ. 2018, 628, 1121–1129. [Google Scholar] [CrossRef]
- Hughes, S.R.; Kay, P.; Brown, L.E. Global synthesis and critical evaluation of pharmaceutical data sets collected from river systems. Environ. Sci. Technol. 2012, 47, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Van den Brink, P.J.; Boxall, A.B.; Maltby, L.; Brooks, B.W.; Rudd, M.A.; Backhaus, T.; Spurgeon, D.; Verougstraete, V.; Ajao, C.; Ankley, G.T.; et al. Toward sustainable environmental quality: Priority research questions for Europe. Environ. Toxicol. Chem. 2018, 37, 2281–2295. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Nakamichi, S.; Habibullah-Al-Mamun, M.; Tani, K.; Masunaga, S.; Matsuda, H. Occurrence and ecological risk of pharmaceuticals in river surface water of Bangladesh. Environ. Res. 2018, 165, 258–266. [Google Scholar] [CrossRef]
- Furlong, E.T.; Noriega, M.C.; Kanagy, C.J.; Kanagy, L.K.; Coffey, L.J.; Burkhardt, M.R. Determination of Human-Use Pharmaceuticals in Filtered Water by Direct Aqueous Injection: High-Performance Liquid Chromatography/Tandem Mass Spectrometry. U.S. Geological Survey Techniques and Methods 5-B10. 2014. Available online: https://pubs.usgs.gov/tm/5b10/pdf/tm10-b5.pdf (accessed on 8 March 2019).
- Campos-Mañas, M.C.; Plaza-Bolaños, P.; Sánchez-Pérez, J.A.; Malato, S.; Agüera, A. Fast determination of pesticides and other contaminants of emerging concern in treated wastewater using direct injection coupled to highly sensitive ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1507, 84–94. [Google Scholar] [CrossRef]
- Chiaia, A.C.; Banta-Green, C.; Field, J. Eliminating solid phase extraction with large-volume injection LC/MS/MS: Analysis of illicit and legal drugs and human urine indicators in US wastewaters. Environ. Sci. Technol. 2008, 42, 8841–8848. [Google Scholar] [CrossRef]
- Bayen, S.; Yi, X.; Segovia, E.; Zhou, Z.; Kelly, B.C. Analysis of selected antibiotics in surface freshwater and seawater using direct injection in liquid chromatography electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2014, 1338, 38–43. [Google Scholar] [CrossRef]
- Boxall, A.B.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and personal care products in the environment: What are they key questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef]
- Wellington, E.M.; Boxall, A.B.; Cross, P.; Feil, E.J.; Gaze, W.H.; Hawkey, P.M.; Johnson-Rollings, A.S.; Jones, D.L.; Lee, N.M.; Otten, W.; et al. The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect. Dis. 2013, 13, 155–165. [Google Scholar] [CrossRef]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological effects of pharmaceuticals in aquatic systems—Impacts through behavioural alterations. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130580. [Google Scholar] [CrossRef]
- Sallach, J.B.; Snow, D.; Hodges, L.; Li, X.; Bartelt-Hunt, S. Development and comparison of four methods for the extraction of antibiotics from a vegetative matrix. Environ. Toxicol. Chem. 2016, 35, 889–897. [Google Scholar] [CrossRef]
- Bisceglia, K.J.; Jim, T.Y.; Coelhan, M.; Bouwer, E.J.; Roberts, A.L. Trace determination of pharmaceuticals and other wastewater-derived micropollutants by solid phase extraction and gas chromatography/mass spectrometry. J. Chromatogr. A 2010, 1217, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.S.; Murphy, M.; Mendola, N.; Wong, V.; Carlson, D.; Waring, L. Characterization of pharmaceuticals and personal care products in hospital effluent and waste water influent/effluent by direct-injection LC-MS-MS. Sci. Total Environ. 2015, 518, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Hermes, N.; Jewell, K.S.; Wick, A.; Ternes, T.A. Quantification of more than 150 micropollutants including transformation products in aqueous samples by liquid chromatography-tandem mass spectrometry using scheduled multiple reaction monitoring. J. Chromatogr. A 2018, 1531, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Gurke, R.; Rossmann, J.; Schubert, S.; Sandmann, T.; Rößler, M.; Oertel, R.; Fauler, J. Development of a SPE-HPLC–MS/MS method for the determination of most prescribed pharmaceuticals and related metabolites in urban sewage samples. J. Chromatogr. B 2015, 990, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Paiga, P.; Santos, L.H.M.L.M.; Delerue-Matos, C. Development of a multi-residue method for the determination of human and veterinary pharmaceuticals and some of their metabolites in aqueous environmental matrices by SPE-UHPLC–MS/MS. J. Pharm. Biomed. Anal. 2017, 135, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Ma, R.; Wang, B.; Yuan, H.; Yu, G. The degradation and persistence of five pharmaceuticals in an artificial climate incubator during a one year period. RSC Adv. 2017, 7, 8280–8287. [Google Scholar] [CrossRef]
- Kwon, J.W.; Armbrust, K.L. Laboratory persistence and fate of fluoxetine in aquatic environments. Environ. Toxicol. Chem. 2006, 25, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Song, W.; Cooper, W.J.; Jung, J.; Greaves, J. Degradation of tetracycline antibiotics: Mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 2010, 78, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Leusch, F.D.L.; Prochazka, E.; Tan, B.L.L.; Carswell, S.; Neale, P.; Escher, B.I. Optimising micropollutants extraction for analysis of water samples: Comparison of different solid phase materials and liquid-liquid extraction. In Proceedings of the Science Forum and Stakeholder Engagement: Building Linkages, Collaboration and Science quality, Brisbane, QL, Australia, 19–20 June 2012; pp. 191–195. [Google Scholar]
- Bradley, P.M.; Barber, L.B.; Duris, J.W.; Foreman, W.T.; Furlong, E.T.; Hubbard, L.E.; Hutchinson, K.J.; Keefe, S.H.; Kolpin, D.W. Riverbank filtration potential of pharmaceuticals in a wastewater-impacted stream. Environ. Pollut. 2014, 193, 173–180. [Google Scholar] [CrossRef] [PubMed]
| Therapeutic Class | Compound | Associated Internal Standard | |
|---|---|---|---|
| Analgesic | Lidocaine | Lidocaine D6 | |
| Naproxen | Naproxen D3 | ||
| Paracetamol | Paracetamol D4 | ||
| Anti-epileptic | Carbamazepine | Carbamazepine D10 | |
| Gabapentin | Gabapentin D10 | ||
| Pregabalin | Atrazine D5 | ||
| Antibiotics | Fluoroquinolones | Ciprofloxacin | Ciprofloxacin D8 |
| Enrofloxacin * | Atrazine D5 | ||
| Lincosamide | Lincomyacin * | Atrazine D5 | |
| Macrolides | Clarithromycin | Atrazine D5 | |
| Erythromycin | Atrazine D5 | ||
| Tylosin * | Atrazine D5 | ||
| Nitroimidazole | Metronidazole | Metronidazole D3 | |
| Penicillin | Cloxacillin | Atrazine D5 | |
| Sulfonamides | Sulfadiazine * | Atrazine D5 | |
| Sulfamethoxazole | Sulfamethoxazole D4 | ||
| Trimethoprim | Trimethoprim D9 | ||
| Tetracyclines | Oxytetracycline | Atrazine D5 | |
| Tetracycline | Atrazine D5 | ||
| Antidepressant | Amitriptyline | Amitriptyline D3 | |
| Citalopram | Citalopram D6 | ||
| Desvenlafaxine | Desvenlafaxine D6 | ||
| Fluoxetine | Atrazine D5 | ||
| Sertraline | Sertraline D3 | ||
| Venlafaxine | Venlafaxine D6 | ||
| Norfluoxetine | Norfluoxetine D6 | ||
| Antifungal | Clotrimazole | Atrazine D5 | |
| Fluconazole | Atrazine D5 | ||
| Itraconazole | Itraconazole D4 | ||
| Ketoconazole | Atrazine D5 | ||
| Miconazole | Atrazine D5 | ||
| Thiabendazole | Atrazine D5 | ||
| Antihistamine | Cetirizine | Atrazine D6 | |
| Diphenhydramine | Diphenhydramine D3 | ||
| Fexofenadine | Atrazine D5 | ||
| Ketotifen | Atrazine D5 | ||
| Loratadine | Atrazine D5 | ||
| Antihyperglycemic | Metformin | Metformin D6 | |
| Sitagliptin | Sitagliptin D4 | ||
| Antimalarial | Artemisinin | Atrazine D5 | |
| Antiviral/-retroviral | Nevirapine | Atrazine D5 | |
| Oseltamivir | Atrazine D5 | ||
| Benzodiazepine | Diazepam | Diazepam D5 | |
| Oxazepam | Oxazepam D5 | ||
| Temazepam | Temazepam D5 | ||
| Beta-blocker | Atenolol | Atenolol D7 | |
| Propranolol | Propranolol D7 | ||
| Calcium channel blocker | Diltiazem | Diltiazem D3 | |
| Verapamil | Verapamil D7 | ||
| Diuretic | Triamterene | Triamterene D5 | |
| Histamine H2 receptor antagonist | Cimetidine | Atrazine D5 | |
| Ranitidine | Atrazine D5 | ||
| Opioid pain medication | Codeine | Codeine D6 | |
| Hydrocodone | Hydrocodone D3 | ||
| Tramadol | Atrazine D5 | ||
| Oral contraceptive | Noreistherone | Atrazine D5 | |
| Selective estrogen receptor modulator | Raloxifene | Ralixifene D4 | |
| Stimulant | Caffeine | Atrazine D5 | |
| Cotinine | Cotinine D3 | ||
| Nicotine | Atrazine D5 | ||
| β2 adrenergic receptor agonist (anti-asthma) | Salbutamol | Salbutamol D9 | |
| Validation Parameter | Mean ± SD | Within Acceptable Range * | |
|---|---|---|---|
| Linearity | r2 | 0.984 ± 0.02 | |
| Intra-day Repeatability (%) | 10 ng/L | 29 ± 19 | 46% |
| 100 ng/L | 11 ± 9 | 82% | |
| 1000 ng/L | 8 ± 6 | 95% | |
| Intermediate/Inter-day Precision (%) | 10 ng/L | 42 ± 41 | 34% |
| 100 ng/L | 16 ± 12 | 72% | |
| 1000 ng/L | 9 ± 6 | 93% | |
| Analyte Response in LCMS-grade water (%) | 10 ng/L | 95 ± 32 | 75% |
| 100 ng/L | 104 ± 22 | 90% | |
| 1000 ng/L | 94 ± 15 | 97% | |
| Analyte Response in tap water (%) | 10 ng/L | 110 ± 27 | 59% |
| 100 ng/L | 101 ± 31 | 80% | |
| 1000 ng/L | 106 ± 28 | 90% | |
| Analyte Response in surface water (%) | 10 ng/L | 101 ± 88 | 56% |
| 100 ng/L | 106 ± 26 | 82% | |
| 1000 ng/L | 106 ± 18 | 92% | |
| Analyte Response in WWTP effluent (%) | 10 ng/L | 195 ± 212 | 39% |
| 100 ng/L | 117 ± 38 | 59% | |
| 1000 ng/L | 108 ± 18 | 92% | |
| Analyte Response WWTP influent (%) | 10 ng/L | 465 ± 992 | 20% |
| 100 ng/L | 168 ± 81 | 38% | |
| 1000 ng/L | 145 ± 48 | 54% | |
| Limit of Detection (ng/L) | LCMS-grade water (ng/L) | 9.16 | |
| Drinking water (ng/L) | 9.72 | ||
| Surface water (ng/L) | 11.79 | ||
| WWTP effluent (ng/L) | 20.22 | ||
| WWTP influent (ng/L) | 54.45 | ||
| Limit of Quantification (ng/L) | LCMS-grade water (ng/L) | 18.32 | |
| Drinking water (ng/L) | 19.44 | ||
| Surface water (ng/L) | 23.57 | ||
| WWTP effluent (ng/L) | 40.43 | ||
| WWTP influent (ng/L) | 108.89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W. A Novel Method to Characterise Levels of Pharmaceutical Pollution in Large-Scale Aquatic Monitoring Campaigns. Appl. Sci. 2019, 9, 1368. https://doi.org/10.3390/app9071368
Wilkinson JL, Boxall ABA, Kolpin DW. A Novel Method to Characterise Levels of Pharmaceutical Pollution in Large-Scale Aquatic Monitoring Campaigns. Applied Sciences. 2019; 9(7):1368. https://doi.org/10.3390/app9071368
Chicago/Turabian StyleWilkinson, John L., Alistair B.A. Boxall, and Dana W. Kolpin. 2019. "A Novel Method to Characterise Levels of Pharmaceutical Pollution in Large-Scale Aquatic Monitoring Campaigns" Applied Sciences 9, no. 7: 1368. https://doi.org/10.3390/app9071368
APA StyleWilkinson, J. L., Boxall, A. B. A., & Kolpin, D. W. (2019). A Novel Method to Characterise Levels of Pharmaceutical Pollution in Large-Scale Aquatic Monitoring Campaigns. Applied Sciences, 9(7), 1368. https://doi.org/10.3390/app9071368






