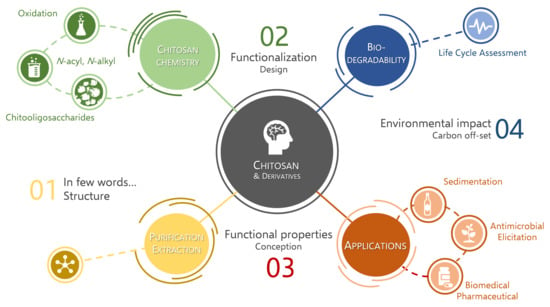
Modification of Chitosan for the Generation of Functional Derivatives
Abstract
1. Introduction
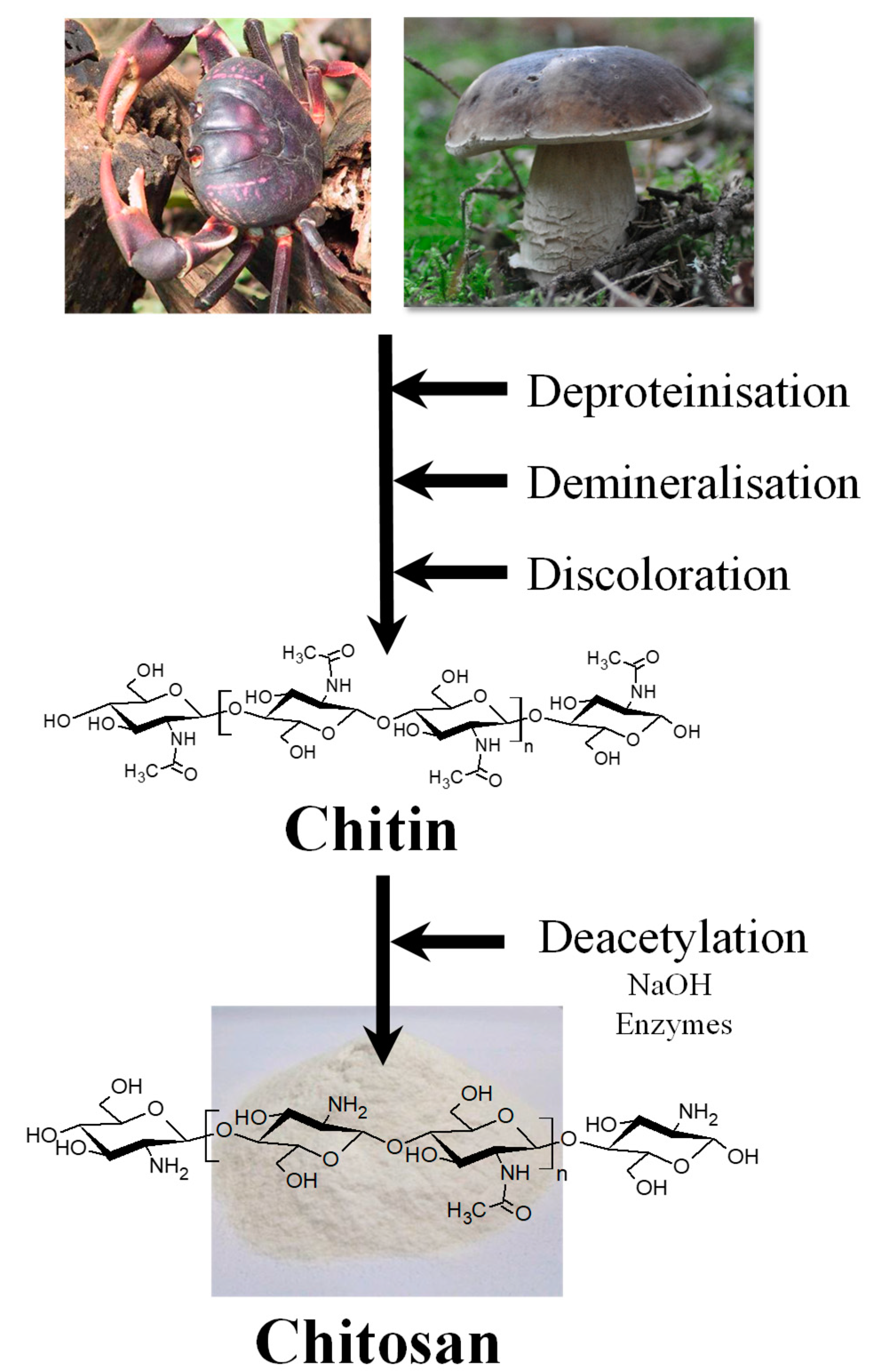
2. Chitosan in Brief
2.1. Extraction and Structure of Chitosan from Natural Sources
2.2. Global Market
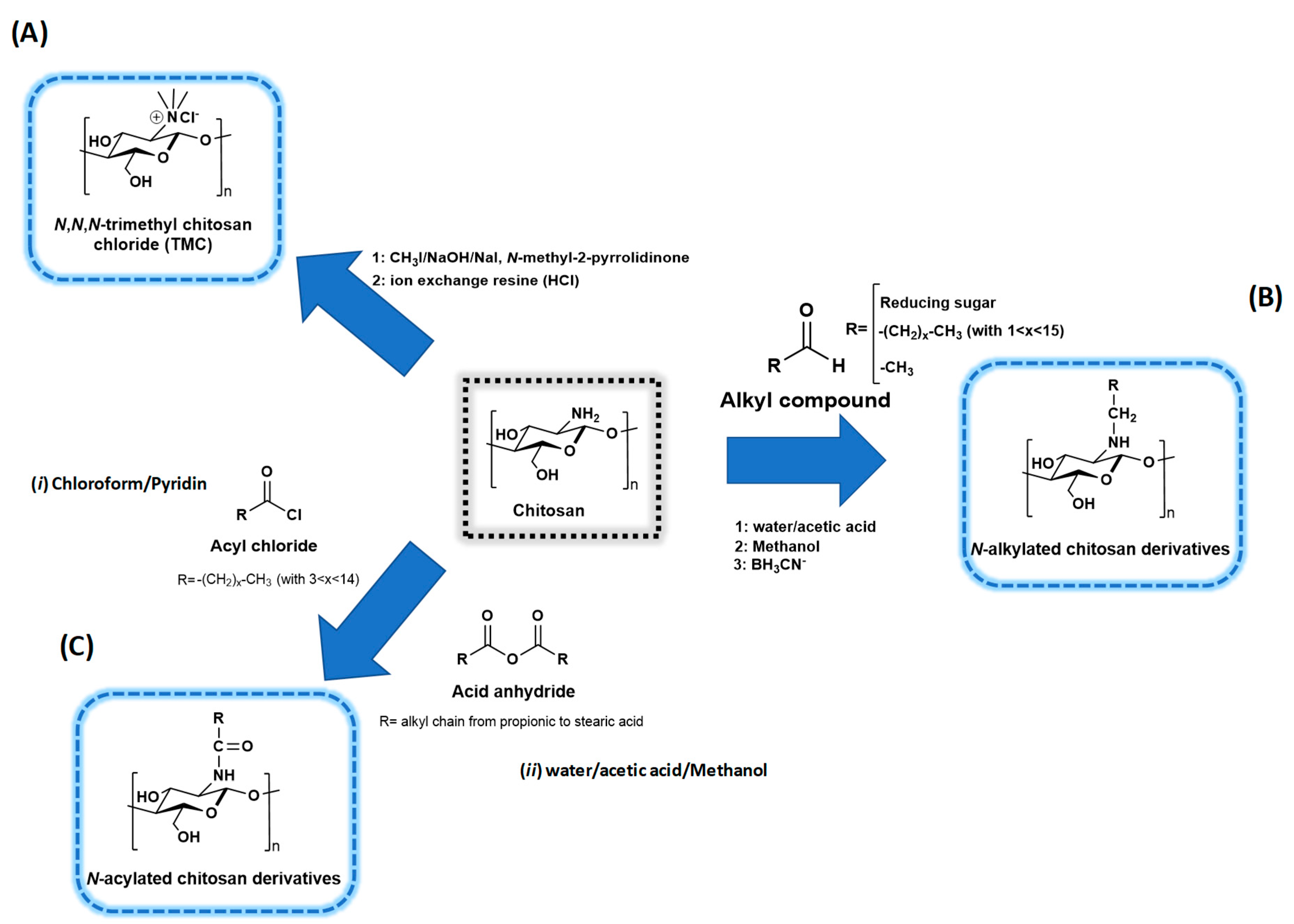
3. Chitosan Modification and Functionalization
3.1. Chitosan Chemistry
3.1.1. Quaternized Chitosan Derivatives
3.1.2. N-acyl Chitosan Derivatives
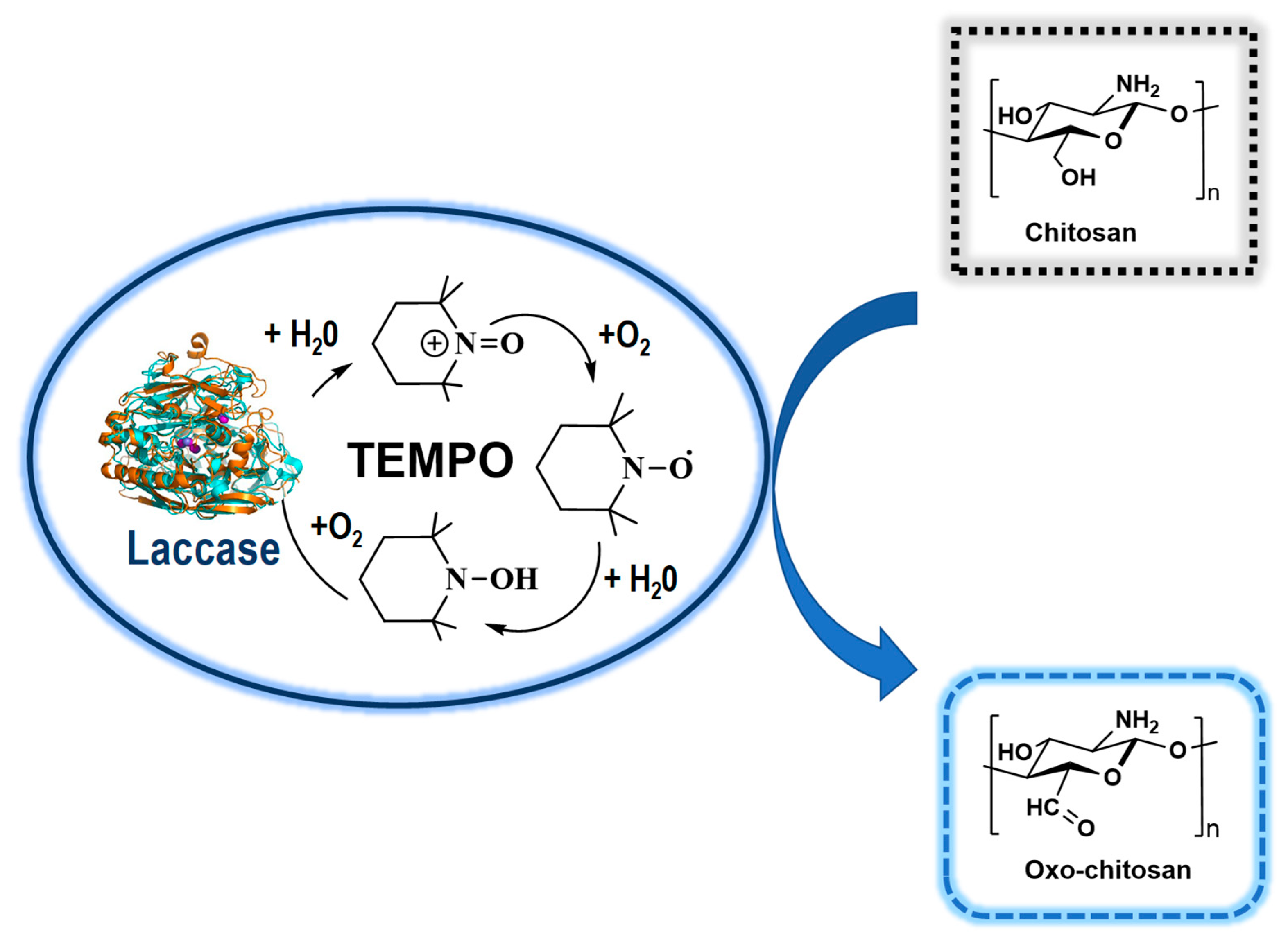
3.1.3. Oxy-Chitosan Derivatives
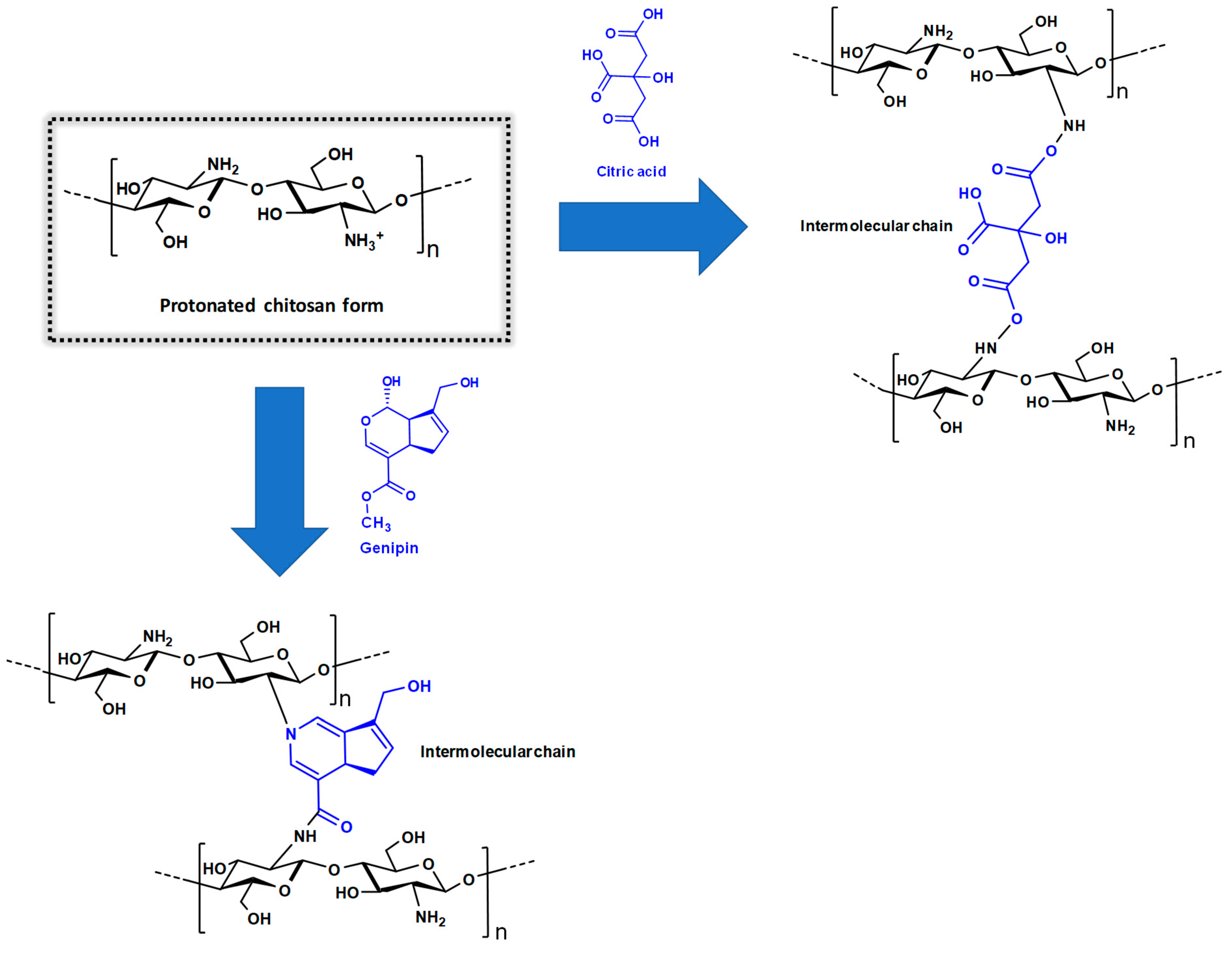
3.1.4. Cross-Linked Chitosan Derivatives
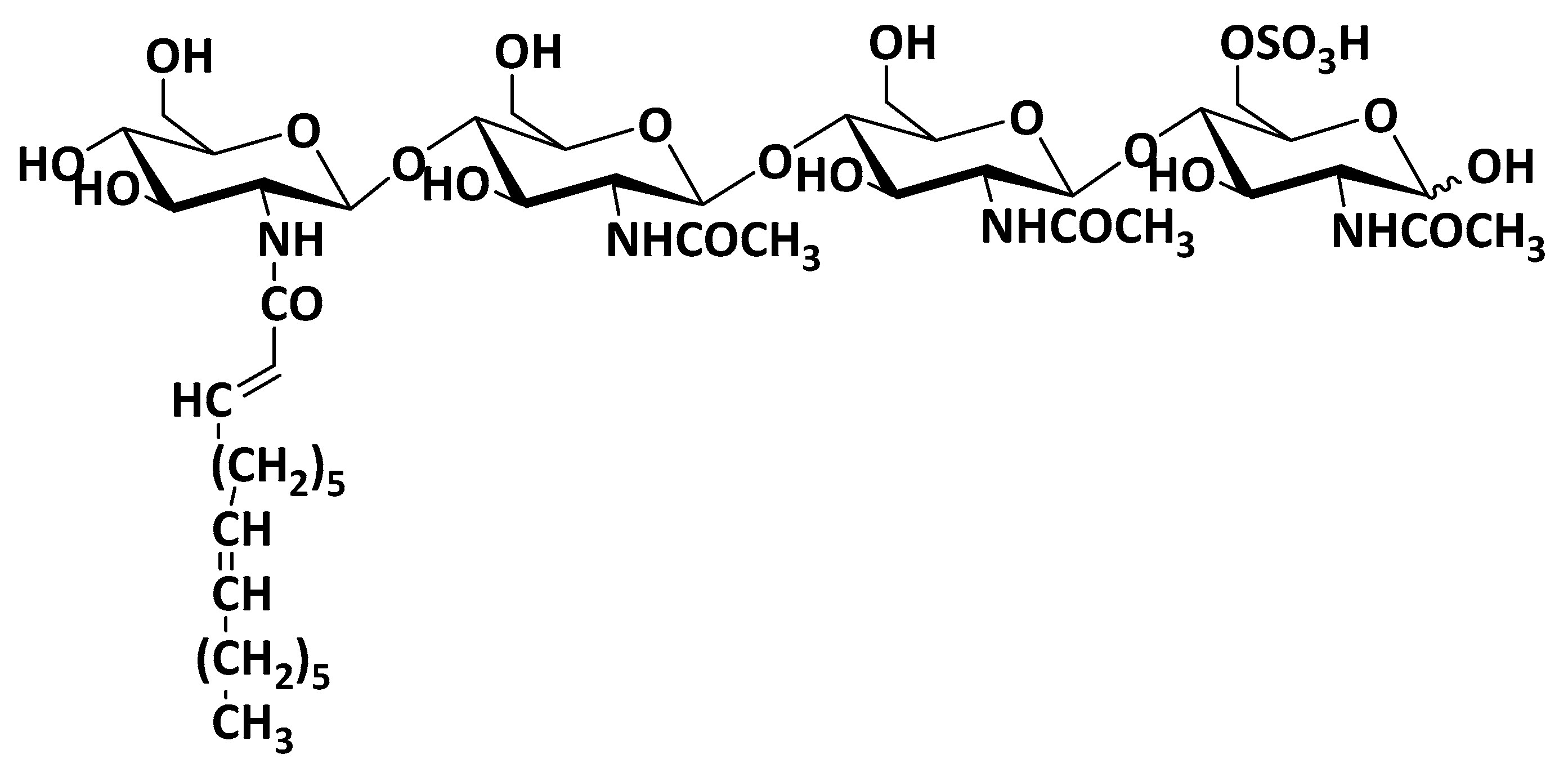
3.2. Chitooligosaccharide (COS) and Low Molecular Weight (LMW) Chitosan
4. Functional Properties of Chitosan and Derivatives
4.1. Sedimentation and Flocculation in the Wine Industry
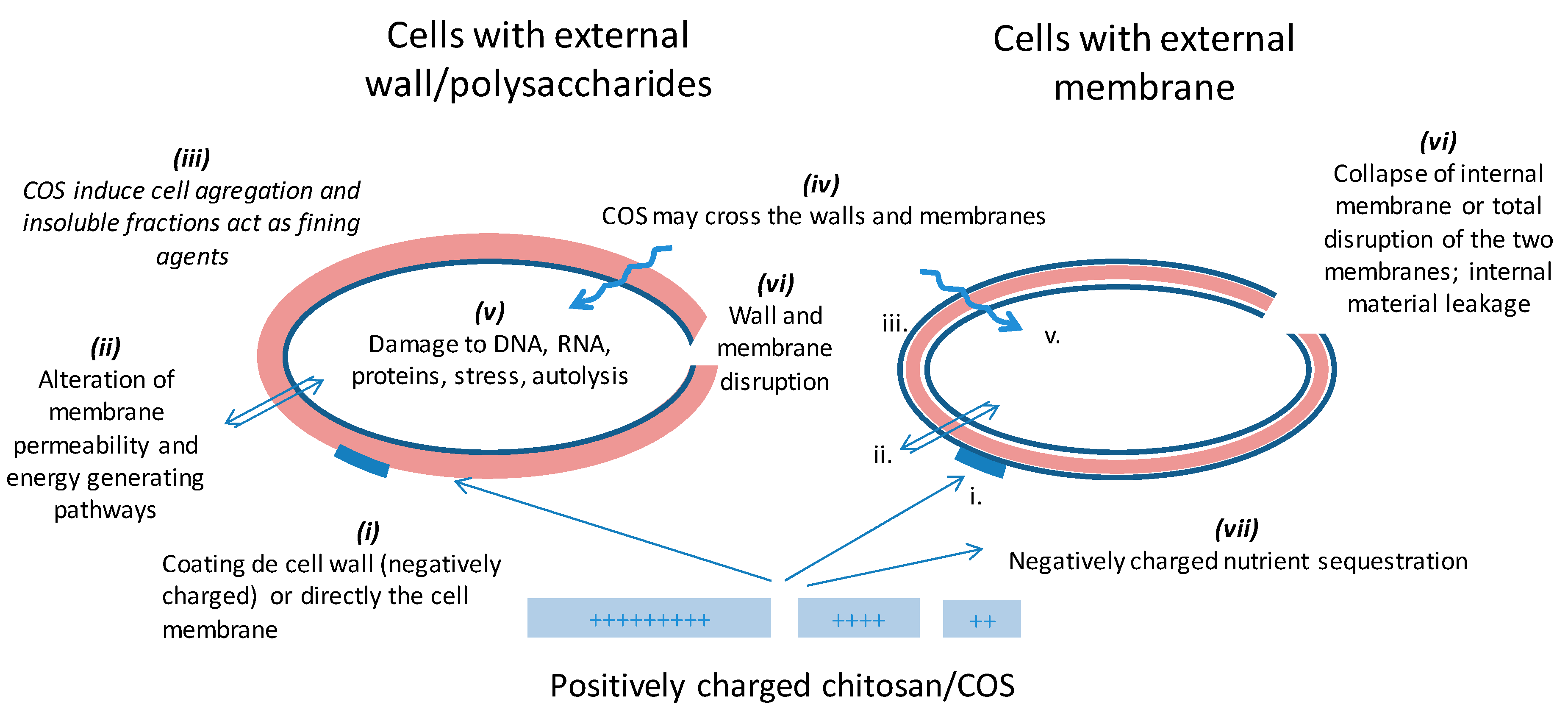
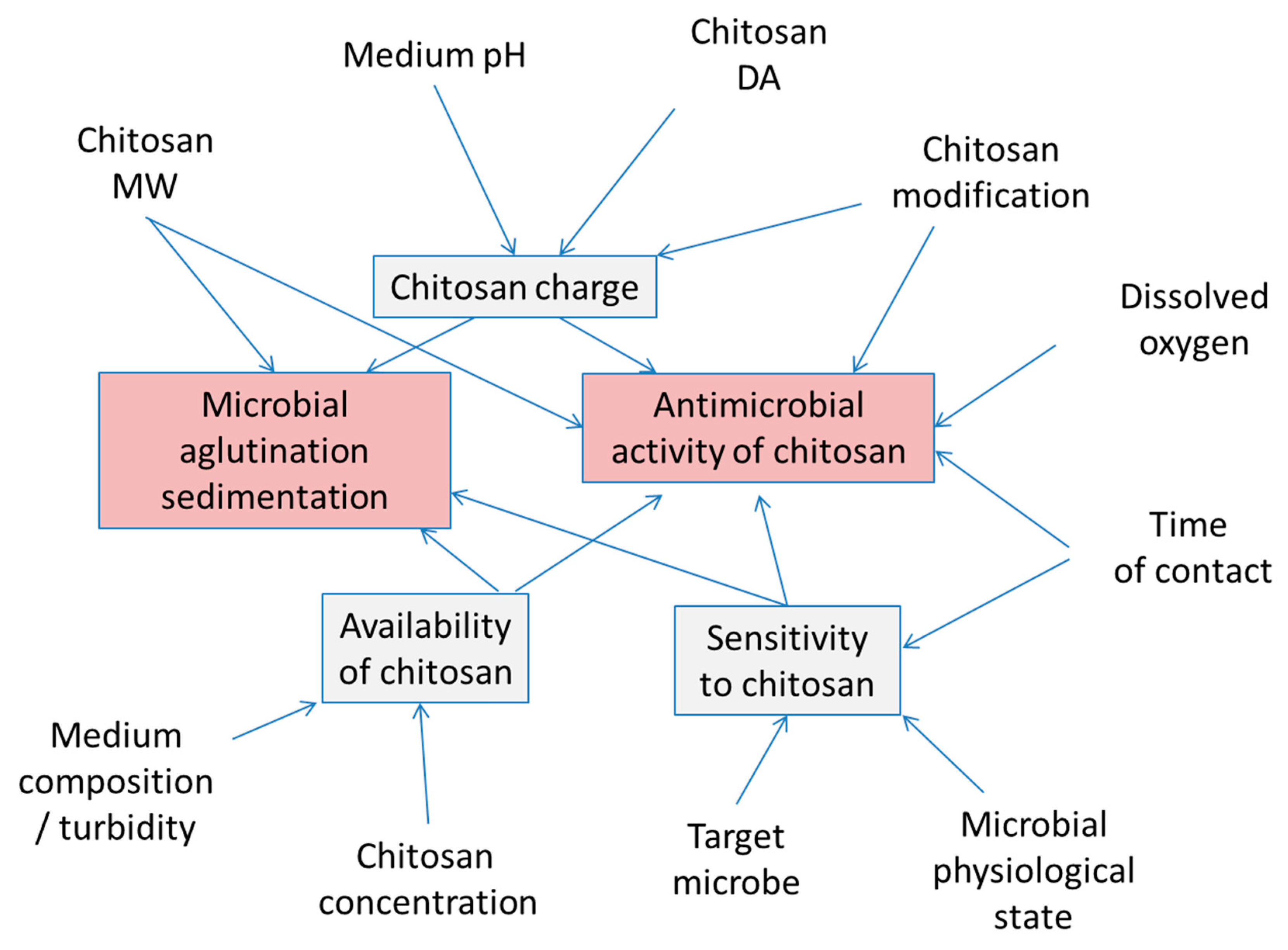
4.2. Antimicrobial Functions
- (i)
- The formation of a physico-chemical barrier (towards oxygen for example) by adhesion to the cell wall, especially on Gram positive bacteria [18,111]. As a result, the microbial envelope, which is known to be highly variable depending on the species and strain, particularly with bacteria, plays an important role in CS initial activity. All the elements, such as teichoic acids or external polysaccharides, that can be negatively charged will favor the interactions with CS. However, the exact nature of the surface components that interact with CS has not been accurately defined [83,103]. Species that contain chitin in their membrane would be less sensitive [82]. The membrane may not be the direct target as liposomes are poorly affected by CS [103,112]. Proteins or elements emerging from the membrane or the wall seem to be more likely to be recognized. However, the membrane composition and fluidity may influence the subsequent consequences of CS treatment [97,112];
- (ii)
- (iii)
- CS also causes agglutination and precipitation of the undesired microorganisms [77,106]. Indeed, E. coli was shown to protect itself by forming aggregates in the presence of chitooligosaccharides (COS), which only displayed a bacteriostatic effect and the bacteria could rapidly grow after separation from the CS by membrane filtration [108,119]. In other studies, high Mw and low DD insoluble CS fractions were shown to act as fining agents, which eliminate such cell aggregates [105,106];
- (iv)
- (v)
- At sublethal doses, an induction of genes involved in stress regulation, arginine or glucose metabolism (energy), protein glycosylation, membrane synthesis, ion transport, wall construction, and autolysis is reported [84,85,110,122,123,124]. S. cerevisiae cells treated with sub-lethal doses of CS strengthen their wall and become resistant to beta-glucanase treatment [122,124];
- (vi)
- Disruption of the membrane and release of cellular components are often reported, especially for Gram negative bacteria and for some yeasts [111], but depending on the dose used, this can or cannot be observed with some Gram positive bacteria, such as S. aureus [68,87,88,103,113,119,125,126,127,128];
- (vii)
- The chelation and sequestration of metal ions and other nutrients in the broth have also been proposed [125].
4.3. Elicitation and Stimulation of Plants
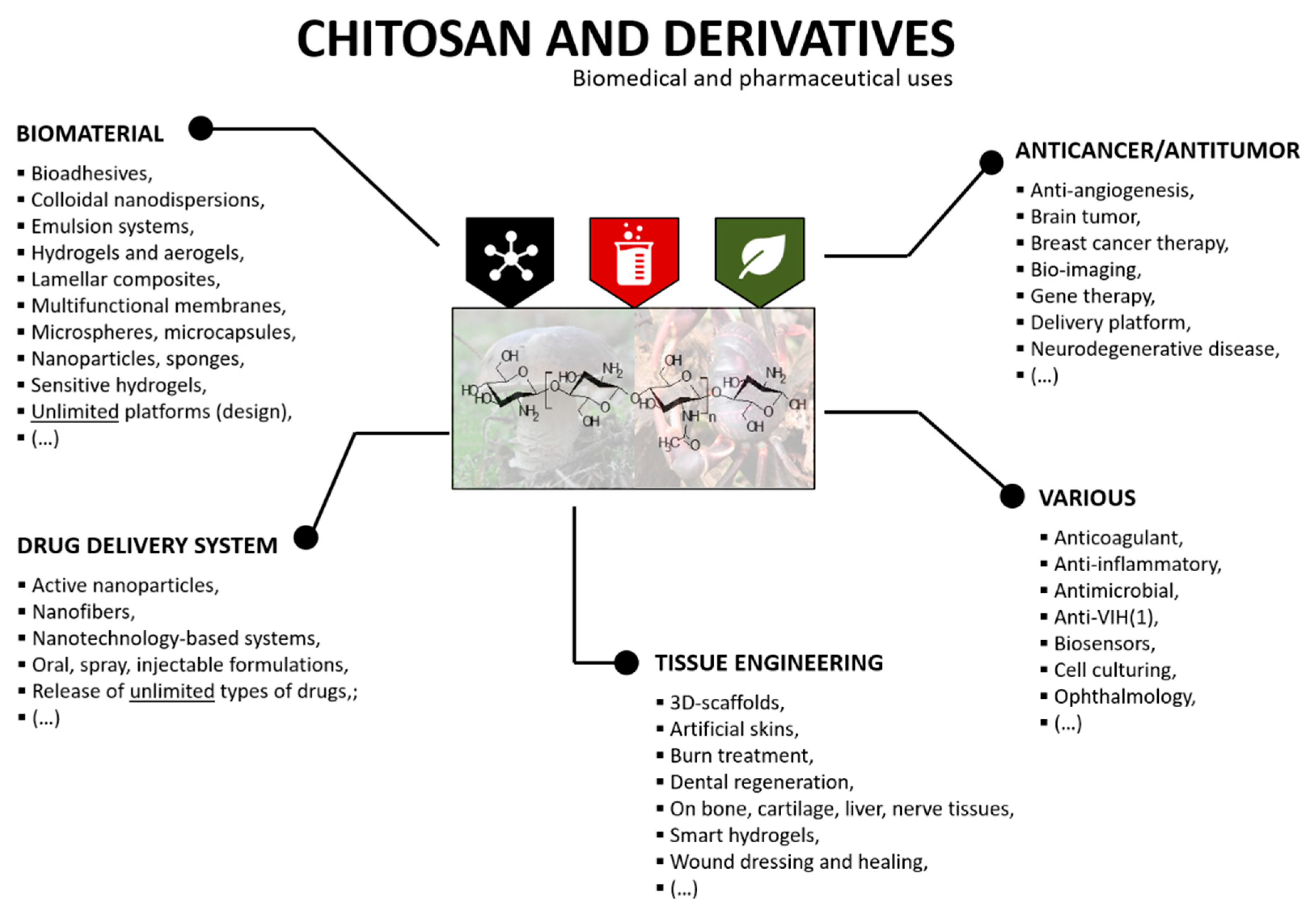
4.4. Biomedical and Pharmaceutical Functions
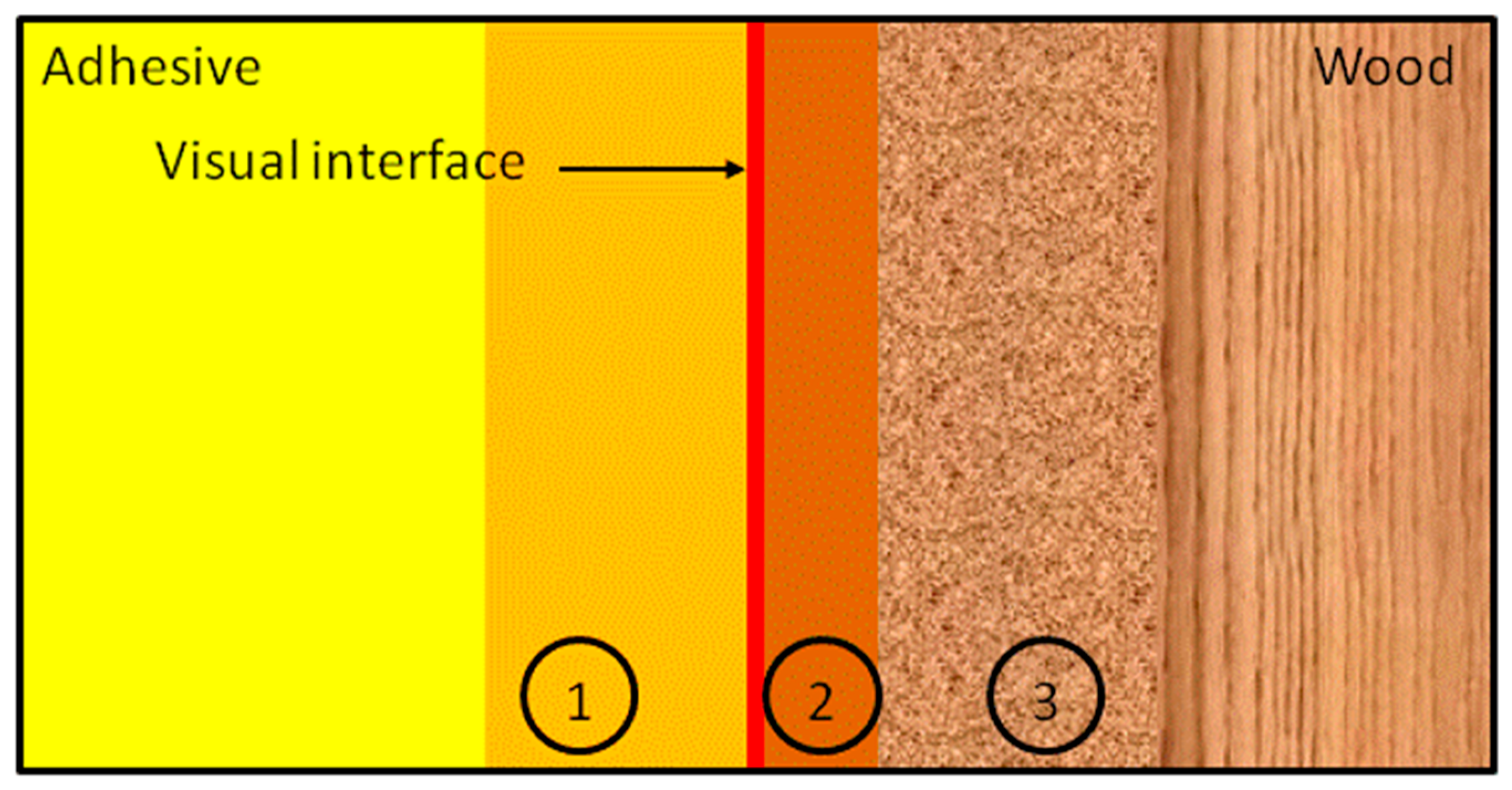
4.5. Adhesive Properties
4.6. Other Potential Applications
5. Biodegradability of Chitosan Derivatives and Life Cycle Assessment (LCA)
6. Conclusions
Information
Author Contributions
Funding
Conflicts of Interest
References
- Mati-Baouche, N.; Elchinger, P.H.; De-Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an adhesive. Eur. Polym. J. 2014, 60, 198–212. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan-based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Laroche, C.; Delattre, C.; Mati-Baouche, N.; Salah, R.; Ursu, A.V.; Moulti-Mati, F.; Michaud, P.; Pierre, G. Bioactivity of chitosan and its derivatives. Curr. Org. Chem. 2017, 21, 1–27. [Google Scholar] [CrossRef]
- Teng, J.Y.; Guo, R.; Xie, J.; Sun, D.J.; Shen, M.Q.; Xu, S.J. Effects of different artificial dermal scaffolds on vascularization and scar formation of wounds in pigs with full-thickness burn. Zhonghua Shao Shang Zazhi 2012, 28, 13–18. [Google Scholar]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Lee, D.W.; Lim, C.; Israelachvili, J.N.; Hwang, D.S. Strong adhesion and cohesion of chitosan in aqueous solutions. Langmuir 2013, 29, 14222–14229. [Google Scholar] [CrossRef]
- Pahwa, R.; Saini, N.; Kumar, V.; Kohli, K. Chitosan-based gastroretentive floating drug delivery technology: An updated review. Expert Opin. Drug Deliv. 2012, 9, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Michelly, C.G.P.; Michele, K.L.T.; Ernandes, T.T.N.; Marcos, R.G.; Edvani, C.M.; Adley, F.R. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef]
- Dimassi, S.; Tabary, N.; Chai, F.; Branchemain, N.; Martel, B. Sulfonated and sulfated chitosan derivatives for biomedical applications: A review. Carbohydr. Polym. 2018, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Laudenslager, M.J.; Schiffman, J.D.; Schauer, C.L. Carboxymethyl chitosan as a matrix material for platinum, gold, and silver nanoparticles. Biomacromolecules 2008, 9, 2682–2685. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, A.; Kathiresann, K.; Nayak, L. Preparation, characterization and antibacterial activity of chitosan and phosphorylated chitosan from cuttlebone of Sepia kobiensis (Hoyle, 1885). Biotechnol. Rep. 2016, 9, 25–30. [Google Scholar] [CrossRef]
- Braconnot, H. Sur la nature des champignons. Ann. Chi. Phys. 1811, 79, 265–304. [Google Scholar]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.M.; Guibal, E. Chitine et Chitosane. Du biopolymère à l’application; Presses Universitaires de Franche Comté: Paris, France, 2009; ISBN 9782848672496. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell. Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef]
- Zhao, Y.; Park, R.D.; Muzzarelli, R.A. Chitin Deacetylases: Properties and Applications. Mar. Drugs 2010, 8, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Versali, M.F.; Clerisse, F.; Bruyere, J.M.; Gautier, S. Cell Wall Derivatives from Biomass and Preparation Thereof. U.S. Patent 7556946 B2, 12 February 2003. [Google Scholar]
- Niederhofer, A.; Muller, B.W. A method for direct preparation of chitosan with low molecular weight from fungi. Eur. J. Pharm. Biopharm. 2004, 57, 101–105. [Google Scholar] [CrossRef]
- Truong, T.O.; Hausler, R.; Monette, F.; Niquette, P. Valorisation des résidus industriels de pêches pour la transformation de chitosane par technique hydrothermo-chimique. Rev. Sci Eau. 2007, 20, 253–321. [Google Scholar] [CrossRef]
- Baxter, S.; Zivanovic, S.; Weiss, J. Molecular weight and degree of acetylation of high-intensity ultrasonicated chitosan. Food Hydrocoll. 2004, 19, 821–830. [Google Scholar] [CrossRef]
- Wu, T. Sonochemical and Hydrophobic Modification of Chitin and Chitosan. Ph.D. Thesis, Univ Tennessee, Knoxville, TN, USA, December 2017. Available online: https://trace.tennessee.edu/utk_graddiss/286/ (accessed on 25 March 2019).
- Al Sagheer, F.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Mahdy-Samar, M.; El-Kalyoubi, M.H.; Khalaf, M.M.; Abd El-Razik, M.M. Physicochemical, functional, antioxidant and antibacterial properties of chitosan extracted from shrimp wastes by microwave technique. Ann. Agric. Sci. 2013, 58, 33–34. [Google Scholar] [CrossRef]
- Birolli, W.G.; Delezuk, J.A.M.; Campana-Filho, S.P. Ultrasound-assisted conversion of alpha-chitin into chitosan. Appl. Acoust. 2016, 103, 239–242. [Google Scholar] [CrossRef]
- El Knidri, H.; El Khalfaouy, R.; Laajeb, A.; Addaou, A.; Lahsini, A. Eco-friendly extraction and characterization of chitin and chitosan from the shrimp shell waste via microwave irradiation. Proc. Saf. Environ. Prot. 2016, 104, 395–405. [Google Scholar] [CrossRef]
- Tsigos, I.; Martinou, A.; Kafetzopoulos, D.; Bouriotis, V. Chitin deacetylases: New, versatile tools in biotechnology. Trends Biotechnol. 2000, 18, 305–312. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotech. 2013, 51, 12–25. [Google Scholar]
- Chitosan Market by Source, Global Opportunity Analysis and Industry Forecast, 2014–2022. Available online: https://www.alliedmarketresearch.com/chitosan-market (accessed on 10 January 2019).
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. The Good, the Bad and the Ugly of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Sabaa, M.W.; El-Ghandour, A.H.; Abdel-Aziz, M.M.; Abdel-Gawad, O.F. Quaternized N-substituted carboxymethyl chitosan derivatives as antimicrobial agents. Int. J. Biol. Macromol. 2013, 60, 156–164. [Google Scholar] [CrossRef]
- Luan, F.; Wei, L.; Zhang, J.; Tan, W.; Chen, Y.; Don, F.; Li, Q.; Guo, Z. Preparation and Characterization of Quaternized Chitosan Derivatives and Assessment of Their Antioxidant Activity. Molecules 2018, 23, 516. [Google Scholar] [CrossRef]
- Desbrières, J.; Martinez, C.; Rinaudo, M. Hydrophobic derivatives of chitosan: Characterization and rheological behaviour. Int. J. Biol. Macromol. 1996, 19, 21–28. [Google Scholar] [CrossRef]
- Rinaudo, M.; Auzely, R.; Vallin, C.; Mullagaliev, I. Specific interactions in modified chitosan systems. Biomacromolecules 2005, 6, 2396–2407. [Google Scholar] [CrossRef] [PubMed]
- Ortona, O.; D’Errico, G.; Mangiapia, G.; Ciccarelli, D. The aggregative behavior of hydrophobically modified chitosans with high substitution degree in aqueous solution. Carbohydr. Polym. 2008, 74, 16–22. [Google Scholar] [CrossRef]
- Hollapa, J.; Nevalainen, T.; Soininen, P.; Másson, M.; Järvinen, T. Synthesis of novel quaternary chitosan derivatives via N-chloroacyl-6-Otriphenylmethylchitosans. Biomacromolecules 2006, 7, 409–410. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Zong, Z.; Kimura, Y.; Takahashi, M.; Yamane, H. Characterization of chemical and solid-state structures of acylated chitosans. Polymer 2000, 41, 899–906. [Google Scholar] [CrossRef]
- Zhang, M.; Hirano, S. Novel N-unsaturated fatty acyl and N-trimethylacetyl derivatives of chitosan. Carbohydr. Polym. 1995, 26, 205–209. [Google Scholar] [CrossRef]
- Hirano, S.; Yamaguchi, Y.; Kamiya, M. Novel N-saturated-fatty-acyl derivatives of chitosan soluble in water and in aqueous acid and alkaline solutions. Carbohydr. Polym. 2002, 48, 203–207. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Miliani, M.; Cartolari, M.; Genta, I.; Perugini, P.; Modena, T.; Pavanetto, F.; Conti, B. Oxychitin-chitosan microcapsules for pharmaceutical use. STP Pharma Sci. 2000, 10, 51–56. [Google Scholar]
- Kato, Y.; Kaminaga, J.; Matsuo, R.; Isogai, A. TEMPO-mediated oxidation of chitin, regenerated chitin and N-acetylated chitosan. Carbohydr. Polym. 2004, 58, 421–426. [Google Scholar] [CrossRef]
- Sun, L.; Du, Y.; Yang, J.; Shi, X.; Li, J.; Wang, X.; Kennedy, J.F. Conversion of crystal structure of the chitin to facilitate preparation of a 6-carboxychitin. Carbohydr. Polym. 2006, 66, 168–175. [Google Scholar] [CrossRef]
- Pierre, G.; Punta, C.; Delattre, C.; Melone, L.; Dubessay, P.; Fiorati, A.; Pastori, N.; Galante, Y.M.; Michaud, P. TEMPO-Mediated Oxidation of Polysaccharides: An Ongoing Story. Carbohydr. Polym. 2017, 165, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Muzzarelli, C.; Cosani, A.; Terbojevich, M. 6-Oxychitins, novel hyaluronan-like regiospecifically carboxylated chitins. Carbohydr. Polym. 1999, 39, 361–367. [Google Scholar] [CrossRef]
- Genta, I.; Perugini, P.; Modena, T.; Pavanetto, F.; Castelli, F.; Muzzarelli, R.A.A.; Muzzarelli, C.; Conti, B. Miconazole-loaded 6-oxychitin-chitosan microcapsules. Carbohydr. Polym. 2003, 52, 11–18. [Google Scholar] [CrossRef]
- Botelho da Silva, S.; Krolicka, M.; van den Broek, L.A.M.; Frissen, A.E.; Boeriu, C.G. Water-soluble chitosan derivatives and pH-responsive hydrogels by selective C-6 oxidation mediated by TEMPO-laccase redox system. Carbohydr. Polym. 2018, 186, 299–309. [Google Scholar] [CrossRef]
- Lusiana, R.A.; Siswanta, D.; Mudasir, M.; Hayashita, T. The Influence of citric acid crosslinked PVA/chitosan membrane hydrophilicity on the transport of creatinine and urea. Indones. J. Chem. 2013, 13, 262–270. [Google Scholar] [CrossRef]
- Casettari, L.; Cespi, M.; Palmieri, G.F.; Bonacucina, G. Characterization of the interaction between chitosan and inorganic sodium phosphates by means of rheological and optical microscopy studies. Carbohydr. Polym. 2013, 91, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Delattre, C.; Vijayalakshmi, M.A. Monolith enzymatic microreactor at the frontier of glycomic toward a new route for the production of bioactive oligosaccharides. J. Mol. Catal. B 2009, 60, 97–105. [Google Scholar] [CrossRef]
- Kasaai, M.R.; Arul, J.; Charlet, G. Fragmentation of Chitosan by Acids. Sci. World J. 2013, 2013, 508540. [Google Scholar] [CrossRef]
- Cabrera, J.C.; Van Cutsem, P. Preparation of Chitooligosaccharides with Degree of Polymerization Higher than 6 by Acid or Enzymatic Degradation of Chitosan. Biochem. Eng. J. 2005, 25, 165–172. [Google Scholar] [CrossRef]
- Chang, K.L.B.; Tai, M.C.; Cheng, F.H. Kinetics and Products of the Degradation of Chitosan by Hydrogen Peroxide. J. Agric. Food Chem. 2001, 49, 4845–4851. [Google Scholar] [CrossRef]
- Hsu, S.C.; Don, T.M.; Chiu, W.Y. Free Radical Degradation of Chitosan with Potassium Persulfate. Polym. Degrad. Stab. 2002, 75, 73–83. [Google Scholar] [CrossRef]
- Choi, W.S.; Ahn, K.J.; Lee, D.W.; Byun, M.W.; Park, H.J. Preparation of Chitosan Oligomers by Irradiation. Polym. Degrad. Stab. 2002, 78, 533–538. [Google Scholar] [CrossRef]
- García, M.A.; Pérez, L.; de la Paz, N.; González, J.; Rapado, M.; Casariego, A. Effect of Molecular Weight Reduction by Gamma Irradiation on Chitosan Film Properties. Mater. Sci. Eng. C Mater Biol. Appl. 2015, 55, 174–180. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Nah, J.W.; Meyers, S.P. Effect of Time/Temperature Treatment Parameters on Depolymerization of Chitosan. J. Appl. Polym. Sci. 2003, 87, 1890–1894. [Google Scholar] [CrossRef]
- Zhang, D.; Bland, J.M.; Xu, D.; Chung, S. Degradation of Chitin and Chitosan by a Recombinant Chitinase Derived from a Virulent Aeromonas hydrophila Isolated from Diseased Channel Catfish. Adv. Microbiol. 2015, 5, 611–619. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Li, Y.K. An Aspergillus chitosanase with potential for large-scale preparation of chitosan oligosaccharides. Biotechnol. Appl. Biochem. 2000, 32, 197. [Google Scholar] [CrossRef]
- Gohi, B.; Zeng, H.Y.; Pan, A. Optimization and Characterization of Chitosan Enzymolysis by Pepsin. Bioengineering 2016, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, Y.; Yang, J.; Feng, T.; Li, A.; Chen, P. Preparation and Characterisation of Low Molecular Weight Chitosan and Chito-Oligomers by a Commercial Enzyme. Polym. Degrad. Stab. 2005, 87, 441–448. [Google Scholar] [CrossRef]
- Tsai, G.J.; Wu, Z.Y.; Su, W.H. Antibacterial Activity of a Chitooligosaccharide Mixture Prepared by Cellulase Digestion of Shrimp Chitosan and Its Application to Milk Preservation. J. Food Prot. 2000, 63, 747–752. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, T.; Yun, H.Y.; Kim, H.H. Depolymerization of Chitosan Using a High-Pressure Homogenizer. Int. J. Pure Appl. Sci. Technol. 2014, 25, 35–42. [Google Scholar]
- Pornsunthorntawee, O.; Katepetch, C.; Vanichvattanadecha, C.; Saito, N.; Rujiravanit, R. Depolymerization of chitosan-metal complexes via a solution plasma technique. Carbohydr. Polym. 2014, 102, 504–512. [Google Scholar] [CrossRef]
- Ibrahim, K.; El-Eswed, B.; Abu-Sbeih, K.; Arafat, T.; Al Omari, M.; Darras, F.; Badwan, A. Preparation of Chito-Oligomers by Hydrolysis of Chitosan in the Presence of Zeolite as Adsorbent. Mar. Drugs 2016, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.C.; Wang, G.; Hu, J.Z.; Tian, Y.W.; Weng, C.; Gao, X. Electrochemical depolymerization of chitosans using the IrO2 electrode with interlayers as anode. Mater. Sci. Forum 2016, 847, 281–286. [Google Scholar] [CrossRef]
- Bornet, A.; Teissedre, P.L. Chitosan, chitin-glucan and chitin effects on minerals (iron, lead, cadmium) and organic (Ochratoxin A) contaminants in wines. Eur. Food Res. Technol. 2008, 226, 681–689. [Google Scholar] [CrossRef]
- Varum, K.M.; Ottoy, M.H.; Smidsrod, O. Water-solubility of partially N-acetylated chitosans as a function of pH: Effect of chemical composition and depolymerisation. Carbohydr. Polym. 1994, 25, 65–70. [Google Scholar] [CrossRef]
- Colangelo, D.; Dante, F.; De Faveri, M.; Lambri, M. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Data on changes in red wine phenolic compounds and headspace aroma compounds after treatment of red wines with chitosans with different structures. Data Brief 2018, 17, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Taillandier, P.; Joannis-Cassan, C.; Jentzer, J.B.; Gautier, S.; Sieczkowski, N.; Granes, D.; Brandam, C. Effect of fungal chitosan preparation on Brettanomyces bruxellensis, a wine contaminant. J. Appl. Microbiol. 2015, 118, 123–131. [Google Scholar] [CrossRef]
- Petrova, B.; Cartwright, Z.M.; Edwards, C.G. Effectiveness of chitosan preparations against Brettanomyces bruxellensis grown in culture media and red wines. J. Int. Sci. Vigne Vin. 2016, 50, 49–56. [Google Scholar] [CrossRef]
- Avramova, M.; Vallet-Courbin, A.; Maupeu, J.; Masneuf-Pomarède, I.; Albertin, W. Molecular Diagnosis of Brettanomyces bruxellensis’ Sulfur Dioxide Sensitivity Through Genotype Specific Method. Front. Microbiol. 2018, 9, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Nardi, T.; Vagnoli, P.; Minacci, A.; Gautier, S.; Sieczkowski, N. Evaluating the impact of a fungal-origin chitosan preparation on Brettanomyces bruxellensis in the context of wine aging. Wine Stud. 2014, 3, 13–15. [Google Scholar] [CrossRef]
- Aliasghari, A.; Rabbani Khorasgani, M.; Vaezifar, S.; Rahimi, F.; Younesi, H.; Khoroushi, M. Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: An in vitro study. Iran J. Microbiol. 2016, 8, 93–100. [Google Scholar]
- Alan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 285–287. [Google Scholar] [CrossRef]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef]
- Mellegård, H.; From, C.; Christensen, B.E.; Granum, P.E. Antibacterial activity of chemically defined chitosans: Influence of molecular weight, degree of acetylation and test organism. Int. J. Food Microbiol. 2011, 148, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mellegård, H.; From, C.; Christensen, B.E.; Granum, P.E. Inhibition of Bacillus cereus spore outgrowth and multiplication by chitosan. Int. J. Food Microbiol. 2011, 149, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.M.; Zhu, B.; Muhammad, I.; Li, B.; Xie, G.L.; Wang, Y.L.; Li, H.Y.; Sun, G.C. Antibacterial activity and mechanism of action of chitosan solutions against apricot fruit rot pathogen Burkholderia seminalis. Carbohydr. Res. 2011, 346, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Je, J.Y. Gallic acid-grafted-chitosan inhibits foodborne pathogens by a membrane damage mechanism. J. Agric. Food Chem. 2013, 61, 6574–6579. [Google Scholar] [CrossRef]
- Bagder Elmaci, S.; Gülgör, G.; Tokatlı, M.; Erten, H.; İşci, A.; Özçelik, F. Effectiveness of chitosan against wine-related microorganisms. Antonie Leeuwenhoek. 2015, 107, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Moreira, D.; Silva, S.; Costa, E.; Pintado, M.; Couto, J.A. The Antimicrobial Action of Chitosan Against the Wine Spoilage Yeast Brettanomyces/Dekkera. J. Chitin Chitosan Sci. 2013, 1, 1–6. [Google Scholar] [CrossRef]
- Wu, T.; Wu, C.; Fu, S.; Wang, L.; Yuan, C.; Chen, S.; Hu, Y. Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity. Carbohydr. Polym. 2017, 155, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Balay, D.; Hu, Y.; McMullen, L.M.; Gänzle, M.G. Effect of chitosan, and bacteriocin—Producing Carnobacterium maltaromaticum on survival of Escherichia coli and Salmonella Typhimurium on beef. Int. J. Food Microbiol. 2018, 290, 68–75. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Wang, T.; Guo, X.; Luan, J.; Sun, Y.; Li, X. The use of chitoologosaccharides in beer brewing for protection against beer spoilage bacteria and its influence on beer performance. Biotechnol. Lett. 2013, 38, 629–635. [Google Scholar] [CrossRef]
- Kiskó, G.; Sharp, R.; Roller, S. Chitosan inactivates spoilage yeasts but enhances survival of Escherichia coli O157:H7 in apple juice. J. Appl. Microbiol. 2005, 98, 872–880. [Google Scholar] [CrossRef]
- Liburdi, K.; Benucci, I.; Palumbo, F.; Esti, M. Lysozyme immobilized on chitosan beads: Kinetic characterization and antimicrobial activity in white wines. Food Control 2016, 63, 46–52. [Google Scholar] [CrossRef]
- Xing, K.; Shen, X.; Zhu, X.; Ju, X.; Miao, X.; Tian, J.; Feng, Z.; Peng, X.; Jiang, J.; Qin, S. Synthesis and in vitro antifungal efficacy of oleoyl-chitosan nanoparticles against plant pathogenic fungi. Int. J. Biol. Macromol. 2016, 82, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Roller, S.; Covill, N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food. Microbiol. 1999, 47, 67–77. [Google Scholar] [CrossRef]
- Campana, R.; Biondo, F.; Mastrotto, F.; Baffone, W.; Casettari, L. Chitosans as new tools against biofilms formation on the surface of silicone urinary catheters. Int. J. Biol. Macromol. 2018, 118, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Rivas, G.; Escudero-Abarca, B.I.; Aguilar-Uscanga, M.G.; Hayward-Jones, P.M.; Mendoza, P.; Ramírez, M. Selective antimicrobial action of chitosan against spoilage yeasts in mixed culture fermentations. J. Ind. Microbiol. Biotechnol. 2004, 31, 16–22. [Google Scholar] [CrossRef]
- Gil, G.; del Mónaco, S.; Cerrutti, P.; Galvagno, M. Selective antimicrobial activity of chitosan on beer spoilage bacteria and brewing yeasts. Biotechnol. Lett. 2004, 26, 569–574. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Tavaria, F.K.; Pintado, M.M. Insights into chitosan antibiofilm activity against methicillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2017, 122, 1547–1557. [Google Scholar] [CrossRef]
- Raafat, D.; von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Chávez de Paz, L.E.; Resin, A.; Howard, K.A.; Sutherland, D.S.; Wejse, P.L. Antimicrobial effect of chitosan nanoparticles on Streptococcus mutans biofilms. Appl. Environ. Microbiol. 2011, 77, 3892–3895. [Google Scholar] [CrossRef] [PubMed]
- Strand, S.P.; Vandvik, M.S.; Vårum, K.M.; Østgaard, K. Screening of chitosans and conditions for vbacterial flocculation. Biomacromolecules 2001, 2, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Strand, S.P.; Nordengen, T.; Ostgaard, K. Efficiency of chitosans applied for flocculation of different bacteria. Water Res. 2002, 36, 4745–4752. [Google Scholar] [CrossRef]
- Valera, M.J.; Sainz, F.; Mas, A.; Torija, M.J. Effect of chitosan and SO2 on viability of Acetobacter in wine. Int. J. Food Microbiol. 2017, 246, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Dutta, J.; Tripathi, S.; Dutta, P.K. Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: A systemic study needs for food applications. Food Sci. Technol. Int. 2012, 18, 3–34. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Li, Y.; Yu, H.; Liu, G. Advances in characterization and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2015, 190, 1174–1181. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2013, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.Y.; Zhu, J.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Palma Guerreo, J.; Lopez-Jimenez, J.A.; Pérez-Berná, A.J.; Huang, I.C.; Jansson, H.B.; Salinas, J.; Villalaín, J.; Read, N.D.; Lopez-Llorca, L.V. Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Mol. Microbiol. 2010, 75, 1021–1032. [Google Scholar] [CrossRef]
- Didenko, L.V.; Gerasimenko, D.V.; Konstantinova, N.D.; Silkina, T.A.; Avdienko, I.D.; Bannikova, G.E.; Varlamov, V.P. Ultrastructural study of chitosan effects on Klebsiella and staphylococci. Bull. Exp. Biol. Med. 2005, 140, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, F.; Yang, R. Synthesis and characterization of chitosan derivatives with dual-antibacterial functional groups. Int. J. Biol. Macromol. 2015, 75, 378–387. [Google Scholar] [CrossRef]
- Park, S.C.; Nam, J.P.; Kim, J.H.; Kim, Y.M.; Nah, J.W.; Jang, M.K. Antimicrobial action of water-soluble β-chitosan against clinical multi- drug resistant bacteria. Int. J. Mol. Sci. 2015, 16, 7995–8007. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S.; Chang, D. Insight into chitosan multiple functional properties: The role of chitosan conformation in the behavior of liposomal membrane. Food Funct. 2015, 6, 3702–3711. [Google Scholar] [CrossRef]
- Xing, K.; Chen, X.G.; Liu, C.S.; Cha, D.S.; Park, H.J. Oleoyl-chitosan nanoparticles inhibits Escherichia coli and Staphylococcus aureus by damaging the cell membrane and putative binding to extracellular or intracellular targets. Int. J. Food Microbiol. 2009, 132, 127–133. [Google Scholar] [CrossRef]
- Xing, K.; Chen, X.G.; Kong, M.; Liu, C.S.; Cha, D.S.; Park, H.J. Effect of oleoyl-chitosan nanoparticles as a novel antibacterial dispersion system on viability, membrane permeability and cell morphology of Escherichia coli and Staphylococcus aureus. Carbohydr. Polym. 2009, 76, 17–22. [Google Scholar] [CrossRef]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Xavier Malcata, F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2018, 108, 1128–1134. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control 2015, 59, 818–823. [Google Scholar] [CrossRef]
- Zakrzewska, A.; Boorsma, A.; Delneri, D.; Brul, S.; Oliver, S.G.; Klis, F.M. Cellular processes and pathways that protect Saccharomyces cerevisiae cells against the plasma membrane-perturbing compound chitosan. Eukaryot. Cell 2007, 6, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Jaime, M.D.; Lopez-Llorca, L.V.; Conesa, A.; Lee, A.Y.; Proctor, M.; Heisler, L.E.; Gebbia, M.; Giaever, G.; Westwood, J.T.; Nislow, C. Identification of yeast genes that confer resistance to chitosan oligosaccharides (COS) using chemogenomics. BMC Genom. 2012, 13, 267. [Google Scholar] [CrossRef]
- Zakrzewska, A.; Boorsma, A.; Brul, S.; Hellingwerf, K.J.; Klis, F.M. Transcriptional response of Saccharomyces cerevisiae to the plasma membrane perturbing compound chitosan. Eukaryot. Cell 2015, 4, 703–705. [Google Scholar] [CrossRef]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Li, X.; Feng, X.; Yang, S.; Fu, G.; Wang, T.; Su, Z. Chitosan kills Escherichia coli through damage to be of cell membrane mechanism. Carbohydr. Polym. 2010, 79, 493–499. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.; Tarsi, R.; Filippini, O.; Giovanetti, E.; Biagini, G.; Varaldo, P.E. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob. Agents Chemother. 1990, 34, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Chen, X.G.; Park, H.J.; Liu, C.G.; Liu, C.S.; Meng, X.H.; Yu, L.J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2005, 64, 60–65. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Sekigushi, S.; Miura, Y.; Kaneko, H.; Nishimura, S.I.; Nishi, N.; Iwase, M.; Tokura, S. Molecular weight dependency of antimicrobial activity by chitosan oligomers. In Food Hydrocolloids: Structure, Properties and Functions; Nishinari, K., Doi, E., Eds.; Springer: Boston, MA, USA, 1994; pp. 71–76. ISBN 9781461524861. [Google Scholar]
- Wu, T.; Zivanovic, S.; Draughon, F.A.; Conway, W.S.; Sams, C.E. Physicochemical properties and bioactivity of fungal chitin and chitosan. J. Agric. Food Chem. 2005, 53, 3888–3894. [Google Scholar] [CrossRef] [PubMed]
- Pierre, G.; Salah, R.; Gardarin, C.; Traikia, M.; Petit, E.; Delort, A.M.; Mameri, N.; Moulti-Mati, F.; Michaud, P. Enzymatic degradation and bioactivity evaluation of C-6 oxidized chitosan. Int. J. Biol. Macromol. 2013, 60, 383–392. [Google Scholar] [CrossRef]
- Teisseidre, P.L.; Bornet, A.; Bruyere, J.M.; Gauthier, S. Use of a Fungal Biomass Extract as Technological Additive for Treating Food-Grade Liquids. Patent WO 2007003863A2, 17 January 2007. [Google Scholar]
- Gylienė, O.; Servienė, E.; Vepštaitė, I.; Binkienė, R.; Baranauskas, M.; Lukša, J. Correlation between the sorption of dissolved oxygen onto chitosan and its antimicrobial activity against Esherichia coli. Carbohydr. Polym. 2015, 20, 218–223. [Google Scholar] [CrossRef]
- Rhoades, J.; Roller, S. Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl. Environ. Microbiol. 2000, 66, 80–86. [Google Scholar] [CrossRef]
- Meng, X.; Xing, R.; Liu, S.; Yu, H.; Li, K.; Qin, Y.; Li, P. Molecular weight and pH effects of aminoethyl modified chitosan on antibacterial activity in vitro. Int. J. Biol. Macromol. 2012, 50, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: A review. Carbohydr. Polym. 2017, 174, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic antimicrobial activities of natural essential oils with chitosan films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Sohal, B.S. Role of Elicitors in Inducing Resistance in Plants against Pathogen Infection: A Review. ISRN Biochem. 2013, 762412, 1–10. [Google Scholar] [CrossRef]
- Chaliha, C.; Rugen, M.D.; Field, R.A.; Kalita, E. Glycans as Modulators of Plant Defense Against Filamentous Pathogens. Front. Plant Sci. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Albersheim, P.; Darvill, A.; Augur, C.; Cheong, J.; Eberhard, S.; Hahn, M.G.; Marfa, V.; Mohnen, D.; O’Neill, M.A.; Spiro, M.D.; et al. Oligosaccharins: Oligosaccharide regulatory molecules. Acc. Chem. Res. 1992, 25, 77–83. [Google Scholar] [CrossRef]
- Darvill, A.; Augur, C.; Bergmann, C.; Carlson, R.W.; Cheong, J.J.; Eberhard, S.; Hahn, M.G.; Lo, V.M.; Marfa, V.; Meyer, B.; et al. Oligosaccharins-Oligosaccharides that regulate growth, development and defence responses in plants. Glycobiology 1992, 2, 181–198. [Google Scholar] [CrossRef]
- Aldington, S.; Fry, S. Oligosaccharins. Adv. Bot. Res. 1993, 19, 1–101. [Google Scholar] [CrossRef]
- Côté, F.; Hahn, M.G. Oligosaccharins: Structure and signal transduction. Plant Mol. Biol. 1994, 12, 1379–1411. [Google Scholar] [CrossRef]
- Warder, E.R.; Ukned, S.J.; Williams, S.C.; Dincher, S.S.; Wiederhold, D.L.; Alexander, D.C.; Ahl-Goy, P.; Métraux, J.P.; Ryals, J.A. Coordinate gene activity in response to agent that induce systemic acquired resistance. Plant Cell 1991, 3, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Klarzynski, O.; Fritig, B. Stimulation des défenses naturelles des plantes. C. R. Acad. Sci. Ser. III Sci. 2001, 324, 953–963. [Google Scholar] [CrossRef]
- Yamada, A.; Shibuya, N.; Kodama, O.; Akatsuka, T. Induction of phytoalexin formation in suspension cultured rice cells by N-acetylchitooligosaccharides. Biosci. Biotechnol. Biochem. 1993, 57, 405–409. [Google Scholar] [CrossRef]
- Barber, M.S.; Bertram, R.E.; Ride, J.P. Chitin oligosaccharides elicit lignification in wounded wheat leaves. Physiol. Mol. Plant Pathol. 1989, 34, 3–12. [Google Scholar] [CrossRef]
- Hadwige, L.A.; Beckman, J. Chitosan as a component of pea-Fusarium solani interactions. Plant Physiol. 1980, 66, 205–211. [Google Scholar] [CrossRef]
- Walker-Simmons, M.; Ryan, C.A. Proteinase inhibitor synthesis in tomato leaves. Plant Physiol. 1984, 76, 787–790. [Google Scholar] [CrossRef]
- Kohle, H.; Jeblick, W.; Poten, F.; Blaschek, W.; Kauss, H. Chitosan elicited callose synthesis in soybean cells as a Ca2C-dependent process. Plant Physiol. 1985, 77, 54–551. [Google Scholar] [CrossRef]
- Conrath, U.; Domard, A.; Kauss, H. Chitosan-elicited synthesis of callose and of coumarin derivatives in parsley cell suspension cultures. Plant Cell Rep. 1989, 8, 152–155. [Google Scholar] [CrossRef]
- Burkhanova, G.F.; Yarullina, L.G.; Maksimov, I.V. The control of wheat defense responses during infection with Bipolaris sorokiniana by chitooligosaccharides. Russ. J. Plant Physiol. 2007, 54, 104–110. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent Advances of Chitosan Applications in Plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef]
- Lerouge, P.; Roche, P.; Faucher, C.; Maillet, F.; Trichet, G.; Promé, J.C.; Dénarié, J. Symbiotic host specificity of Rhizobium meliloti is determined by a sulphated end acetylated glucosamine oligosaccharide signal. Nature 1990, 344, 781–784. [Google Scholar] [CrossRef]
- Roche, P.; Lerouge, P.; Ponthus, C.; Promé, J.C. Structural determination of bacterial nodulation factors involved in the Rhizobium meliloti-alfalfa symbiosis. J. Biol. Chem. 1991, 266, 10933–10940. [Google Scholar] [PubMed]
- Schultze, M.; Quiclet-Sire, B.; Kondorosi, E.; Virelizier, H.; Glushka, J.N.; Endre, G.; Géro, S.D.; Kondorosi, A. Rhizobium meliloti produces a family of sulfated lipooligosaccharides exhibiting different degrees of plant host specificity. Proc. Natl. Acad. Sci. USA 1992, 89, 192–196. [Google Scholar] [CrossRef]
- Dénarié, J.; Debellé, F.; Promé, J.C. Rhizobium lipochitooligosaccharide nodulation factors: Signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem. 1996, 65, 503–535. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.F.; Long, S.R. Rhizobium-plant exchange. Nature 1992, 357, 655–660. [Google Scholar] [CrossRef]
- Spaink, H.P. Rhizobial lipo-oligosaccharides: Answers and questions. Plant Mol. Biol. 1992, 20, 977–986. [Google Scholar] [CrossRef]
- Dénarié, J.; Cullimore, J. Lipo-oligosaccharide nodulation factors: A new class of signaling molecules mediating recognition and morphogenesis. Cell 1993, 74, 951–954. [Google Scholar] [CrossRef]
- Rajitha, P.; Gopinath, D.; Biswas, R.; Sabitha, M.; Jayakumar, R. Chitosan nanoparticles in drug therapy of infectious and inflammatory diseases. Expert Opin. Drug Deliv. 2016, 13, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, L.A.; Knoop, R.J.; Kappen, F.H.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Hsieh, W.C.; Liau, J.-J.; Li, Y.-J. Characterization and Cell Culture of a Grafted Chitosan Scaffold for Tissue Engineering. Int. J. Polym. Sci. 2015, 2015, 935305. [Google Scholar] [CrossRef]
- Hermenean, A.; Codreanu, A.; Herman, H.; Balta, C.; Rosu, C.; Mihali, C.V.; Ivan, A.; Dinescu, S.; Ionita, M.; Costache, M. Chitosan-Graphene Oxide 3D scaffolds as Promising Tools for Bone Regeneration in Critical-Size Mouse Calvarial Defects. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nordback, P.H.; Miettinen, S.; Kääriäinen, M.; Haaparanta, A.M.; Kellomäki, M.; Kuokkanen, H.; Seppänen, R. Chitosan membranes in a rat model of full-thickness cutaneous wounds: Healing and IL-4 levels. J. Wound Care 2015, 26, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Bucatariu, S.-M.; Doroftei, F.; Fundueanu, G. Smart composite materials based on chitosan microspheres embedded in thermosensitive hydrogel for controlled delivery of drugs. Carbohydr. Polym. 2017, 157, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Assa, F.; Jafarizadeh-Malmiri, H.; Ajamein, H.; Vaghari, H.; Anarjan, N.; Ahmadi, O.; Berenjian, A. Chitosan magnetic nanoparticles for drug delivery systems. Crit. Rev. Biotechnol. 2017, 37, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Vongchan, P.; Sajomsang, W.; Subyen, D.; Kongtawelert, P. Anticoagulant activity of a sulfated chitosan. Carbohydr. Res. 2002, 337, 1239–1242. [Google Scholar] [CrossRef]
- Nam, J.P.; Nah, J.W. Target gene delivery from targeting ligand conjugated chitosan-PEI copolymer for cancer therapy. Carbohydr. Res. 2016, 135, 153–161. [Google Scholar] [CrossRef]
- Mittal, H.; Ray, S.S.; Kaith, B.S.; Bhatia, J.K.; Sukriti; Sharma, J.; Alhassan, S.M. Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur. Polym. J. 2018, 109, 402–434. [Google Scholar] [CrossRef]
- Khan, F.I.; Rahman, S.; Queen, A.; Ahamad, S.; Ali, S.; Kim, J.; Hassan, M.I. Implications of molecular diversity of chitin and its derivatives. Appl. Microbiol. Biotechnol. 2017, 101, 3513–3536. [Google Scholar] [CrossRef] [PubMed]
- Phil, L.; Naveed, M.; Mohammad, I.S.; Bo, L.; Bin, D. Chitooligosaccharide: An evaluation of physicochemical and biological properties with the proposition for determination of thermal degradation products. Biomed. Pharmacother. 2018, 102, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, G.; Kim, Y.-S.; Hwang, J.-W.; Kim, S.-K.; Jeon, Y.-J.; Je, J.-Y.; Ahn, C.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 654913. [Google Scholar] [CrossRef]
- Soudararajan, A. Recent Research in the Applications of Chitin, Chitosan and Oligosaccharides. In Green Polymers and Environmental Pollution Control; Khalaf, M.N., Ed.; Apple Academic Press: Palm Bay, Fl, USA, 2015; ISBN 9781771881395. [Google Scholar]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Kim, M.-M. Applications of Chitin and Its Derivatives in Biological Medicine. Int. J. Mol. Sci. 2010, 11, 5152–5164. [Google Scholar] [CrossRef] [PubMed]
- Artan, M.; Karadeniz, F.; Karagozlu, M.Z.; Kim, M.-M.; Kim, S.-K. Anti-HIV-1 activity of low molecular weight sulfated chitooligosaccharides. Carbohydr. Res. 2010, 345, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Patel, H.M.; Surana, S.J.; Vanjari, Y.H.; Belgamwar, V.S.; Pardeshi, C.V. N,N,N-Trimethyl chitosan: An advanced polymer with myriad of opportunities in nanomedicine. Carbohydr. Polym. 2017, 157, 875–902. [Google Scholar] [CrossRef]
- Tan, H.; Shen, Q.; Jia, X.; Yuan, Z.; Xiong, D. Injectable Nanohybrid Scaffold for Biopharmaceuticals Delivery and Soft Tissue Engineering. Macromol. Rapid Commun. 2012, 33, 2015–2022. [Google Scholar] [CrossRef]
- Sheng, J.; He, H.; Han, L.; Qin, J.; Chen, S.; Ru, G.; Li, R.; Yang, P.; Wang, J.; Yang, V.C. Enhancing insulin oral absorption by using mucoadhesive nanoparticles loaded with LMWP-linked insulin conjugates. J. Control. Release 2016, 233, 181–190. [Google Scholar] [CrossRef]
- Hu, X.-J.; Liu, Y.; Zhou, X.-F.; Zhu, Q.-L.; Bei, Y.-Y.; You, B.-G.; Zhang, C.-G.; Chen, W.-L.; Wang, Z.-L.; Zhu, A.-J.; et al. Synthesis and characterization of low-toxicity N-caprinoyl-N-trimethyl chitosan as self-assembled micelles carriers for osthole. Int. J. Nanomed. 2013, 8, 3543–3558. [Google Scholar] [CrossRef]
- Bei, Y.Y.; Zhou, X.F.; You, B.G.; Yuan, Z.Q.; Chen, W.L.; Xia, P.; Liu, Y.; Jin, Y.; Hu, X.J.; Zhu, Q.L.; et al. Application of the central composite design to optimize the preparation of novel micelles of harmine. Int. J. Nanomed. 2013, 8, 1795–1808. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mater. Sci. Eng. C 2017, 77, 1349–1362. [Google Scholar] [CrossRef]
- Hakimi, S.; Mortazavian, E.; Mohammadi, Z.; Samadi, F.Y.; Samadikhah, H.; Taheritarigh, S.; Tehrani, N.R.; Rafiee-Tehranig, M. Thiolated methylated dimethylaminobenzyl chitosan: A novel chitosan derivative as a potential delivery vehicle. Int. J. Biol. Macromol. 2017, 95, 574–581. [Google Scholar] [CrossRef]
- Chetouani, A.; Follain, N.; Marais, S.; Rihouey, C.; Elkolli, M.; Bounekhel, M.; Benachour, D.; Le Cerf, D. Physicochemical properties and biological activities of novel blend films using oxidized pectin/chitosan. Int. J. Biol. Macromol. 2017, 97, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.R.; Burns, A.T.H.; Radecka, I.; Kowalczuk, M.; Khalaf, T.; Adamus, G.; Johnston, B.; Khechara, M.P. Bacterial-Derived Polymer Poly-y-Glutamic Acid (y-PGA)-Based Micro/Nanoparticles as a Delivery System for Antimicrobials and Other Biomedical Applications. Int. J. Mol. Sci. 2017, 18, 313. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; Oliveira, J.L.; Fraceto, L.F. Poly(ethylene glycol) and Cyclodextrin-Grafted Chitosan: From Methodologies to Preparation and Potential Biotechnological Applications. Front. Chem. 2017, 5, 93. [Google Scholar] [CrossRef]
- Shelley, H.; Babu, R.J. Role of Cyclodextrins in Nanoparticle-Based Drug Delivery Systems. J. Pharm. Sci. 2018, 107, 1741–1753. [Google Scholar] [CrossRef]
- Xu, W.; Xiao, Y.; Luo, P.; Fan, L. Preparation and characterization of C-phycocyanin peptide grafted N-succinyl chitosan by enzyme method. Int. J. Biol. Macromol. 2018, 113, 841–848. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Naeem, S.; Ramesh, S.; Ramesh, K. pH responsive N-succinyl chitosan/Poly (acrylamide-co-acrylic acid) hydrogels and in vitro release of 5-fluorouracil. PLoS ONE 2017, 12, e0179250. [Google Scholar] [CrossRef]
- Sorlier, P.; Viton, C.; Domard, A. Relation between solution properties and degree of acetylation of chitosan: Role of aging. Biomacromolecules 2002, 3, 1336–1342. [Google Scholar] [CrossRef]
- Lehr, C.M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48. [Google Scholar] [CrossRef]
- Patel, A.K.; Mathias, J.D.; Michaud, P. Polysaccharides as adhesives: A critical review. Rev. Adhes. Adhes. 2013, 1, 312–345. [Google Scholar] [CrossRef]
- Mati-Baouche, N.; de Baynast, H.; Vial, C.; Audonnet, F.; Sun, S.; Petit, E.; Pennec, F.; Prevost, V.; Michaud, P. Physicochemical, thermal and mechanical approaches for the characterization of solubilized and solid state chitosans. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Rotta, J.; Ozorio, R.A.; Kehrwald, A.M.; de Oliveira Barra, G.M.; de Melo Castanho Amboni, R.D.; Barreto, P.L.M. Parameters of color, transparency, water solubility, wettability and surface free energy of chitosan/hydroxypropylmethylcellulose (HPMC) films plasticized with sorbitol. Mater. Sci. Eng. C 2009, 29, 619–623. [Google Scholar] [CrossRef]
- Kutnar, A.; Akambe, F.; Petric, M.; Sernak, M. The influence of viscoelastic thermal compression on the chemistry and surface energeties of wood. Colloids Surf. A 2008, 329, 82–86. [Google Scholar] [CrossRef]
- Marra, A.A. Technology of Wood Bonding: Principles in Practice, 1st ed.; Van Nostrand Reinhold: New York, NY, USA, 1992; pp. 76–80. ISBN 0442007973. [Google Scholar]
- Patel, A.; Michaud, P.; Petit, E.; de Baynast, H.; Grédiac, M.; Mathias, J.D. Development of a chitosan-based adhesive, application to wood bonding. J. Appl. Polym. Sci. 2013, 127, 5014–5021. [Google Scholar] [CrossRef]
- Mati-Baouche, N.; de Baynast, H.; Lebert, A.; Sun, S.; Sacristan Lopez-Mingo, C.J.; Leclaire, P.; Michaud, P. Mechanical, thermal and acoustical characterizations of an insulating bio-based composite made from sunflower stalks particles and chitosan. Ind. Crop. Prod. 2014, 58, 244–250. [Google Scholar] [CrossRef]
- Kambe, F.; Lee, J. Adhesive penetration in wood—A review. Wood Fiber Sci. 2007, 39, 205–220. [Google Scholar]
- Pizzi, A. Advanced Wood Adhesives Technology; Marcel Dekker, Inc.: New York, NY, USA, 1994; 289p. [Google Scholar]
- Umemura, K.; Inoue, A.; Kawai, S. Development of new natural polymer-based wood adhesives I: Dry bond strength and water resistance of konjac glucomannan, chitosan, and their composites. J. Wood Sci. 2003, 49, 221–226. [Google Scholar] [CrossRef]
- Umemura, K.; Mihara, A.; Kawai, S. Development of new natural polymer-based wood adhesives III: Effects of glucose addition on properties of chitosan. J. Wood Sci. 2010, 56, 387–394. [Google Scholar] [CrossRef]
- Paiva, D.; Gonçalves, C.; Vale, I.; Bastos, M.; Magalhães, F.D. Oxidized Xanthan Gum and Chitosan as Natural Adhesives for Cork. Polymers 2016, 8, 259. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan-starch composite film: Preparation and characterization. Ind. Crop. Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Ji, X.; Li, B.; Yuan, B.; Guo, M. Preparation and characterizations of a chitosan-based medium-density fiberboard adhesive with high bonding strength and water resistance. Carbohydr. Polym. 2017, 176, 273–280. [Google Scholar] [CrossRef]
- Ji, X.; Guo, M. Preparation and properties of a chitosan-lignin wood adhesive. Int. J. Adhes. Adhes. 2018, 82, 8–13. [Google Scholar] [CrossRef]
- Patel, A.; Michaud, P.; de Baynast, H.; Mathias, J.D. Preparation of chitosan-based adhesives and assessment of their mechanical properties. J. Appl. Polym. Sci. 2013, 127, 3869–3876. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Cheng, Z. Removal of Heavy Metal Ions Using Chitosan and Modified Chitosan: A Review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Charles, J.; Morin-Crini, N.; Badot, P.M.; Winterton, P.; Crini, G. Chitosan Flocculation of Cardboard-Mill Secondary Biological Wastewater. Chem. Eng. J. 2009, 155, 775–783. [Google Scholar] [CrossRef]
- Nechita, P. Applications of Chitosan in Wastewater Treatment. In Biological Activities and Application of Marine Polysaccharides; Shalaby, E., Ed.; InTechOpen: London, UK, 2017; pp. 210–228. ISBN 978-953-51-2860. [Google Scholar]
- Leceta, I.; Guerrero, P.; de la Caba, K. Functional Properties of Chitosan-Based Films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G.H. Production of Chitooligosaccharides and Their Potential Applications in Medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef]
- Bathnagar, A.; Sillanpää, M. Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater—A short review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef]
- Einbu, A.; Vårum, K.M. Depolymerization and de-N-acetylation of chitin oligomers in hydrochloric acid. Biomacromolecules 2007, 8, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Trombotto, S.; Ladavière, C.; Delolme, F.; Domard, A. Chemical preparation and structural characterization of a homogeneous series of chitin/chitosan oligomers. Biomacromolecules 2008, 9, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Park, P.J.; Kim, S.K. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polym. 2001, 44, 71–76. [Google Scholar] [CrossRef]
- Thadathyl, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Viens, P.; Lacombe-Harvey, M.-È.; Brzezinski, R. Chitosanases from Family 46 of Glycoside Hydrolases: From Proteins to Phenotypes. Mar. Drugs 2015, 13, 6566–6587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Neau, S.H. In vitro degradation of chitosan by a commercial enzyme preparation: Effect of molecular weight and degree of deacetylation. Biomaterials 2001, 22, 1653–1658. [Google Scholar] [CrossRef]
- Chiang, C.L.; Chang, Y.M.; Chang, C.T.; Sung, H.Y. Characterization of a chitosanase isolated from a commercial ficin preparation. J. Agric. Food Chem. 2005, 53, 7579–7585. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Liu, J. Advance in chitosan hydrolysis by non-specific cellulases. Biores. Technol. 2008, 99, 6751–6762. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Yu, P.; Chen, S.; Li, Z.L. Studies on the catalytic degradation of chitosan by lipase. J. Fujian Med. Univ. 2003, 36, 302–305. [Google Scholar]
- Sardar, M.; Roy, I.; Gupta, M.N. A smart bioconjugate of alginate and pectinase with unusual biological activity toward chitosan. Biotechnol. Prog. 2003, 19, 1654–1658. [Google Scholar] [CrossRef]
- Pan, A.D.; Zeng, H.Y.; Foua, G.B.; Alain, C.; Li, Y.Q. Enzymolysis of chitosan by papain and its kinetics. Carbohydr. Polym. 2016, 135, 199–206. [Google Scholar] [CrossRef]
- Picart, C.; Schneider, A.; Etienne, O.; Mutterer, J.; Schaaf, P.; Egles, C.; Jessel, N.; Voegel, J.C. Controlled degradability of polysaccharide multilayer films in vitro and in vivo. Adv. Funct. Mater. 2005, 15, 1771–1780. [Google Scholar] [CrossRef]
- Mawad, D.; Warren, C.; Barton, M.; Mahns, D.; Morley, J.; Pham, B.T.T.; Pham, N.T.H.; Kueh, S.; Lauto, A. Lysozyme depolymerization of photo-activated chitosan adhesive films. Carbohydr. Polym. 2015, 121, 56–63. [Google Scholar] [CrossRef]
- Lee, D.X.; Xia, W.S.; Zhang, J.L. Enzymatic preparation of chitooligosaccharides by commercial lipase. Food Chem. 2008, 111, 291–295. [Google Scholar] [CrossRef]
- Je, J.Y.; Kim, S.K. Chitooligosaccharides as potential nutraceuticals: Production and bioactivities. Adv. Food Nutr. Res. 2012, 65, 321–336. [Google Scholar] [CrossRef]
- Gao, X.A.; Ju, W.T.; Jung, W.J.; Park, R.D. Purification and characterization of chitosanase from Bacillus cereus D-11. Carbohydr. Polym. 2007, 72, 513–520. [Google Scholar] [CrossRef]
- Ogura, J.; Toyoda, A.; Kurosawa, T.; Chong, A.L.; Chohnan, S.; Masaki, T. Purification, characterization and gene analysis of cellulase (Cel8A) from Lysobacter sp. IB-9374. Biosci. Biotechnol. Biochem. 2006, 70, 2420–2428. [Google Scholar] [CrossRef]
- Liu, J.; Xia, W.S. Purification and characterization of a bifunctional enzyme with chitosanase and cellulase activity from commercial cellulase. Biochem. Eng. J. 2006, 30, 82–87. [Google Scholar] [CrossRef]
- Su, C.X.; Wang, D.M.; Yao, L.M.; Yu, Z.L. Purification, characterization, and gene cloning of a chitosanase from Bacillus species strain S65. J. Agri. Food Chem. 2006, 54, 4208–4214. [Google Scholar] [CrossRef] [PubMed]
- Kurakake, M.; Yo-U, S.; Nakagawa, K.; Sugihara, M.; Komaki, T. Properties of chitosanase from Bacillus cereus S1. Curr. Microbiol. 2000, 40, 6–9. [Google Scholar] [CrossRef]
- Kim, P.; Tae, K.H.; Chung, K.J.; Kim, S.; Chung, K. Purification of a constitutive chitosanase produced by Bacillus sp. MET 1299 with cloning and expression of the gene. FEMS Microbiol. Lett. 2004, 240, 31–39. [Google Scholar] [CrossRef]
- Ike, M.; Ko, Y.; Yokoyama, K.; Sumitani, J.I.; Kawaguchi, T.; Ogasawara, W.; Okada, H.; Morikawa, Y. Cellobiohydrolase I (Cel7A) from Trichoderma reesei has chitosanase activity. J. Mol. Catal. B Enzym. 2007, 47, 159–163. [Google Scholar] [CrossRef]
- Fukamizo, T.; Fleury, A.; Côté, N.; Mitsutomi, M.; Brzezinski, R. Exo-b-d-glucosaminidase from Amycolatopsis orientalis: Catalytic residues, sugar recognition specificity, kinetics, and synergism. Glycobiology 2006, 16, 1064–1072. [Google Scholar] [CrossRef]
- Côté, N.; Fleury, A.; Dumont-Blanchette, E.; Fukamiso, T.; Mitsutomi, M.; Brzezinsky, R. Two exo-β-d-glucosaminidases/exochitosanases from actinomycetes define a new subfamily within family 2 of glycoside hydrolases. Biochem. J. 2006, 15, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Shinya, S.; Ohnuma, T.; Yamashiro, R.; Kimoto, H.; Kusaoke, H.; Anbazhagan, P.; Juffer, A.H.; Fukamizo, T. The first identification of carbohydrate binding modules specific to chitosan. J. Biol. Chem. 2013, 288, 30042–30053. [Google Scholar] [CrossRef]
- Shinya, S.; Nishimura, S.; Kitaoku, Y.; Numata, T.; Kimoto, H.; Kusaoke, H.; Ohnuma, T.; Fukamizo, T. Mechanism of chitosan recognition by CBM32 carbohydrate-binding modules from a Paenibacillus sp. IK-5 chitosanase/glucanase. Biochem. J. 2016, 473, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Mitsutomi, M.; Isono, M.; Uchiyama, A.; Nikaidou, N.; Ikegami, T.; Watanabe, T. Chitosanase activity of the enzyme previousreported as b-1,3/b-1,4-glucanase from Bacillus circulans WL-12. Biosci. Biotechnol. Biochem. 1998, 62, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Pechsrichuang, P.; Yoohat, K.; Yamabhai, M. Production of recombinant Bacillus subtilis chitosanase, suitable for biosynthesis of chitosan-oligosaccharides. Biores. Technol. 2013, 127, 407–414. [Google Scholar] [CrossRef]
- Kidibule, P.E.; Santos-Moriano, P.; Jiménez-Ortega, E.; Ramírez-Escudero, M.; Limón, M.C.; Remacha, M.; Plou, F.J.; Sanz-Aparicio, J.; Fernández-Lobato, M. Use of chitin and chitosan to produce new chitooligosaccharides by chitinase Chit42: Enzymatic activity and structural basis of protein specificity. Microb. Cell Fact. 2018, 22, 17–47. [Google Scholar] [CrossRef] [PubMed]
- Leceta, I.; Guerrero, P.; Cabezudo, S.; De la Caba, K. Environmental assessment of chitosan-based films. J. Cleaner Prod. 2013, 41, 312–318. [Google Scholar] [CrossRef]
- Kittur, F.S.; Vishu Kumar, A.B.; Varadaraj, M.C.; Tharanathan, R.N. Chitooligosaccharides-preparation with the aid of pectinase isoenzyme from Aspergillus niger and their antibacterial activity. Carbohydr. Res. 2005, 340, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Bonina, P.; Petrova, T.; Manolova, N.; Rashkov, I.; Naydenov, M. pH sensitive hydrogels composed of chitosan and polyacrylamide: Enzymatic degradation. J. Bioact. Compat. Polym. 2004, 19, 197–208. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Khor, E.; Lim, L.Y. Implantable applications of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]

| Type of Method | Depolymerization Methods | Conditions | Mw(1) DP(2) | References |
|---|---|---|---|---|
| PHYSICAL | High pressure homogenization | 1500 bars 1% CS in 1% acetic acid | 30 kDa | [68] |
| Sonication | Sonication at 35.2 W/cm2, 30 min | 140–143 kDa | [26] | |
| Gamma radiations | 2% CS in 2% acetic acid, 200 KGy | 3–5 kDa | [60] | |
| 1% CS, 0.1% Tween 80 irradiation 50 kGy | 75–77 kDa | [61] | ||
| Autoclave | 1% CS, 1% acetic acid, 121 °C, 60 min, 1 bar | 313 kDa | [62] | |
| CHEMICAL | Acid hydrolysis | 0.5 M HCl, 1% CS, 30 h, 65 °C | - | [56] |
| 2% CS, 1.8 M HCl reflux 100 °C, 2 h | DP < 40 | [70] | ||
| 0.976% CS, 50 mM HCl, 3.89 mM HNO3, 35 °C, 30 min | < 16 kDa | [57] | ||
| 1% CS in HCl 1.8 M, 100 °C, 2 h | DP > 6 | [57] | ||
| Free radical methods | 2% CS, 2% acetic acid, 1.5% H2O2 (final) pH 3.0, 6 h | 9.9 kDa | [58] | |
| 1.5% CS in 2% acetic acid solution, 1.08 g KPS, 70 °C | 17.4 kDa | [59] | ||
| ENZYMATIC | Specific enzymes | Chitosanase from Aspergillus sp. 5U in 5.5% CS solution 45–50 °C, 68 h | DP < 10 | [64] |
| Chitinase from Aeromonas hydrophila | DP 1 to 5 | [63] | ||
| Non-specific enzymes | 1% CS in 100 mM sodium acetate pH 4 with 1:100 Pepsin ratio, 2 h | 9–13 kDa | [65] | |
| 4% CS 1% acetic acid 50 °C E/S protease ratio 1:20 | DP 1 to 8 | [67] | ||
| 4.5% CS in 0.5 M acetic acid bicarbonate pH 5.6, cellulase, 50 °C, 14 h | DP 3 to 8 | [66] |
| Effect | Medium/Method | Chitosan Form or Derivative | Microbial Species Targeted | References |
|---|---|---|---|---|
| Microbial growth inhibition | Liquid model medium (MIC) | Nanoparticles, many Mw/DA | Many species | [81,82,83,84,85,86,87,88,89,90,91,92,93] |
| Beef slices | [93] | |||
| Beer, wine | [94,95,96] | |||
| Solid agar plates | [81,83,92,97,98] | |||
| Medical catheter | Diverse viscosity | K. pneumoniae E. coli | [99] | |
| Liquid media | Distinct concentrations | Microbials cultures | [95,100] | |
| Metabolism modification | Liquid medium | Distinct concentrations | S cerevisiae | [101] |
| Biofilm inhibition | Liquid medium | Nanoparticles | S. aureus | [81,102] |
| Microbial elimination | Liquid medium, minimal lethal concentration | Many Mw/DA | Many species | [77,83,84,85,87,88,89,103] |
| Biofilm elimination | Elimination of biofilms, in flow cells/polystyrene wells | Nanoparticles | S. mutans S. aureus | [102,104] |
| Floculation/sedimentation | Liquid medium | Many Mw/DA | Distinct species | [77,87,101,105,106,107] |
| Plants | Effects | References |
|---|---|---|
| Rice | Induction of phytoalexin | [149] |
| Wheat | Increase phenolic compounds | [150,155] |
| Pea | phytoalexin production | [151] |
| Tomato | Proteinase inhibitor synthesis | [152] |
| Soybean | Synthesis of callose | [153] |
| Parsley | Synthesis of callose | [154] |
| Potato | Enhance tuber size | [156] |
| Strawberry | Increase fruits yields | [156] |
| Barley | Increase phenolic compounds | [156] |
| Maize | Increase seed weight | [156] |
| Rape | Increase chlorophyll | [156] |
| Basil | Increase phenolic compounds | [156] |
| Enzyme/Microorganism | Mode of Action on Chitosan | Distribution of Reaction Products | Substrate Specificity | References |
|---|---|---|---|---|
| Cellulase | ||||
| Bacillus cereus D-11 | GlcN-GlcNAc, GlcNAc-GlcN, GlcN-GlcN | Chitobiose, chitotriose and chitobiose | CMC, CS | [235] |
| Bacillus sp. 65 | GlcN-GlcN | ND | CMC, CS | [238] |
| Bacillus cereus S1 | GlcN-GlcN | Dimer, trimer and tetramer | CMC, Colloidal and soluble CS | [239] |
| Lysobacter sp. IB-9374 | Endo-type cleavage | Chitobiose, chitotriose, chitotetraose | CMC, Colloidal CS, CS, Glycol CS | [236] |
| Trichoderma reesei | GlcN-GlcN | Oligomers | CMC, Avicel, CS | [241] |
| Trichoderma viride | GlcN-GlcNAc, GlcNAc-GlcN, GlcN-GlcN cleavage from the non-reducing end | Oligomers | CMC, CS | [227] |
| Chitosanase | ||||
| Bacillus circulans WL-12 | GlcN-GlcN, GlcN-GlcNAc | (GlcN)2, (GlcN)3, (GlcN)4, oligomers | Lichenan, colloidal CS | [246] |
| Bacillus subitilis str168 | NA | (GlcN)2 to (GlcN)6 | Low weight CS | [247] |
| Amycolatopsis orientalis | Exo-type chitosanase (Exo-β-d-glucosaminidase) | NA | CS | [248] |
| Chitinase | Ramdom hydrolysis GlcNAc | Oligomers | CS | [249] |
| Lipase | NA | Mainly (GlcN)2 to (GlcN)6, complete hydrolysis (GlcM) when increasing reaction time | CS | [237] |
| Papain | GlcN-GlcN, GlcN-GlcNAc | GlcN, (GlcN)3, (GlcN)4 in soluble fraction, and oligomers in insoluble fraction | CS | [230] |
| Pectinase | ||||
| Aspergillus niger | NA | Dimer to hexamer with predominance of dimer, oligomers | CS | [229,250] |
| Lysozyme | GlcNAc-GlcNAc | NA | CS film | [231,232] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of Chitosan for the Generation of Functional Derivatives. Appl. Sci. 2019, 9, 1321. https://doi.org/10.3390/app9071321
Brasselet C, Pierre G, Dubessay P, Dols-Lafargue M, Coulon J, Maupeu J, Vallet-Courbin A, de Baynast H, Doco T, Michaud P, et al. Modification of Chitosan for the Generation of Functional Derivatives. Applied Sciences. 2019; 9(7):1321. https://doi.org/10.3390/app9071321
Chicago/Turabian StyleBrasselet, Clément, Guillaume Pierre, Pascal Dubessay, Marguerite Dols-Lafargue, Joana Coulon, Julie Maupeu, Amélie Vallet-Courbin, Hélène de Baynast, Thierry Doco, Philippe Michaud, and et al. 2019. "Modification of Chitosan for the Generation of Functional Derivatives" Applied Sciences 9, no. 7: 1321. https://doi.org/10.3390/app9071321
APA StyleBrasselet, C., Pierre, G., Dubessay, P., Dols-Lafargue, M., Coulon, J., Maupeu, J., Vallet-Courbin, A., de Baynast, H., Doco, T., Michaud, P., & Delattre, C. (2019). Modification of Chitosan for the Generation of Functional Derivatives. Applied Sciences, 9(7), 1321. https://doi.org/10.3390/app9071321