Applications of Continuous Wave Free Precession Sequences in Low-Field, Time-Domain NMR
Abstract
1. Introduction
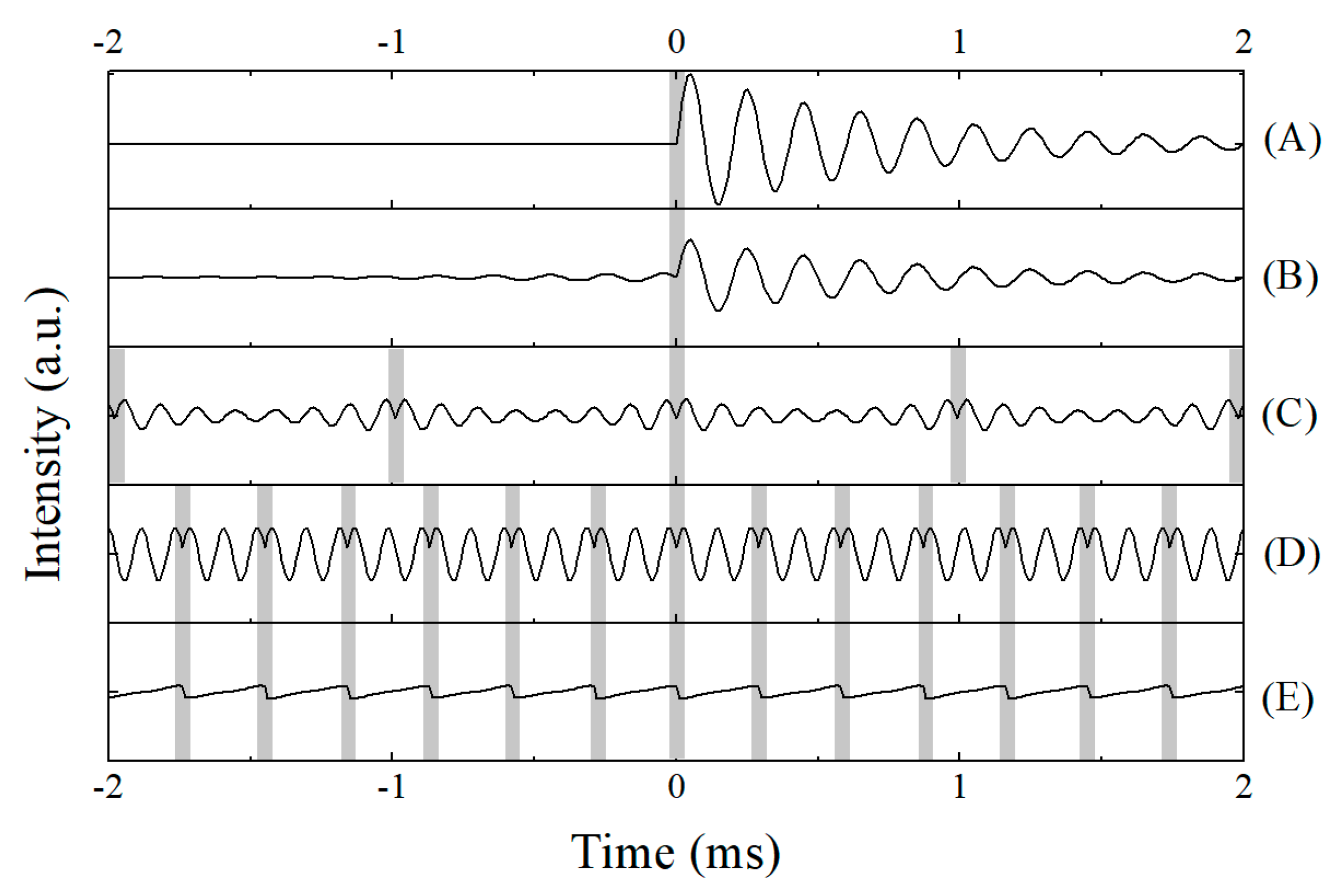
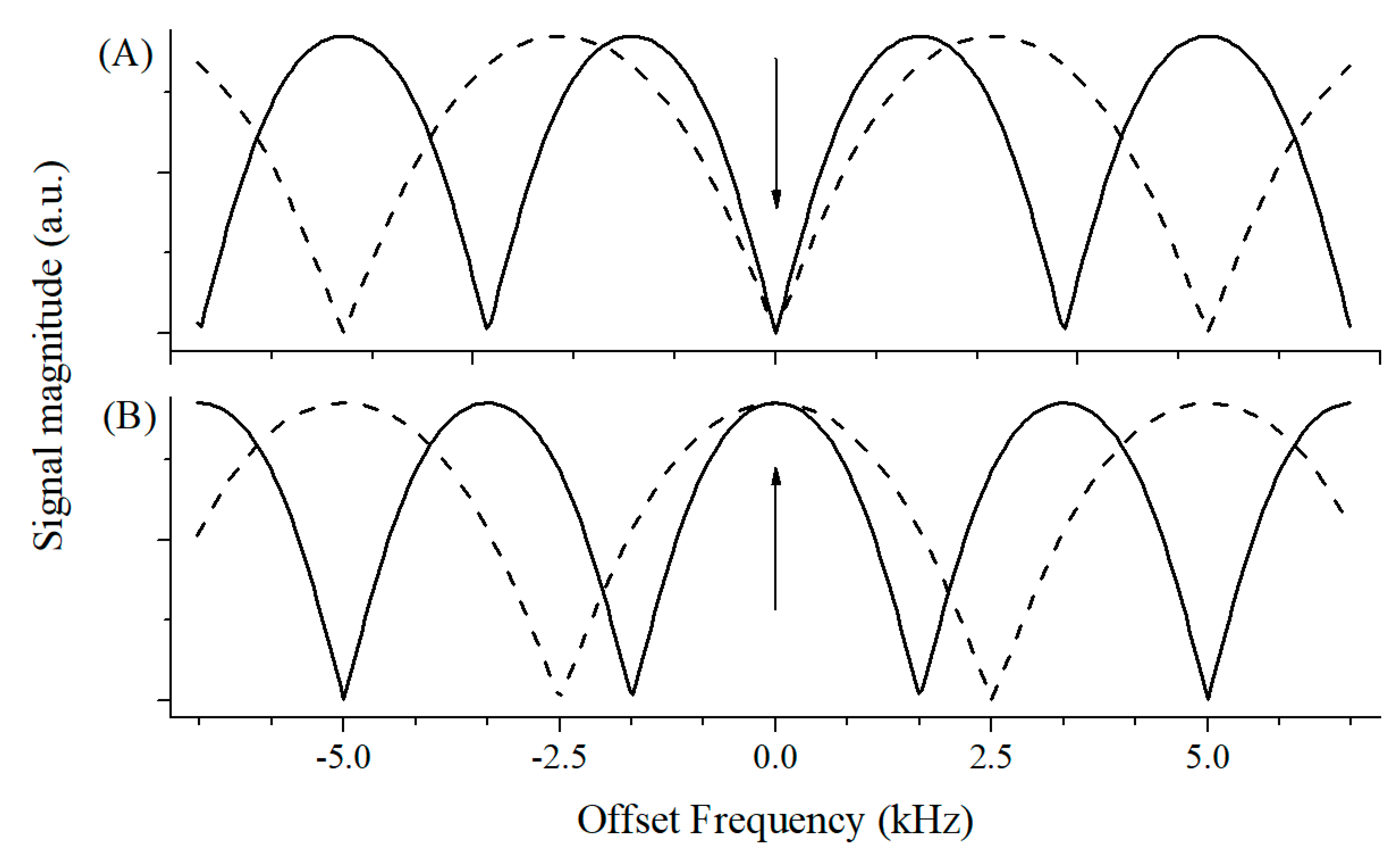
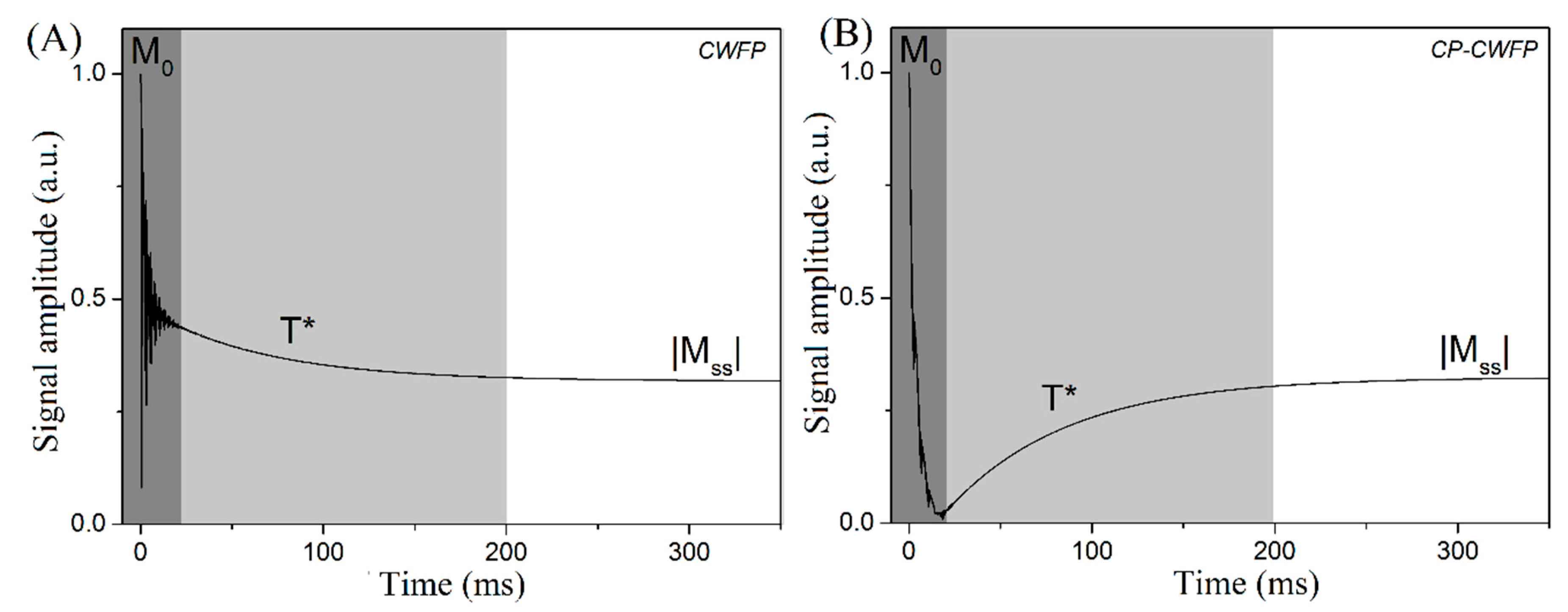
2. SSFP/CWFP Theory
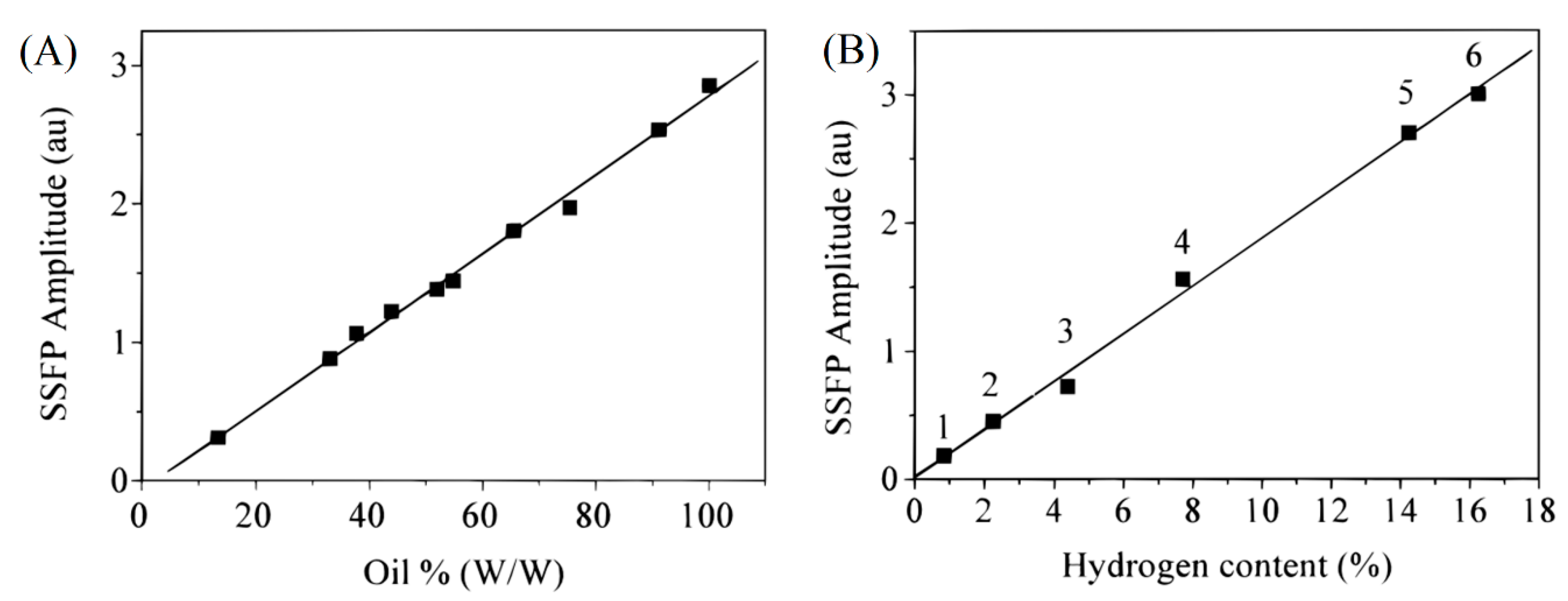
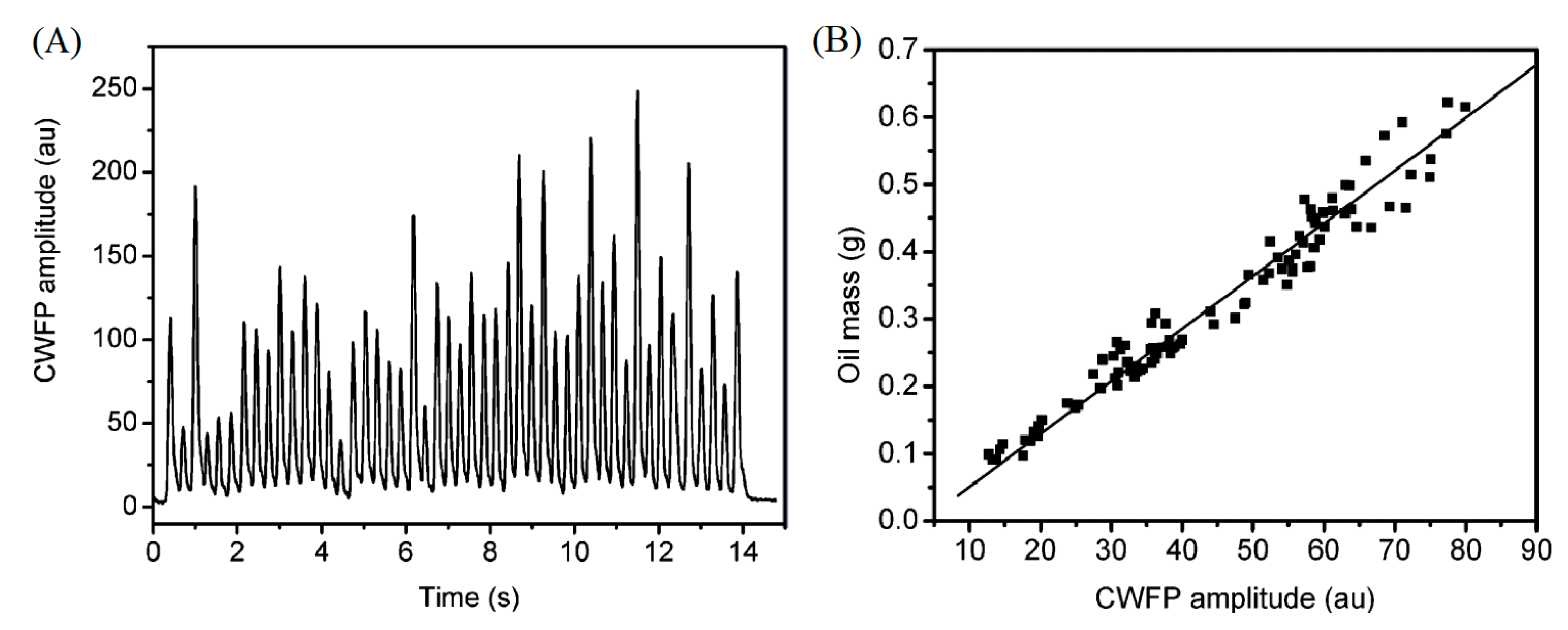
3. Applications of CWFP in Steady State Regime
4. Applications of the Transient Regime
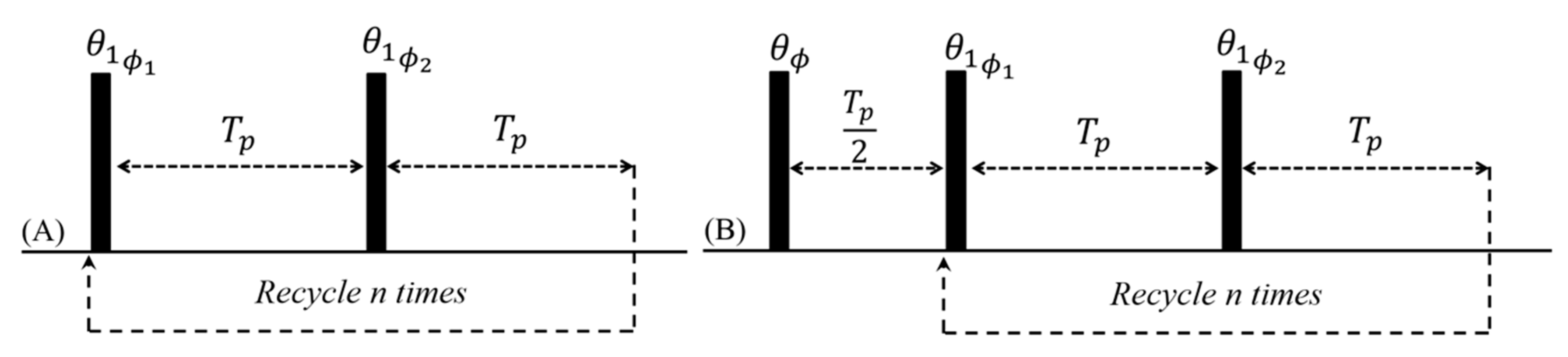
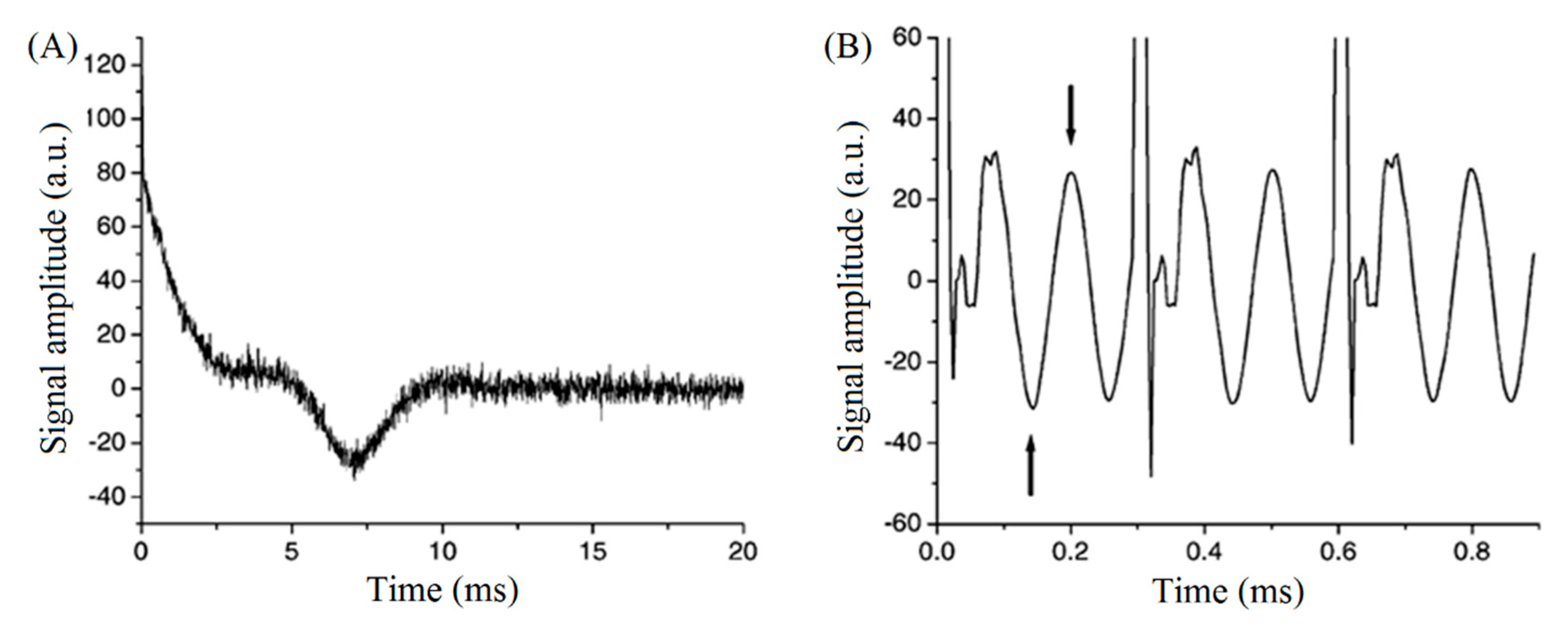
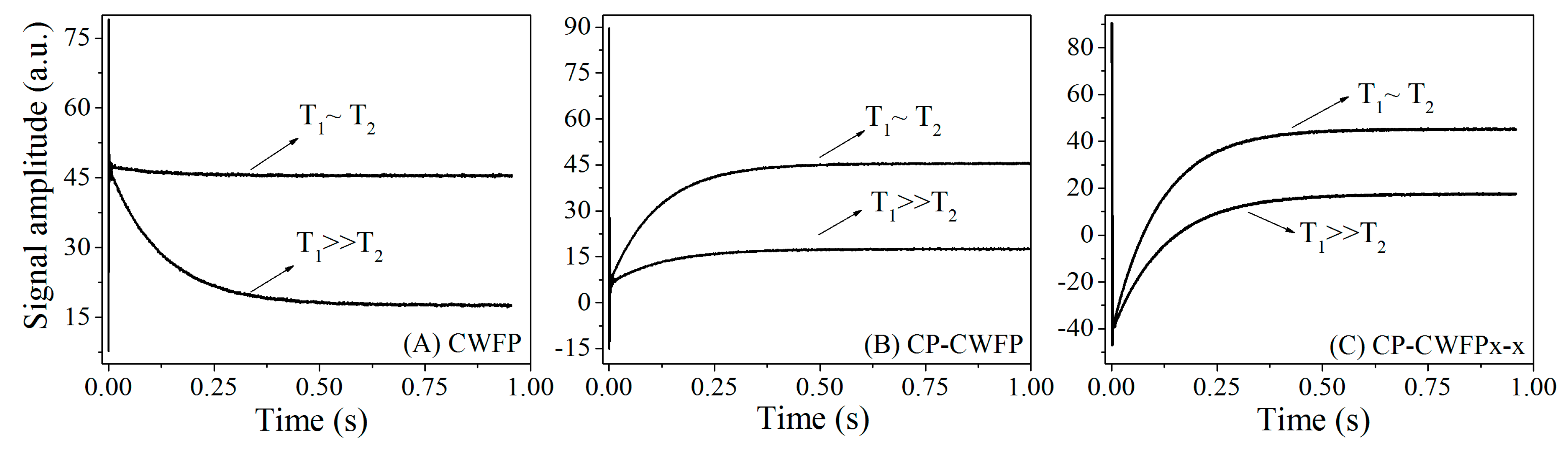
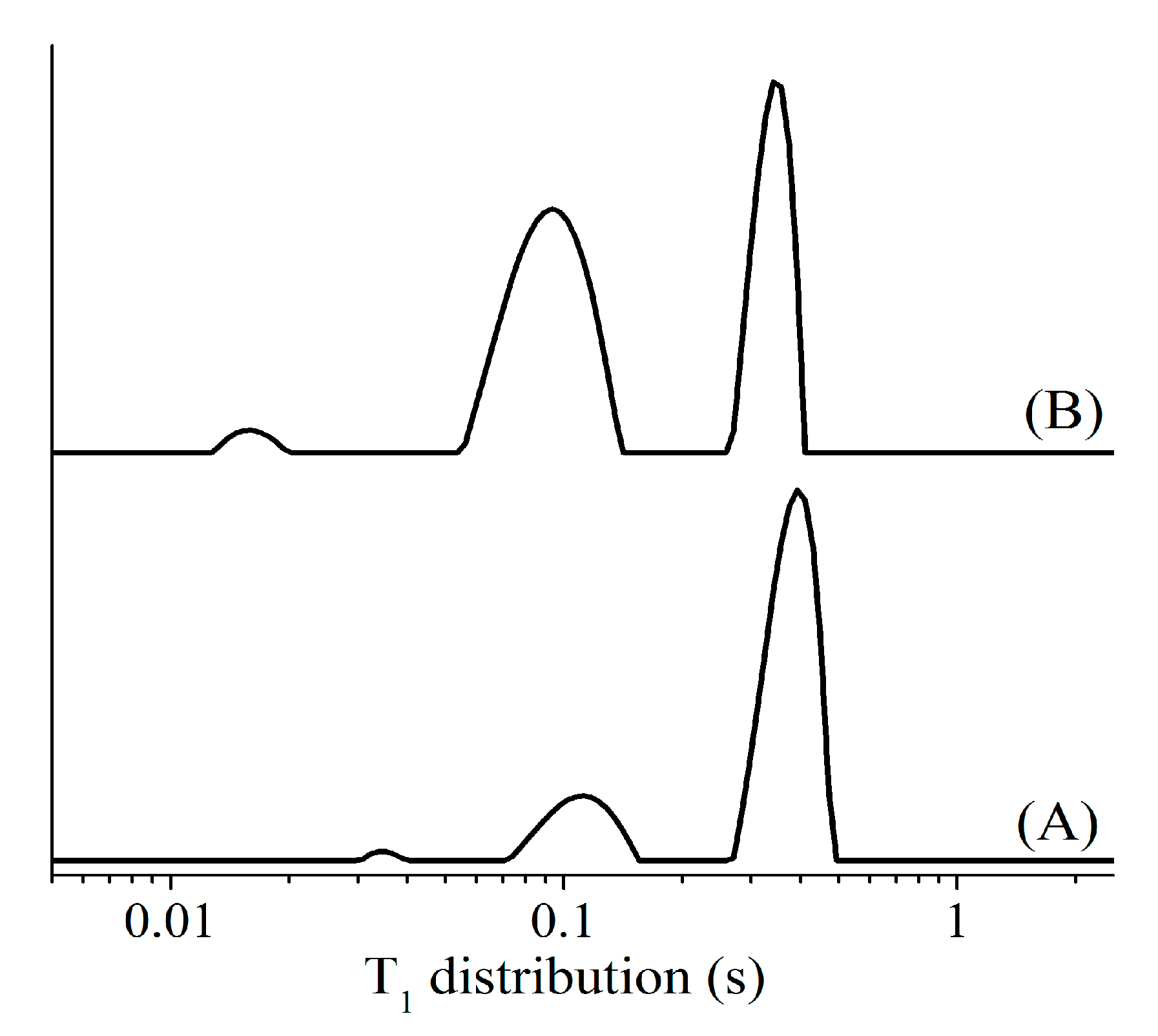
4.1. Fast and Simultaneous T1 and T2 Measurements
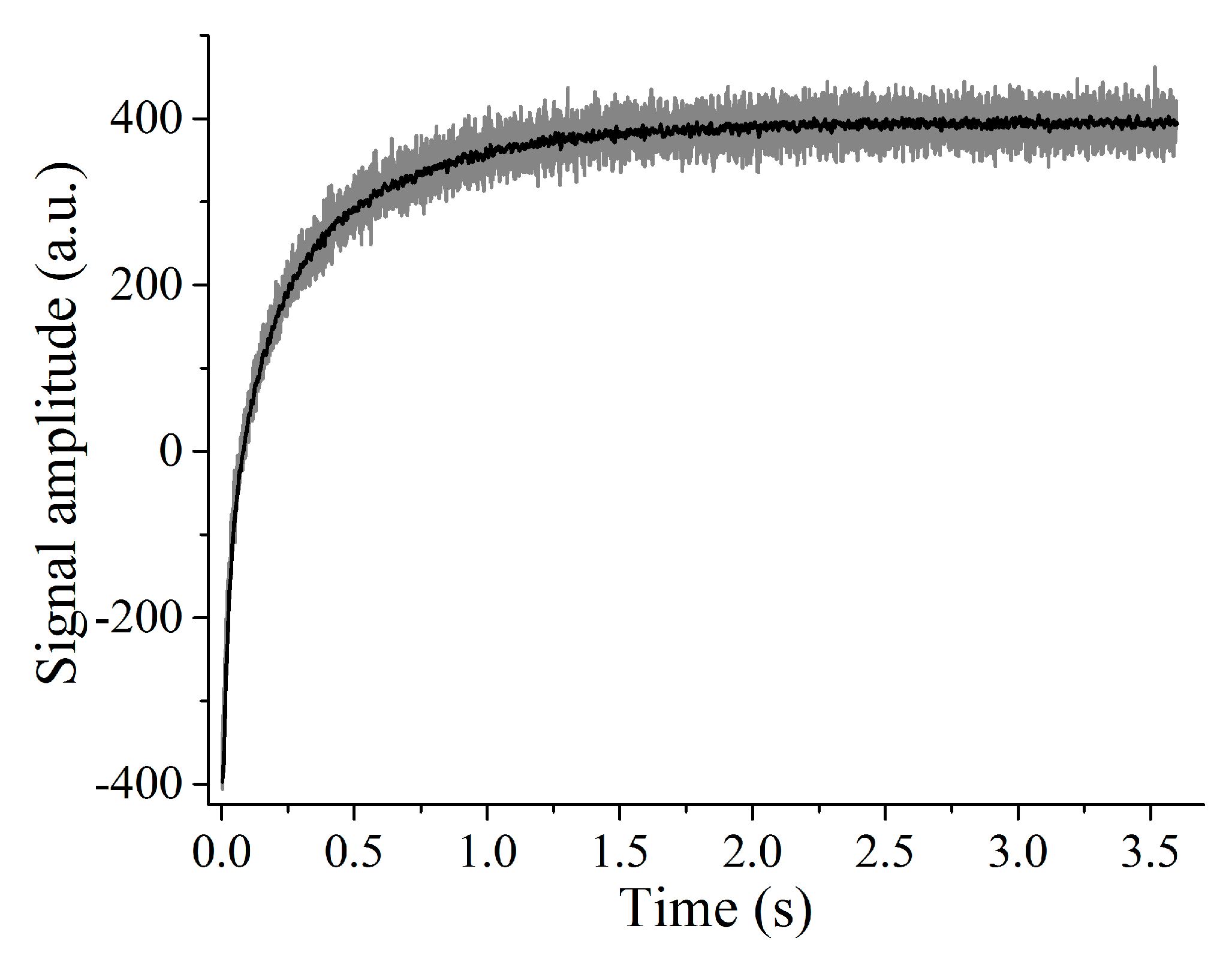
4.2. Analysis of Products Using Transient Cwfp Signals
4.2.1. Agri-Food Products
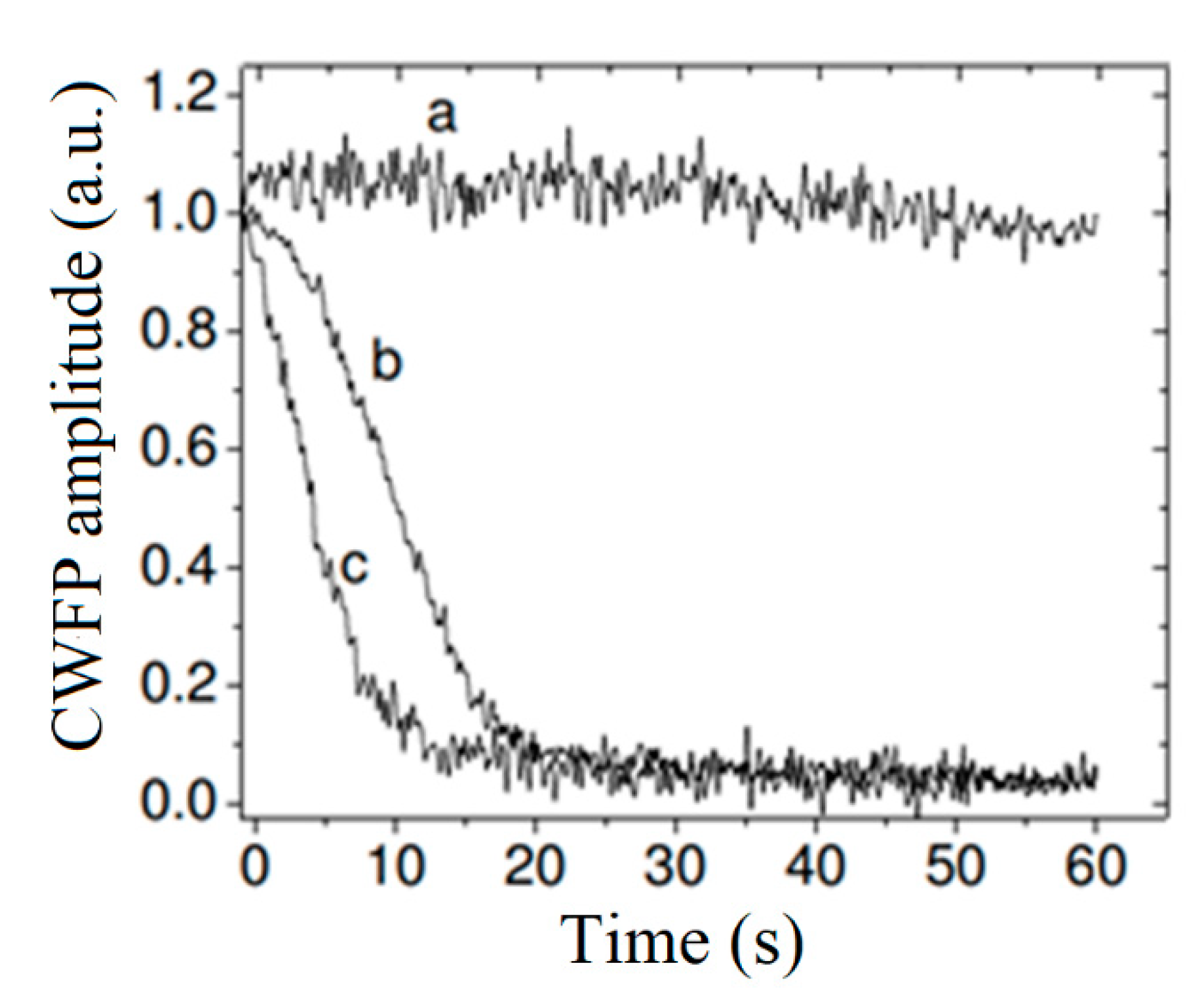
4.2.2. Polymers
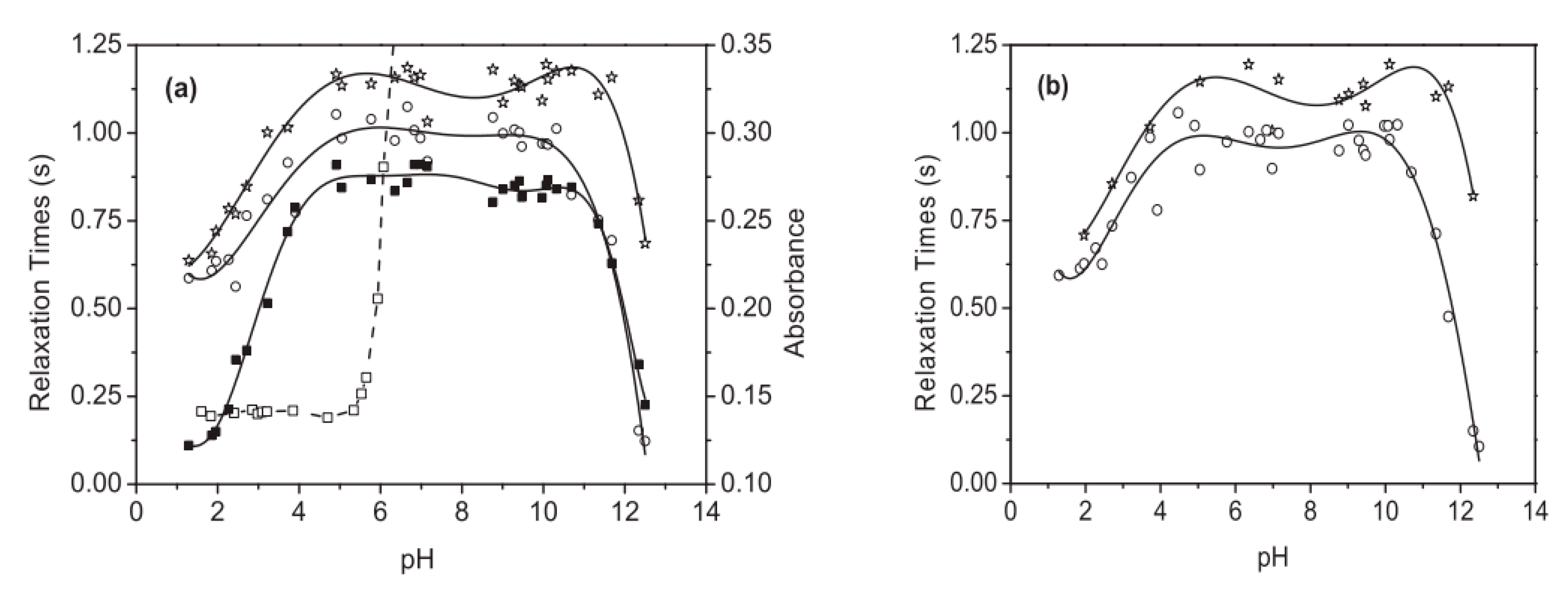
4.2.3. Paramagnetic Complexes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carr, H.Y. Steady-State Free Precession in Nuclear Magnetic Resonance. Phys. Rev. 1958, 112, 1693–1701. [Google Scholar] [CrossRef]
- Ernst, R.R.; Anderson, W.A. Application of Fourier Transform Spectroscopy to Magnetic Resonance. Rev. Sci. Instrum. 1966, 37, 93–102. [Google Scholar] [CrossRef]
- Freeman, R.; Hill, H.D.W. Phase and intensity anomalies in fourier transform NMR. J. Magn. Reson. 1969 1971, 4, 366–383. [Google Scholar] [CrossRef]
- Schwenk, A. NMR pulse technique with high sensitivity for slowly relaxing systems. J. Magn. Reson. 1969 1971, 5, 376–389. [Google Scholar] [CrossRef]
- Dos Santos, P.M.; de Souza, A.A.; Colnago, L.A. Fast Acquisition of 13C NMR Spectra using the Steady-state Free Precession Sequence. Appl. Magn. Reson. 2011, 40, 331. [Google Scholar] [CrossRef]
- Moraes, T.B.; Santos, P.M.; Magon, C.J.; Colnago, L.A. Suppression of spectral anomalies in SSFP-NMR signal by the Krylov Basis Diagonalization Method. J. Magn. Reson. 2014, 243, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bangerter, N.K.; Hargreaves, B.A.; Vasanawala, S.S.; Pauly, J.M.; Gold, G.E.; Nishimura, D.G. Analysis of multiple-acquisition SSFP. Magn. Reson. Med. 2004, 51, 1038–1047. [Google Scholar] [CrossRef]
- Miller, K.L. FMRI using balanced steady-state free precession (SSFP). NeuroImage 2012, 62, 713–719. [Google Scholar] [CrossRef]
- Venâncio, T.; Engelsberg, M.; Azeredo, R.B.V.; Alem, N.E.R.; Colnago, L.A. Fast and simultaneous measurement of longitudinal and transverse NMR relaxation times in a single continuous wave free precession experiment. J. Magn. Reson. 2005, 173, 34–39. [Google Scholar] [CrossRef]
- Freed, D.E.; Scheven, U.M.; Zielinski, L.J.; Sen, P.N.; Hürlimann, M.D. Steady-state free precession experiments and exact treatment of diffusion in a uniform gradient. J. Chem. Phys. 2001, 115, 4249–4258. [Google Scholar] [CrossRef]
- Azeredo, R.B.d.V.; Engelsberg, M.; Colnago, L.A. Flow sensitivity and coherence in steady-state free spin precession. Phys. Rev. E 2001, 64, 016309. [Google Scholar] [CrossRef]
- Bagueira de Vasconcelos Azeredo, R.; Colnago, L.A.; Engelsberg, M. Quantitative Analysis Using Steady-State Free Precession Nuclear Magnetic Resonance. Anal. Chem. 2000, 72, 2401–2405. [Google Scholar] [CrossRef]
- Azeredo, R.B.V.; Colnago, L.A.; Souza, A.A.; Engelsberg, M. Continuous wave free precession: Practical analytical tool for low-resolution nuclear magnetic resonance measurements. Anal. Chim. Acta 2003, 478, 313–320. [Google Scholar] [CrossRef]
- Hills, B.P. Applications of Low-Field NMR to Food Science. Annu. Rep. NMR Spectrosc. 2006, 58, 177–230. [Google Scholar] [CrossRef]
- Marigheto, N.; Venturi, L.; Hills, B. Two-dimensional NMR relaxation studies of apple quality. Postharvest Biol. Technol. 2008, 48, 331–340. [Google Scholar] [CrossRef]
- Van Duynhoven, J.; Voda, A.; Witek, M.; Van As, H.; Webb, G. Time-Domain NMR Applied to Food Products. Annu. Rep. NMR Spectrosc. 2010, 69, 145–197. [Google Scholar] [CrossRef]
- Zalesskiy, S.S.; Danieli, E.; Blümich, B.; Ananikov, V.P. Miniaturization of NMR Systems: Desktop Spectrometers, Microcoil Spectroscopy, and “NMR on a Chip” for Chemistry, Biochemistry, and Industry. Chem. Rev. 2014, 114, 5641–5694. [Google Scholar] [CrossRef]
- Maiwald, M.; Klas, M.; Zientek, N.; Kern, S. Process control with compact NMR. TrAC Trends Anal. Chem. 2016, 83, 39–52. [Google Scholar] [CrossRef]
- Stueber, D.; Jehle, S. Quantitative Component Analysis of Solid Mixtures by Analyzing Time Domain 1H and 19F T1 Saturation Recovery Curves (qSRC). J. Pharm. Sci. 2017, 106, 1828–1838. [Google Scholar] [CrossRef]
- Ge, X.; Chen, H.; Fan, Y.; Liu, J.; Cai, J.; Liu, J. An improved pulse sequence and inversion algorithm of T 2 spectrum. Comput. Phys. Commun. 2017, 212, 82–89. [Google Scholar] [CrossRef]
- Kirtil, E.; Cikrikci, S.; McCarthy, M.J.; Oztop, M.H. Recent Advances in Time Domain NMR & MRI Sensors and Their Food Applications. Curr. Opin. Food Sci. 2017, 17, 9–15. [Google Scholar] [CrossRef]
- Rondeau-Mouro, C. 2D TD-NMR Analysis of Complex Food Products. In Mordern Magnetic Resonance; Springer: Berlin, Germany, 2017; pp. 1–20. [Google Scholar] [CrossRef]
- Moraes, T.B.; Monaretto, T.; Colnago, L.A. Rapid and simple determination of T1 relaxation times in time-domain NMR by Continuous Wave Free Precession sequence. J. Magn. Reson. 2016, 270, 1–6. [Google Scholar] [CrossRef]
- Monaretto, T.; Andrade, F.D.; Moraes, T.B.; Souza, A.A.; deAzevedo, E.R.; Colnago, L.A. On resonance phase alternated CWFP sequences for rapid and simultaneous measurement of relaxation times. J. Magn. Reson. 2015, 259, 174–178. [Google Scholar] [CrossRef]
- Carosio, M.G.A.; Fernandes, D.F.; Andrade, F.D.; Moraes, T.B.; Tosin, G.; Colnago, L.A. Measuring thermal properties of oilseeds using time domain nuclear magnetic resonance spectroscopy|Request PDF. J. Food Eng. 2015, 173, 143–149. [Google Scholar] [CrossRef]
- Pereira, F.M.V.; Bertelli Pflanzer, S.; Gomig, T.; Lugnani Gomes, C.; de Felício, P.E.; Alberto Colnago, L. Fast determination of beef quality parameters with time-domain nuclear magnetic resonance spectroscopy and chemometrics. Talanta 2013, 108, 88–91. [Google Scholar] [CrossRef]
- Andrade, F.D.d.; Marchi Netto, A.; Colnago, L.A. Use of Carr–Purcell pulse sequence with low refocusing flip angle to measure T1 and T2 in a single experiment. J. Magn. Reson. 2012, 214, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.J.R.; Sebastião, P.J.O.; Tavares, M.I.B. 1H time domain NMR real time monitoring of polyacrylamide hydrogels synthesis. Polym. Test. 2017, 60, 396–404. [Google Scholar] [CrossRef]
- Rodrigues, E.J.d.R.; Neto, R.P.C.; Sebastião, P.J.O.; Tavares, M.I.B. Real-time monitoring by proton relaxometry of radical polymerization reactions of acrylamide in aqueous solution. Polym. Int. 2018, 67, 675–683. [Google Scholar] [CrossRef]
- Kronenbitter, J.; Schwenk, A. A new technique for measuring the relaxation times T1 and T2 and the equilibrium magnetization M0 of slowly relaxing systems with weak NMR signals. J. Magn. Reson. 1969 1977, 25, 147–165. [Google Scholar] [CrossRef]
- Webb, A. Increasing the Sensitivity of Magnetic Resonance Spectroscopy and Imaging. Anal. Chem. 2012, 84, 9–16. [Google Scholar] [CrossRef]
- Colnago, L.A.; Engelsberg, M.; Souza, A.A.; Barbosa, L.L. High-Throughput, Non-Destructive Determination of Oil Content in Intact Seeds by Continuous Wave-Free Precession NMR. Anal. Chem. 2007, 79, 1271–1274. [Google Scholar] [CrossRef]
- Pusiol, D. Apparatus and Method for Real Time and Real Flow-Rate Measurement of Multi-Phase Fluids. U.S. Patent 20060020403A1, 26 January 2006. [Google Scholar]
- Colnago, L.A.; Azeredo, R.B.V.; Coelho, I.; Tavares, M.I.B.; Engelsberg, M. Analysis of the Photopolymerization Rate of Methacrylate Blends by Continuous Wave Free Precession NMR. Ann. Magn. Reson. 2003, 2, 125–127. [Google Scholar]
- Venâncio, T.; Engelsberg, M.; Azeredo, R.B.V.; Colnago, L.A. Thermal diffusivity and nuclear spin relaxation: A continuous wave free precession NMR study. J. Magn. Reson. 2006, 181, 29–34. [Google Scholar] [CrossRef]
- Duarte, C.J.; Colnago, L.A.; de Vasconcellos Azeredo, R.B.; Venâncio, T. Solvent Suppression in High-Resolution 1H NMR Spectroscopy Using Conventional and Phase Alternated Continuous Wave Free Precession. Appl. Magn. Reson. 2013, 44, 1265–1280. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Kingsley, P.B. Methods of measuring spin-lattice (T1) relaxation times: An annotated bibliography. Concepts Magn. Reson. 1999, 11, 243–276. [Google Scholar] [CrossRef]
- Monaretto, T.; Souza, A.; Moraes, T.B.; Bertucci-Neto, V.; Rondeau-Mouro, C.; Colnago, L.A. Enhancing signal-to-noise ratio and resolution in low-field NMR relaxation measurements using post-acquisition digital filters. Magn. Reson. Chem. 2018. [Google Scholar] [CrossRef]
- Santos, P.M.; Corrêa, C.C.; Forato, L.A.; Tullio, R.R.; Cruz, G.M.; Colnago, L.A. A fast and non-destructive method to discriminate beef samples using TD-NMR. Food Control 2014, 38, 204–208. [Google Scholar] [CrossRef]
- Corrêa, C.C.; Forato, L.A.; Colnago, L.A. High-throughput non-destructive nuclear magnetic resonance method to measure intramuscular fat content in beef. Anal. Bioanal. Chem. 2009, 393, 1357–1360. [Google Scholar] [CrossRef]
- Constantino, A.F.; Lacerda, V., Jr.; Santos, R.B.d.; Greco, S.J.; Silva, R.C.; Neto, Á.; Barbosa, L.L.; Castro, E.V.R.d.; Freitas, J.C.C. Análise do teor e da qualidade dos lipídeos presentes em sementes de oleaginosas por rmn de baixo campo. Quím. Nova 2014, 37, 10–17. [Google Scholar] [CrossRef]
- Colnago, L.A.; Azeredo, R.B.V.; Marchi Netto, A.; Andrade, F.D.; Venâncio, T. Rapid analyses of oil and fat content in agri-food products using continuous wave free precession time domain NMR. Magn. Reson. Chem. 2011, 49, S113–S120. [Google Scholar] [CrossRef]
- Venâncio, T.; Colnago, L.A. Simultaneous measurements of T1 and T2 during fast polymerization reaction using continuous wave-free precession NMR method. Magn. Reson. Chem. 2012, 50, 534–538. [Google Scholar] [CrossRef]
- Kock, F.V.C.; Colnago, L.A. Rapid method for monitoring chitosan coagulation using low-field NMR relaxometry. Carbohydr. Polym. 2016, 150, 1–4. [Google Scholar] [CrossRef]
- Kock, F.V.C.; Colnago, L.A. Rapid and simultaneous relaxometric methods to study paramagnetic ion complexes in solution: An alternative to spectrophotometry. Microchem. J. 2015, 122, 144–148. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno Moraes, T.; Monaretto, T.; Colnago, L.A. Applications of Continuous Wave Free Precession Sequences in Low-Field, Time-Domain NMR. Appl. Sci. 2019, 9, 1312. https://doi.org/10.3390/app9071312
Bueno Moraes T, Monaretto T, Colnago LA. Applications of Continuous Wave Free Precession Sequences in Low-Field, Time-Domain NMR. Applied Sciences. 2019; 9(7):1312. https://doi.org/10.3390/app9071312
Chicago/Turabian StyleBueno Moraes, Tiago, Tatiana Monaretto, and Luiz Alberto Colnago. 2019. "Applications of Continuous Wave Free Precession Sequences in Low-Field, Time-Domain NMR" Applied Sciences 9, no. 7: 1312. https://doi.org/10.3390/app9071312
APA StyleBueno Moraes, T., Monaretto, T., & Colnago, L. A. (2019). Applications of Continuous Wave Free Precession Sequences in Low-Field, Time-Domain NMR. Applied Sciences, 9(7), 1312. https://doi.org/10.3390/app9071312






