Chemical Defence of a Seagrass against Microfoulers and Its Seasonal Dynamics
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results
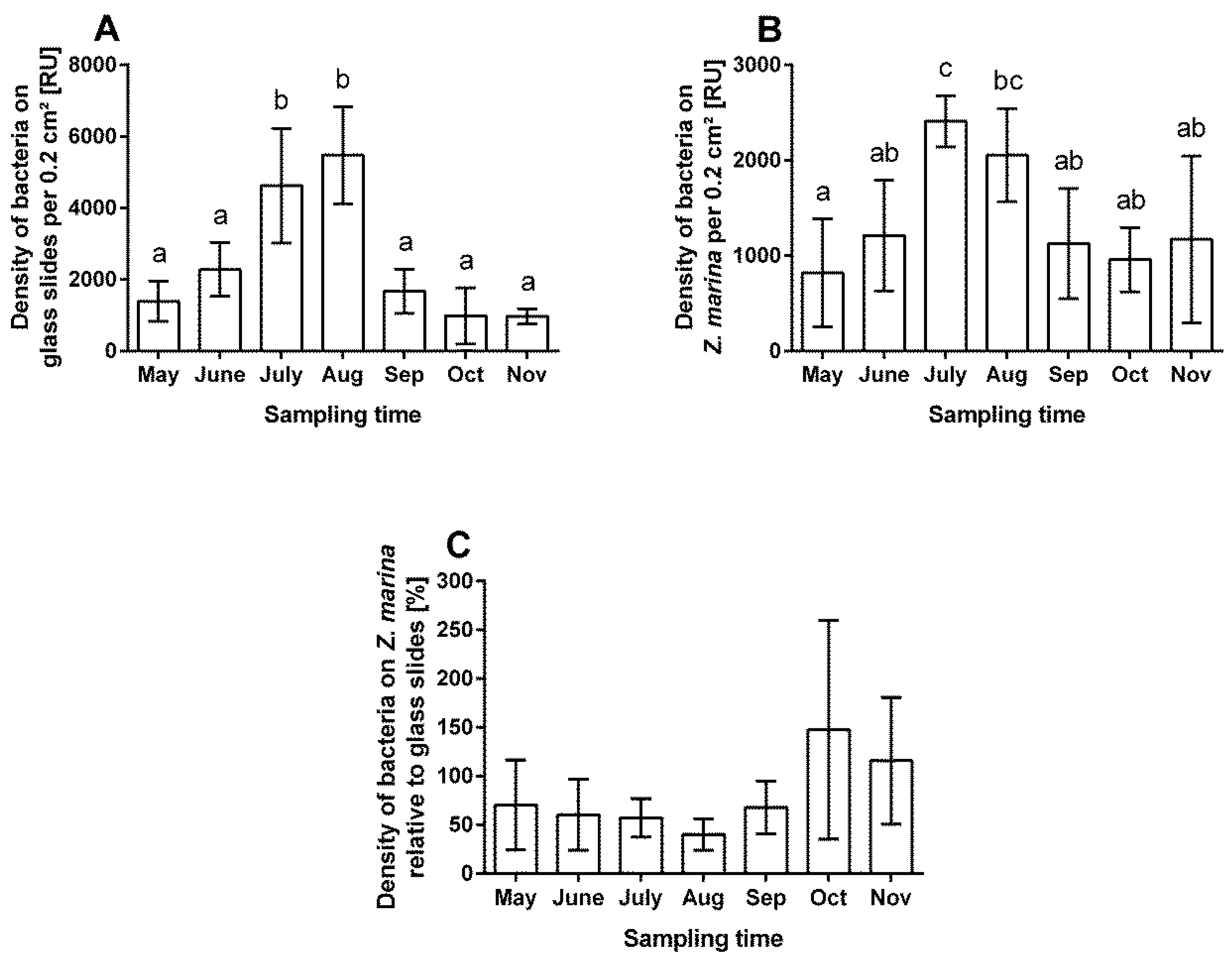
3.1. Fouling Pressure, Settled Bacteria, on Eelgrass and the Ratio
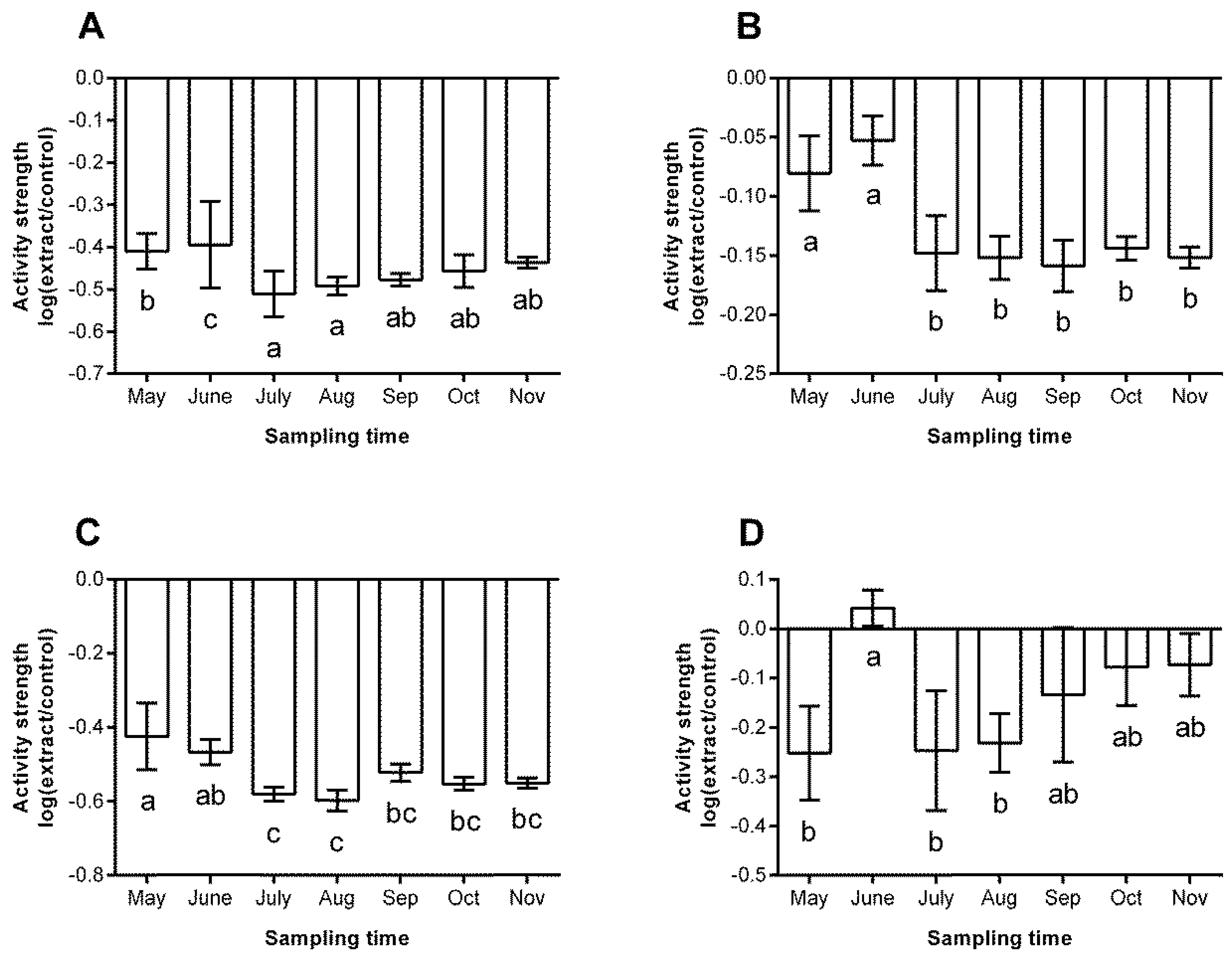
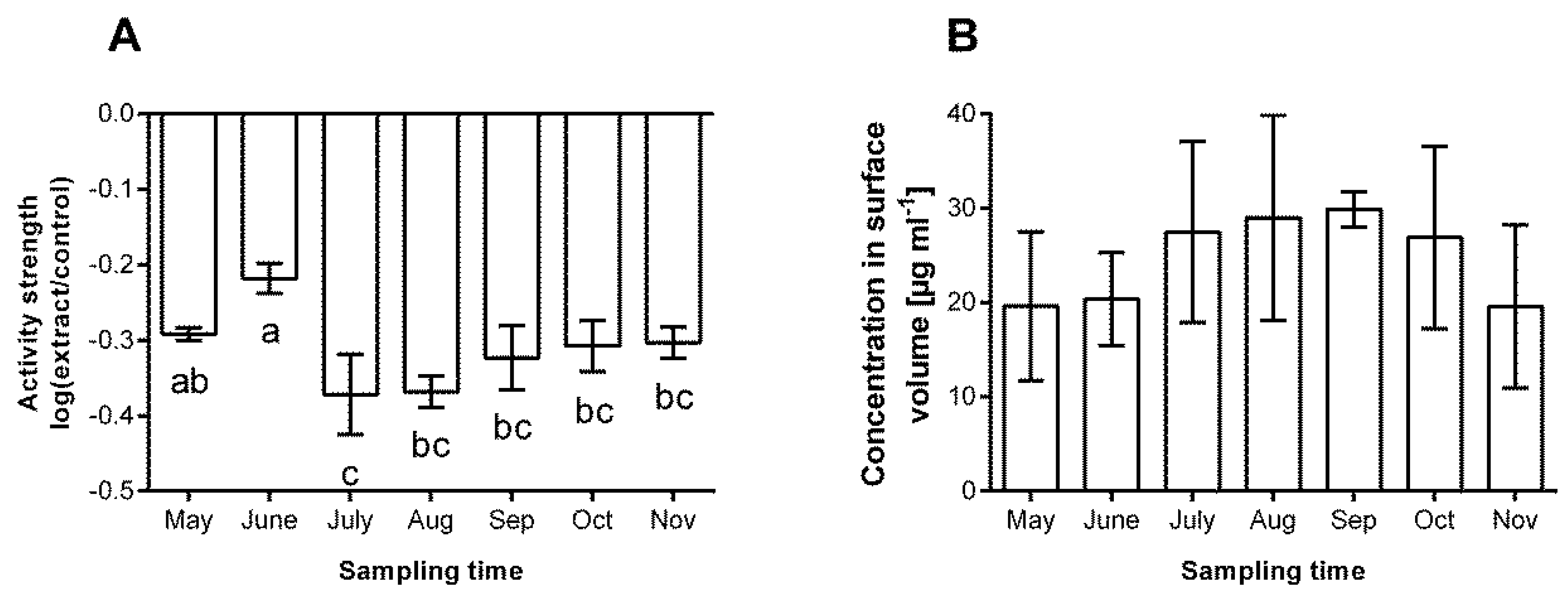
3.2. Chemical Defence
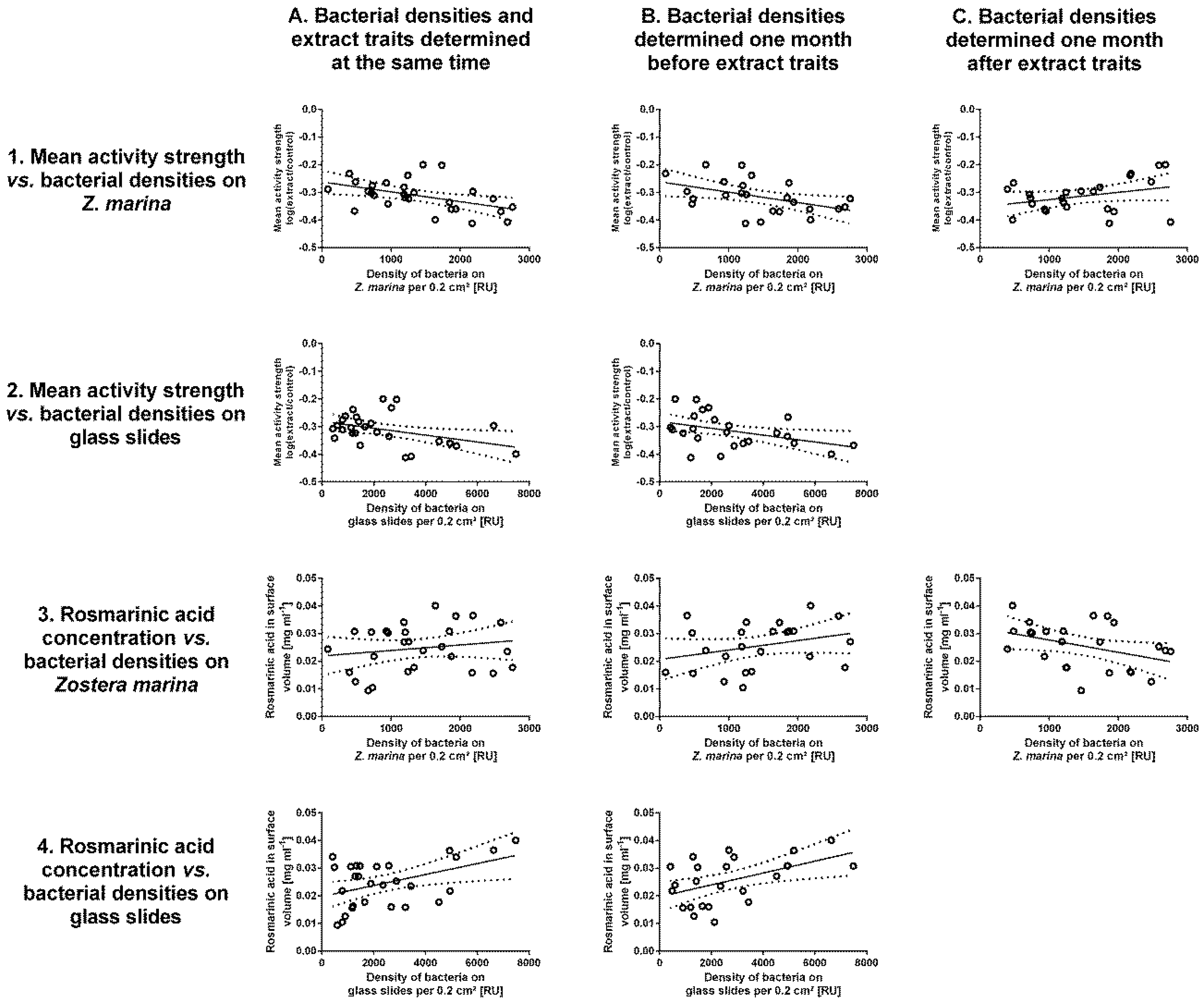
3.3. Correlations between Extract Traits and Bacterial Densities
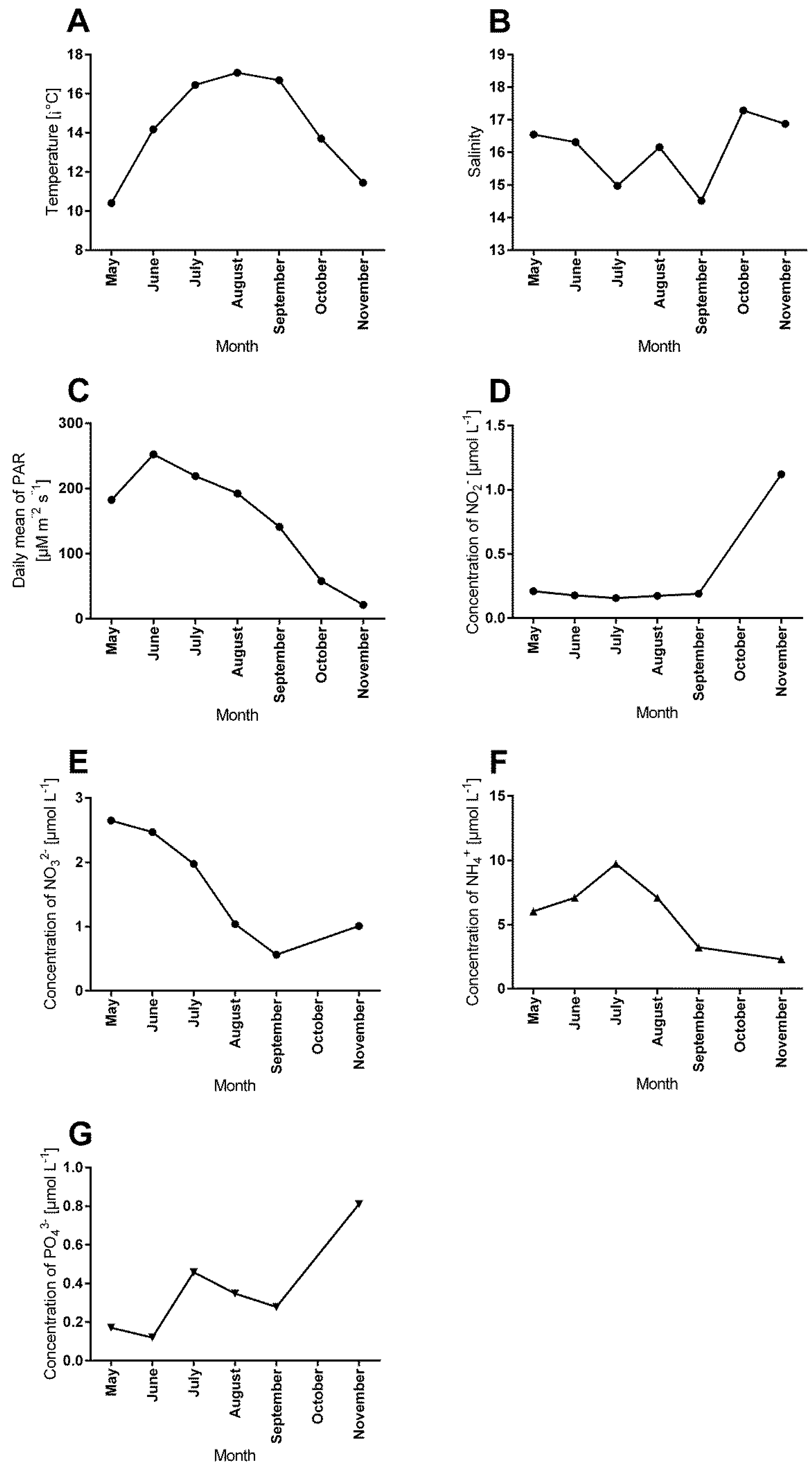
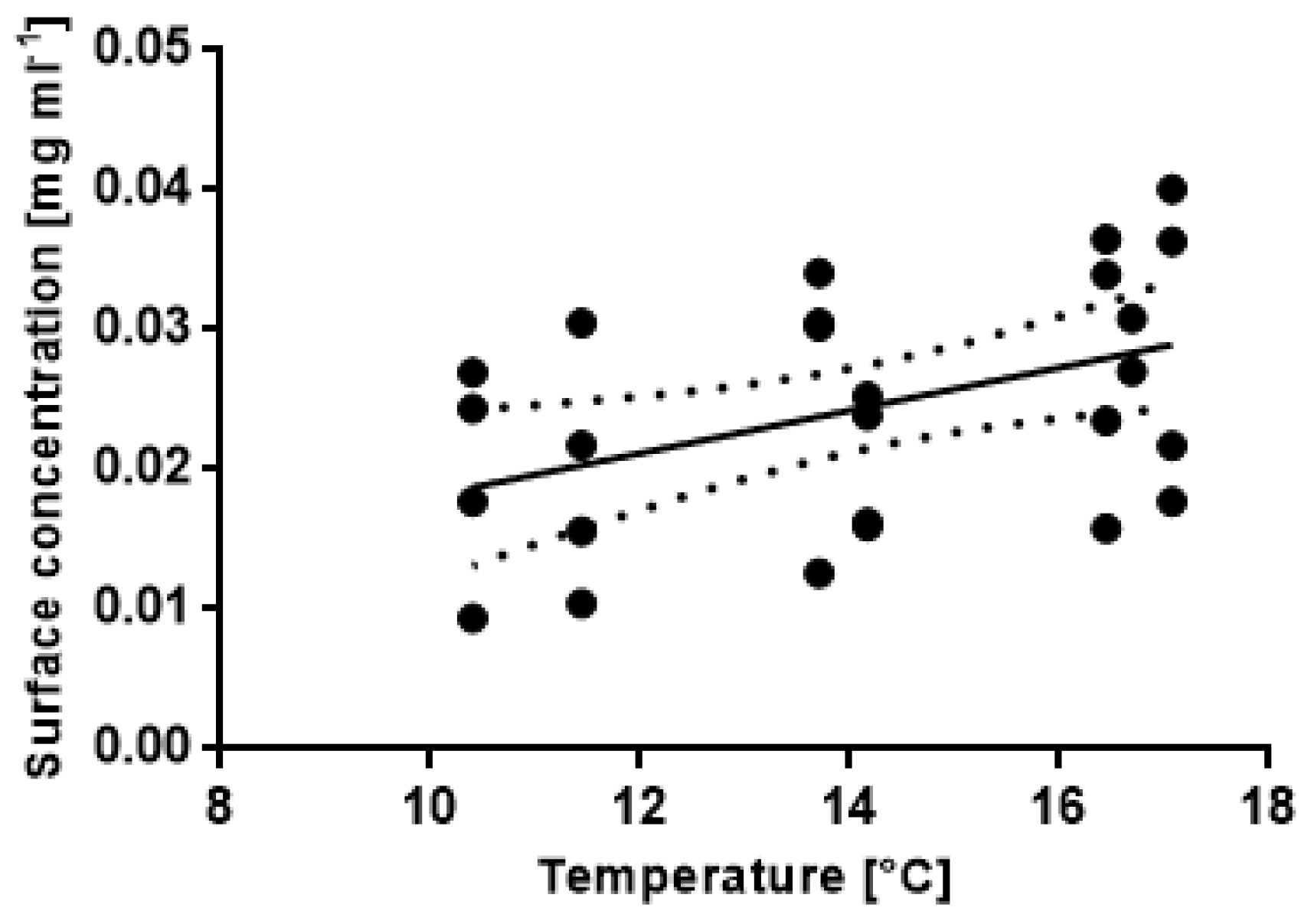
3.4. Correlations between Abiotic Factors and Antifouling Defence Related Variables
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A global crisis for seagrass ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Duarte, C.; Borum, J.; Short, F.; Walker, D. Seagrass ecosystems: Their global status and prospects. In Aquatic Ecosystems; Polunin, N., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 281–294. [Google Scholar]
- Schubert, P.R.; Hukriede, W.; Karez, R.; Reusch, T.B.H. Mapping and modeling eelgrass Zostera marina distribution in the western Baltic Sea. Mar. Ecol. Prog. Ser. 2015, 522, 79–95. [Google Scholar] [CrossRef]
- Bengtsson, M.M.; Buhler, A.; Brauer, A.; Dahlke, S.; Schubert, H.; Blindow, I. Eelgrass leaf surface microbiomes are locally variable and highly correlated with epibiotic eukaryotes. Front. Microbiol. 2017, 8, 1312. [Google Scholar] [CrossRef]
- Fahimipour, A.K.; Kardish, M.R.; Lang, J.M.; Green, J.L.; Eisen, J.A.; Stachowicz, J.J. Global-scale structure of the eelgrass microbiome. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Wahl, M. Epibiosis: Ecology, effects and defences. In Marine Hard Bottom Communities—Patterns, Dynamics, Diversity and Change; Wahl, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 61–72. [Google Scholar]
- Harder, T. Marine epibiosis: Concepts, ecological consequences and host defence. In Marine and Industrial Biofouling; Flemming, H.-C., Murthy, P.S., Venkatesan, R., Cooksey, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 219–231. [Google Scholar]
- Wahl, M.; Hay, M.E. Associational resistance and shared doom: Effects of epibiosis on herbivory. Oecologia 1995, 102, 329–340. [Google Scholar] [CrossRef]
- Barbosa, P.; Hines, J.; Kaplan, I.; Martinson, H.; Szczepaniec, A.; Szendrei, Z. Associational resistance and associational susceptibility: Having right or wrong neighbors. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 1–20. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Biofouling: Lessons from nature. Philos. Trans. R. Soc. A 2011, 370, 2381–2417. [Google Scholar] [CrossRef]
- Bryars, S.; Collings, G.; Miller, D. Nutrient exposure causes epiphytic changes and coincident declines in two temperate Australian seagrasses. Mar. Ecol. Prog. Ser. 2011, 441, 89–103. [Google Scholar] [CrossRef]
- Romero, J.; Lee, K.-S.; Pérez, M.; Mateo, M.A.; Alcoverro, T. Nutrient dynamics in seagrass ecosystems. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W.D., Orth, R.J., Duarte, C.M., Eds.; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Munoz, J.; Fotedar, R. Epiphytism of Gracilaria cliftonii (Withell, Millar & Kraft) from Western Australia. J. Appl. Phycol. 2010, 22, 371–379. [Google Scholar]
- Michetti, K.M.; Miravalles, A.B.; Hughes, M.H.; Leonardi, P.I. Infection process of Ceramium rubrum (Rhodophyta, Ceramiales) on the agarophyte Gracilaria chilensis (Rhodophyta, Gracilariales). Bot. Mar. 2016, 59. [Google Scholar] [CrossRef]
- Goecke, F.; Labes, A.; Wiese, J.; Imhoff, J.F. Chemical interactions between marine macroalgae and bacteria. Mar. Ecol.-Prog. Ser. 2010, 409, 267–299. [Google Scholar] [CrossRef]
- Wang, S.; Wang, G.; Weinberger, F.; Bian, D.; Nakaoka, M.; Lenz, M. Anti-epiphyte defences in the red seaweed Gracilaria vermiculophylla: Non-native algae are better defended than their native conspecifics. J. Ecol. 2017, 105, 445–457. [Google Scholar] [CrossRef]
- Scardino, A.J.; de Nys, R. Mini review: Biomimetic models and bioinspired surfaces for fouling control. Biofouling 2011, 27, 73–86. [Google Scholar] [CrossRef]
- Dobretsov, S.; Abed, R.M.; Teplitski, M. Mini-review: Inhibition of biofouling by marine microorganisms. Biofouling 2013, 29, 423–441. [Google Scholar] [CrossRef]
- Wahl, M.; Kroger, K.; Lenz, M. Non-toxic protection against epibiosis. Biofouling 1998, 12, 205–226. [Google Scholar] [CrossRef]
- Jensen, P.R.; Jenkins, K.M.; Porter, D.; Fenical, W. Evidence that a new antibiotic flavone glycoside chemically defends the sea grass Thalassia testudinum against zoosporic fungi. Appl. Environ. Microbiol. 1998, 64, 1490–1496. [Google Scholar]
- Zidorn, C. Secondary metabolites of seagrasses (Alismatales and Potamogetonales; Alismatidae): Chemical diversity, bioactivity, and ecological function. Phytochemistry 2016, 124, 5–28. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Rajaram, R.; Balasubramanian, V.; Mathivanan, K. Antifouling potentials of extracts from seaweeds, seagrasses and mangroves against primary biofilm forming bacteria. Asian Pac. J. Trop. Biomed. 2012, 2, 316–322. [Google Scholar] [CrossRef]
- Guan, C.; Parrot, D.; Wiese, J.; Sönnichsen, F.D.; Saha, M.; Tasdemir, D.; Weinberger, F. Identification of rosmarinic acid and sulfated flavonoids as inhibitors of microfouling on the surface of eelgrass Zostera marina. Biofouling 2017, 33, 867–880. [Google Scholar] [CrossRef]
- Bryant, J.P.; Chapin, F.S.; Reichardt, P.B.; Clausen, T.P. Response of winter chemical defence in Alaska paper birch and green alder to manipulation of plant carbon/nutrient balance. Oecologia 1987, 72, 510–514. [Google Scholar] [CrossRef]
- Vergeer, L.H.T.; Develi, A. Phenolic acids in healthy and infected leaves of Zostera marina and their growth-limiting properties towards Labyrinthula zosterae. Aquat. Bot. 1997, 58, 65–72. [Google Scholar] [CrossRef]
- Arnold, T.; Mealey, C.; Leahey, H.; Miller, A.W.; Hall-Spencer, J.M.; Milazzo, M.; Maers, K. Ocean acidification and the loss of phenolic substances in marine plants. PLoS ONE 2012, 7, e35107. [Google Scholar] [CrossRef]
- Harrison, P.G.; Durance, C. Seasonal variation in phenolic content of eelgrass shoots. Aquat. Bot. 1989, 35, 409–413. [Google Scholar] [CrossRef]
- Ravn, H.; Pedersen, M.F.; Borum, J.; Andary, C.; Anthoni, U.; Christophersen, C.; Nielsen, P.H. Seasonal variation and distribution of two phenolic compounds, rosmarinic acid and caffeic acid, in leaves and roots-rhizomes of eelgrass (Zostera marina L.). Ophelia 1994, 40, 51–61. [Google Scholar] [CrossRef]
- Saha, M.; Wahl, M. Seasonal variation in the antifouling defence of the temperate brown alga Fucus vesiculosus. Biofouling 2013, 29, 661–668. [Google Scholar] [CrossRef]
- Rickert, E.; Lenz, M.; Barboza, F.R.; Gorb, S.N.; Wahl, M. Seasonally fluctuating chemical microfouling control in Fucus vesiculosus and Fucus serratus from the Baltic Sea. Mar. Biol. 2016, 163. [Google Scholar] [CrossRef]
- Wang, S.; Weinberger, F.; Lenz, M. Fluctuations in the strength of chemical antifouling defences in a red macroalga in response to variations in epibiont colonization pressure. Mar. Biol. 2018, 165. [Google Scholar] [CrossRef]
- Amade, P.; Lemée, R. Chemical defence of the mediterranean alga Caulerpa taxifolia: Variations in caulerpenyne production. Aquat. Toxicol. 1998, 43, 287–300. [Google Scholar] [CrossRef]
- Hellio, C.; Marechal, J.P.; Veron, B.; Bremer, G.; Clare, A.S.; Le Gal, Y. Seasonal variation of antifouling activities of marine algae from the Brittany coast (France). Mar. Biotechnol. 2004, 6, 67–82. [Google Scholar] [CrossRef]
- Marechal, J.P.; Culioli, G.; Hellio, C.; Thomas-Guyon, H.; Callow, M.E.; Clare, A.S.; Ortalo-Magne, A. Seasonal variation in antifouling activity of crude extracts of the brown alga Bifurcaria bifurcata (Cystoseiraceae) against cyprids of Balanus amphitrite and the marine bacteria Cobetia marina and Pseudoalteromonas haloplanktis. J. Exp. Mar. Biol. Ecol. 2004, 313, 47–62. [Google Scholar] [CrossRef]
- Cronin, G. Resource allocation in seaweeds and marine invertebrates: Chemical defence patterns in relation to defence theories. In Marine Chemical Ecology; McClintock, J., Baker, B., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 325–353. [Google Scholar]
- Keen, N.T. Gene-for-gene complementarity in plant-pathogen interactions. Annu. Rev. Genet. 1990, 24, 447–463. [Google Scholar] [CrossRef]
- Ponce de León, I.; Montesano, M. Activation of defence mechanisms against pathogens in mosses and flowering plants. Int. J. Mol. Sci. 2013, 14, 3178–3200. [Google Scholar] [CrossRef]
- Howe, G.; Schaller, A. Direct defences in plants and their induction by wounding and insect herbivores. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer: Berlin, Germany, 2008; pp. 7–29. [Google Scholar]
- Pereira, R.; da Silva Costa, E.; Sudatti, D.; Da Gama, B. Inducible defences against herbivory and fouling in seaweeds. J. Sea Res. 2017, 122, 25–33. [Google Scholar] [CrossRef]
- Wahl, M.; Shahnaz, L.; Dobretsov, S.; Saha, M.; Symanowski, F.; David, K.; Lachnit, T.; Vasel, M.; Weinberger, F. Ecology of antifouling resistance in the bladder wrack Fucus vesiculosus: Patterns of microfouling and antimicrobial protection. Mar. Ecol. Prog. Ser. 2010, 411, 33–48. [Google Scholar] [CrossRef]
- Saha, M.; Rempt, M.; Grosser, K.; Pohnert, G.; Weinberger, F. Surface-associated fucoxanthin mediates settlement of bacterial epiphytes on the rockweed Fucus vesiculosus. Biofouling 2011, 27, 423–433. [Google Scholar] [CrossRef]
- Saha, M.; Wiese, J.; Weinberger, F.; Wahl, M. Rapid adaptation to controlling new microbial epibionts in the invaded range promotes invasiveness of an exotic seaweed. J. Ecol. 2016, 104, 969–978. [Google Scholar] [CrossRef]
- Weinberger, F.; Buchholz, B.; Karez, R.; Wahl, M. The invasive red alga Gracilaria vermiculophylla in the Baltic Sea: Adaptation to brackish water may compensate for light limitation. Aquat. Biol. 2008, 3, 251–264. [Google Scholar] [CrossRef]
- Rao, T.S. Comparative effect of temperature on biofilm formation in natural and modified marine environment. Aquat. Ecol. 2010, 44, 463–478. [Google Scholar] [CrossRef]
- Zaccone, R.; Azzaro di Rosamarina, M.; Azzaro, F.; Bergamasco, A.; Caruso, G.; Leonardi, M.; La Ferla, R.; Maimone, G.; Mancuso, M.; Monticelli, L.; et al. Seasonal dynamics of prokaryotic abundance and activities in relation to environmental parameters in a transitional aquatic ecosystem (Cape Peloro, Italy). Microb. Ecol. 2014, 67, 45–56. [Google Scholar] [CrossRef]
- Barron, C.; Marba, N.; Duarte, C.M.; Pedersen, M.F.; Lindblad, C.; Kersting, K.; Moy, F.; Bokn, T. High organic carbon export precludes eutrophication responses in experimental rocky shore communities. Ecosystems 2003, 6, 144–153. [Google Scholar] [CrossRef]
- Weinberger, F. Pathogen-induced defence and innate immunity in macroalgae. Biol. Bull. 2007, 213, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Nürnberger, T.; Kemmerling, B. Receptor protein kinases - pattern recognition receptors in plant immunity. Trends Plant Sci. 2006, 11, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.L.; Rouze, P.; Verhelst, B.; Lin, Y.C.; Bayer, T.; Collen, J.; Dattolo, E.; De Paoli, E.; Dittami, S.; Maumus, F.; et al. The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature 2016, 530, 331–335. [Google Scholar] [CrossRef]
- Loucks, K.; Waddell, D.; Ross, C. Lipopolysaccharides elicit an oxidative burst as a component of the innate immune system in the seagrass Thalassia testudinum. Plant Physiol. Biochem. 2013, 70, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Piñeiro, Z.; Barroso, C.G. Stability of phenolic compounds during extraction with superheated solvents. J. Chromatogr. A 2001, 921, 169–174. [Google Scholar] [CrossRef]
- Papazian, S.; Parrot, D.; Burýšková, B.; Weinberger, F.; Tasdemir, D. Surface chemical defence of the eelgrass Zostera marina against microbial foulers. Sci. Rep. 2019, 9, 3323. [Google Scholar] [CrossRef]
| SS | DF | MS | F | p | ||
|---|---|---|---|---|---|---|
| A | Intercept | 172,931,151 | 1 | 172,931,151 | 81.22 | 0.0029 |
| Error | 6,387,136 | 3 | 2,129,045 | |||
| Month | 79,883,772 | 6 | 13,313,962 | 19.02 | <0.0001 | |
| Error | 12,596,887 | 18 | 699,827 | |||
| B | Intercept | 54,269,905 | 1 | 54,269,905 | 79.12 | 0.0030 |
| Error | 2,057,806 | 3 | 685,935 | |||
| Month | 8,559,980 | 6 | 1,426,663 | 5.76 | 0.0017 | |
| Error | 4,461,122 | 18 | 247,840 | |||
| C | Intercept | 177,998.3 | 1 | 177,998.3 | 46.37 | 0.0065 |
| Error | 11,517.0 | 3 | 3839.0 | |||
| Month | 34,337.6 | 6 | 5722.9 | 1.92 | 0.1316 | |
| Error | 53,516.4 | 18 | 2973.1 |
| SS | DF | MS | F | p | ||
|---|---|---|---|---|---|---|
| A | Intercept | 5.7717 | 1 | 5.7717 | 4604.62 | <0.0001 |
| Error | 0.0038 | 3 | 0.0013 | |||
| Month | 0.0441 | 6 | 0.0073 | 2.67 | 0.0492 | |
| Error | 0.0494 | 18 | 0.0027 | |||
| B | Intercept | 0.4503 | 1 | 0.4503 | 2517.79 | <0.0001 |
| Error | 0.0005 | 3 | 0.0002 | |||
| Month | 0.0425 | 6 | 0.0071 | 13.06 | <0.0001 | |
| Error | 0.0098 | 18 | 0.0005 | |||
| C | Intercept | 7.8046 | 1 | 7.8046 | 7108.19 | <0.0001 |
| Error | 0.0033 | 3 | 0.0011 | |||
| Month | 0.0933 | 6 | 0.0155 | 8.93 | 0.0001 | |
| Error | 0.0313 | 18 | 0.0017 | |||
| D | Intercept | 0.5419 | 1 | 0.5419 | 68.50 | 0.0037 |
| Error | 0.0237 | 3 | 0.0079 | |||
| Month | 0.2952 | 6 | 0.0492 | 5.97 | 0.0014 | |
| Error | 0.1483 | 18 | 0.0082 |
| SS | DF | MS | F | p | ||
|---|---|---|---|---|---|---|
| A | Intercept | 0.08607 | 1 | 0.08607 | 0.21 | 0.8885 |
| Error | 0.00070 | 3 | 0.000233 | |||
| Month | 0.06532 | 6 | 0.01089 | 9.77 | <0.0001 | |
| Error | 0.02005 | 18 | 0.001114 | |||
| B | Intercept | 0.0171 | 1 | 0.0171 | 118.57 | 0.0017 |
| Error | 0.0004 | 3 | 0.0001 | |||
| Month | 0.0005 | 6 | <0.0001 | 1.58 | 0.2097 | |
| Error | 0.0010 | 18 | <0.0001 |
| A | B | C | ||||
|---|---|---|---|---|---|---|
| Parameters | r | p-Value | R | p-Value | R | p-Value |
| BD to AS | −0.7395 | 0.0116 | −0.7330 | 0.0237 | 0.6368 | 0.123 |
| FP to AS | −0.7075 | 0.0256 | −0.7112 | 0.0376 | ||
| BD to RA | 0.4989 | 0.3709 | 0.6195 | 0.3021 | −0.6913 | 0.0545 |
| FP to RA | 0.7272 | 0.016 | 0.7580 | 0.013 | ||
| A | SS | DF | MS | F | p | |
| NH4+ | 0.003564 | 1 | 0.003564 | 3.25 | 0.0875 | |
| NO32− | 0.036928 | 1 | 0.036928 | 33.64 | 0.0001 | |
| Temperature | 0.014374 | 1 | 0.014374 | 13.10 | 0.0018 | |
| NO2− | 0.006761 | 1 | 0.006761 | 6.16 | 0.0226 | |
| Residuals | 0.020856 | 19 | 0.001098 | |||
| B | SS | DF | MS | F | p | |
| Temperature | 0.0004 | 1 | 0.0004 | 7.54 | 0.0118 | |
| Residuals | 0.0012 | 22 | 0.0001 | |||
| C | SS | DF | MS | F | p | |
| PO43− | 340,743 | 1 | 340,743 | 1.29 | 0.2721 | |
| Salinity | 1,344,377 | 1 | 1,344,377 | 5.08 | 0.0376 | |
| Light | 4,942,563 | 1 | 4,942,563 | 18.69 | 0.0005 | |
| NO2− | 828,775 | 1 | 828,775 | 3.13 | 0.0946 | |
| NH4+ | 223,201 | 1 | 223,201 | 0.84 | 0.3711 | |
| NO32− | 1,686,026 | 1 | 1,686,026 | 6.38 | 0.0218 | |
| Residuals | 4,495,253 | 17 | ||||
| D | SS | DF | MS | F | p | |
| Fouling pressure | 4,800,251 | 1 | 4,800,251 | 12.11 | 0.0022 | |
| Defence strength | 734,242 | 1 | 734,242 | 1.85 | 0.188 | |
| Residuals | 8,326,443 | 21 | 396,497 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, C.; Saha, M.; Weinberger, F. Chemical Defence of a Seagrass against Microfoulers and Its Seasonal Dynamics. Appl. Sci. 2019, 9, 1258. https://doi.org/10.3390/app9061258
Guan C, Saha M, Weinberger F. Chemical Defence of a Seagrass against Microfoulers and Its Seasonal Dynamics. Applied Sciences. 2019; 9(6):1258. https://doi.org/10.3390/app9061258
Chicago/Turabian StyleGuan, Chi, Mahasweta Saha, and Florian Weinberger. 2019. "Chemical Defence of a Seagrass against Microfoulers and Its Seasonal Dynamics" Applied Sciences 9, no. 6: 1258. https://doi.org/10.3390/app9061258
APA StyleGuan, C., Saha, M., & Weinberger, F. (2019). Chemical Defence of a Seagrass against Microfoulers and Its Seasonal Dynamics. Applied Sciences, 9(6), 1258. https://doi.org/10.3390/app9061258





