Micro-Brazing of Stainless Steel Using Ni-P Alloy Plating
Abstract
1. Introduction
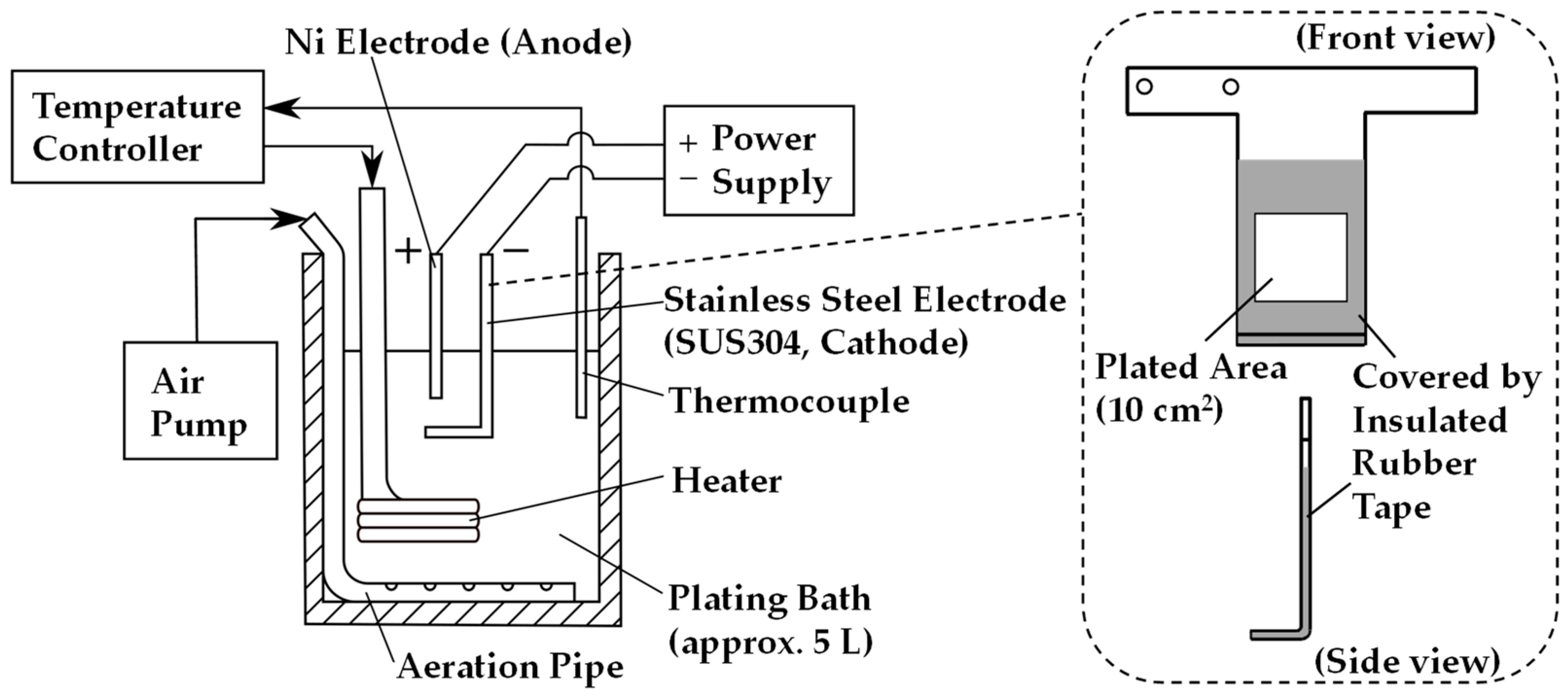
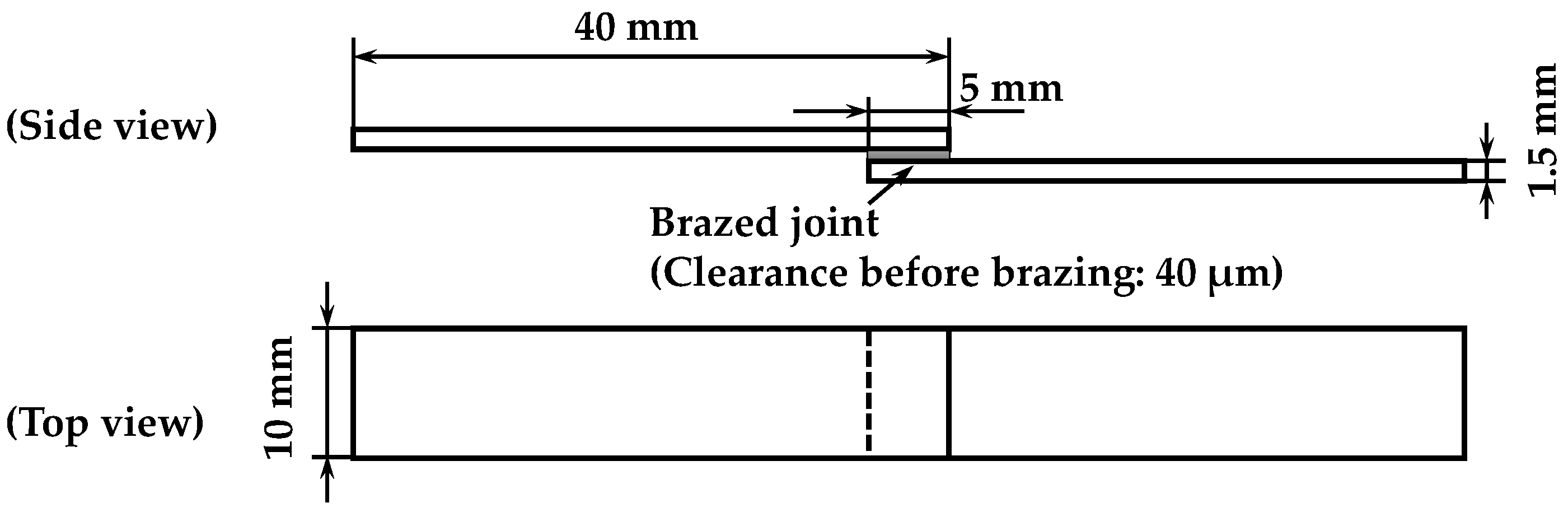
2. Experimental Procedure
3. Results and Discussion
3.1. Formation of Ni-11P Alloy Plated Layer
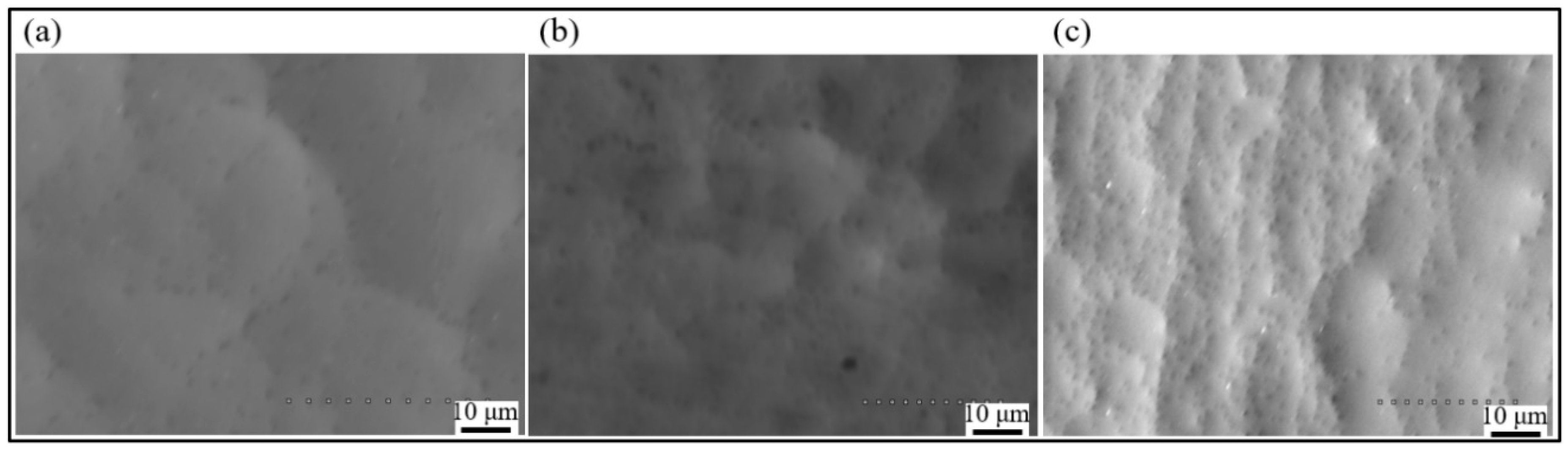
3.2. Effect of Current Density on Surface Morphology of Ni-P Alloy Plated Layer
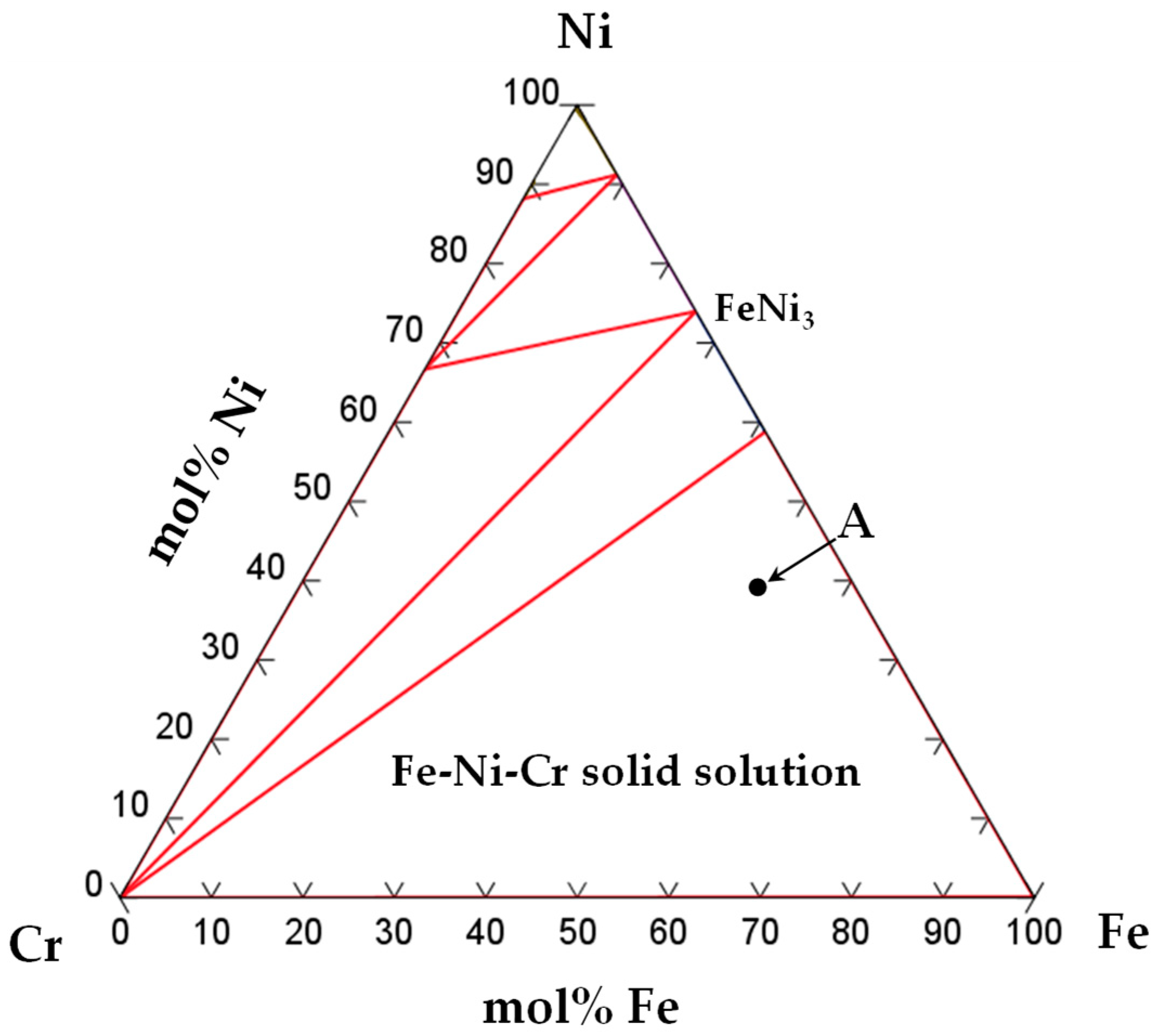
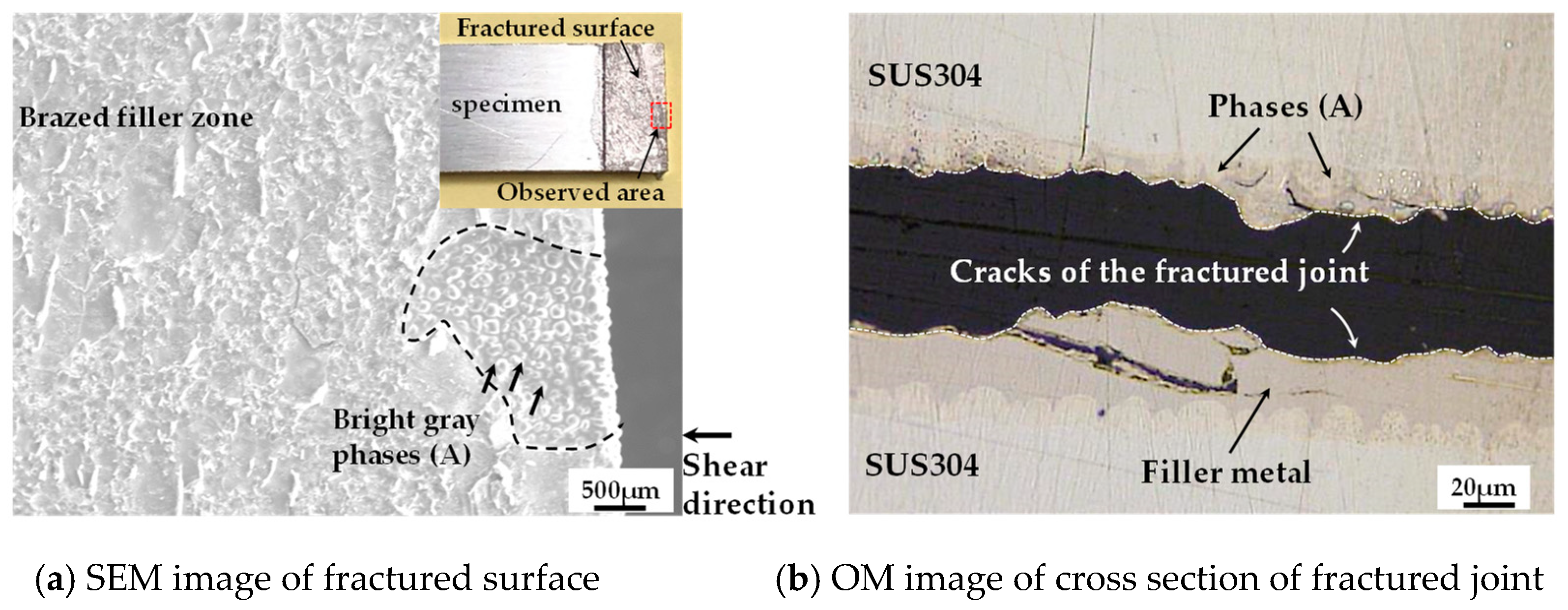
3.3. Microstructure Observation of Brazed Joint
3.4. Shear Test
4. Conclusions
- A Ni-11 wt.% P electroplated layer of 20 μm thickness was formed on the surface of the SUS304 plate in the case of 0.4 M H3PO3 in the bath, a current density of 2.0 A/dm2 and a plating time of 90 min.
- The average shear strength of the brazed joints was determined to be 47.3 MPa.
- Fracture occurred in the brazed filler zone where the brittle P-containing compounds existed.
- The brazing filler metal formed by electroplating was homogeneously distributed between the SUS304 plates after brazing. This means that the electroplated Ni-P layer can be used as a brazing filler metal for SUS304.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yin, Y.-S. Brittleness of brazed seam for nickel-based filler metals and its influence factors. Trans. China Weld. Inst. 1997, 18, 4–10. [Google Scholar]
- Tung, S.K.; Lim, L.C.; Lai, M.O. Solidification Phenomena in nickel base brazes containing boron and silicon. Scr. Mater. 1996, 34, 763–769. [Google Scholar] [CrossRef]
- Zorc, B.; Kosec, L. Comparison of brazed joints made with BNi-1 and BNi-7 nickel-base brazing alloys. Rev. Metal. Madr. 2000, 36, 100–107. [Google Scholar] [CrossRef]
- Matsu, K. Brazing of Ni based brazing filler metals and the latest trend. Weld. Technol. 2011, 59, 54–57. [Google Scholar]
- Shohji, I.; Arai, S.; Kano, N.; Otomo, N.; Uenishi, M. Development of Cu brazing sheet with Cu-P composite plating. Key Eng. Mater. 2007, 353–358, 2025–2028. [Google Scholar] [CrossRef]
- Hamlyn-Harris, J.H.; St. John, D.H.; Sood, D.K. Amorphization of nickel and Ni-P alloys by ion implantation. Mater. Sci. Eng. A 1991, 147, 201–210. [Google Scholar] [CrossRef]
- Bagley, B.G.; Turnbull, D. The preparation and crystallization behaviour of amorphous nickel-phosphorous thin films. Acta Metal. 1970, 18, 857–862. [Google Scholar] [CrossRef]
- Peeters, P.; Hoorn, G.V.D.; Daenen, T.; Kurowski, A.; Staikov, G. Properties of electroless and electroplated Ni-P and its application in microgalvanics. Electrochim. Acta 2001, 47, 161–169. [Google Scholar] [CrossRef]
- Feng, L. Commercial Process for Electroplating Nickel-Phosphorous Coatings. U.S. Patent 20040031694A1, 19 February 2004. [Google Scholar]
- Morikawa, T.; Nakade, T.; Yokoi, M.; Fukumoto, Y.; Iwakura, C. Electrodeposition of Ni-P alloys from Ni-citrate bath. Electrochim. Acta 1997, 42, 115–118. [Google Scholar] [CrossRef]
- Seo, M.H.; Kim, J.S.; Hwang, W.S.; Kim, D.J.; Hwanf, S.S.; Chun, B.S. Characteristics of Ni-P alloy electrodeposited from a sulfamate bath. Surf. Coat. Technol. 2004, 176, 135–140. [Google Scholar] [CrossRef]
- Engelhaupt, D.; Ramsey, B. Nickel Cobalt Phosphorous Low Stress Electroplating. U.S. Patent 20020164262A1, 7 November 2002. [Google Scholar]
- Li, C.S.; Lee, C.Y.; Chen, F.J.; Chie, C.T.; Lin, P.L.; Chung, W.C. Electrodeposition of nickel-phosphorous alloy from sulfamate baths with improved current efficiency. J. Electrochem. Soc. 2006, 153, 387–392. [Google Scholar]
- Ma, C.B.; Cao, F.H.; Zhang, Z.; Zhang, J.Q. Electrodeposition of amorphous Ni-P coatings onto Nd-Fe-B permanent magnet substrates. Appl. Surf. Sci. 2006, 253, 2251–2256. [Google Scholar] [CrossRef]
- Harris, T.M.; Dang, Q.D. The mechanism of phosphorus incorporation during the electrodeposition of nickel phosphorus alloys. J. Electrochem. Soc. 1993, 140, 81–83. [Google Scholar] [CrossRef]
- Uttam, K.C.; Ajay, B.; Sudesna, R.; Soobhankar, P. Evaluation of Ni-Cr-P coatings electrodeposited on low carbon steel bipolar for polymer electrolyte membrane fuel cell. Int. J. Hydrogen Energry 2018, 43, 23430–23440. [Google Scholar]
- Haichuan, S.; Zhishui, Y.; JongRae, C. A study on the microstructure and properties of brazing joint for Cr18-Ni8 steel using a BNi7+9%Cu mixed filler metal. Vacuum 2018, 151, 226–232. [Google Scholar]
- Takayama, S.; Arikura, Y.; Shohji, I.; Nakazawa, T.; Matsumoto, K.; Hikita, M. Joint Strength and Microstructure of SUS304 Brazed with Nickel-base Filler Metal for Heat Exchangers. J. Jpn. Inst. Met. 2004, 68, 130–133. [Google Scholar] [CrossRef]
- Wu, N.; Li, Y.; Wang, J.; Puchkow, U.A. Vaccum brazing of super-Ni/NiCr laminated composite to Cr18-Ni8 steel with NiCrP filler metal. J. Mater. Process. Technol. 2012, 212, 794–800. [Google Scholar] [CrossRef]



| Composition | Concentration |
|---|---|
| NiSO4·6H2O | 1.0 M |
| NiCl2·6H2O | 0.2 M |
| H3BO3 | 0.5 M |
| H3PO3 | 0.12–0.5 M |
| C6H5Na3O7 | 0.5 M |
| Point | Element Composition (mol%) | Possible Phase | |||
|---|---|---|---|---|---|
| Ni | P | Fe | Cr | ||
| A | 41.3 | 2.0 | 48.1 | 8.6 | Fe-Ni-Cr solid solution |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Shohji, I.; Iioka, M.; Hashimoto, A.; Hirohashi, J.; Wake, T.; Arai, S. Micro-Brazing of Stainless Steel Using Ni-P Alloy Plating. Appl. Sci. 2019, 9, 1094. https://doi.org/10.3390/app9061094
Liu S, Shohji I, Iioka M, Hashimoto A, Hirohashi J, Wake T, Arai S. Micro-Brazing of Stainless Steel Using Ni-P Alloy Plating. Applied Sciences. 2019; 9(6):1094. https://doi.org/10.3390/app9061094
Chicago/Turabian StyleLiu, Shubin, Ikuo Shohji, Makoto Iioka, Anna Hashimoto, Junichiro Hirohashi, Tsunehito Wake, and Susumu Arai. 2019. "Micro-Brazing of Stainless Steel Using Ni-P Alloy Plating" Applied Sciences 9, no. 6: 1094. https://doi.org/10.3390/app9061094
APA StyleLiu, S., Shohji, I., Iioka, M., Hashimoto, A., Hirohashi, J., Wake, T., & Arai, S. (2019). Micro-Brazing of Stainless Steel Using Ni-P Alloy Plating. Applied Sciences, 9(6), 1094. https://doi.org/10.3390/app9061094





