A Membrane Modified with Nitrogen-Doped TiO2/Graphene Oxide for Improved Photocatalytic Performance
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Nanomaterials
2.3. Modification of the PVDF Microfiltration Membrane
2.4. Modified Membrane Characterization
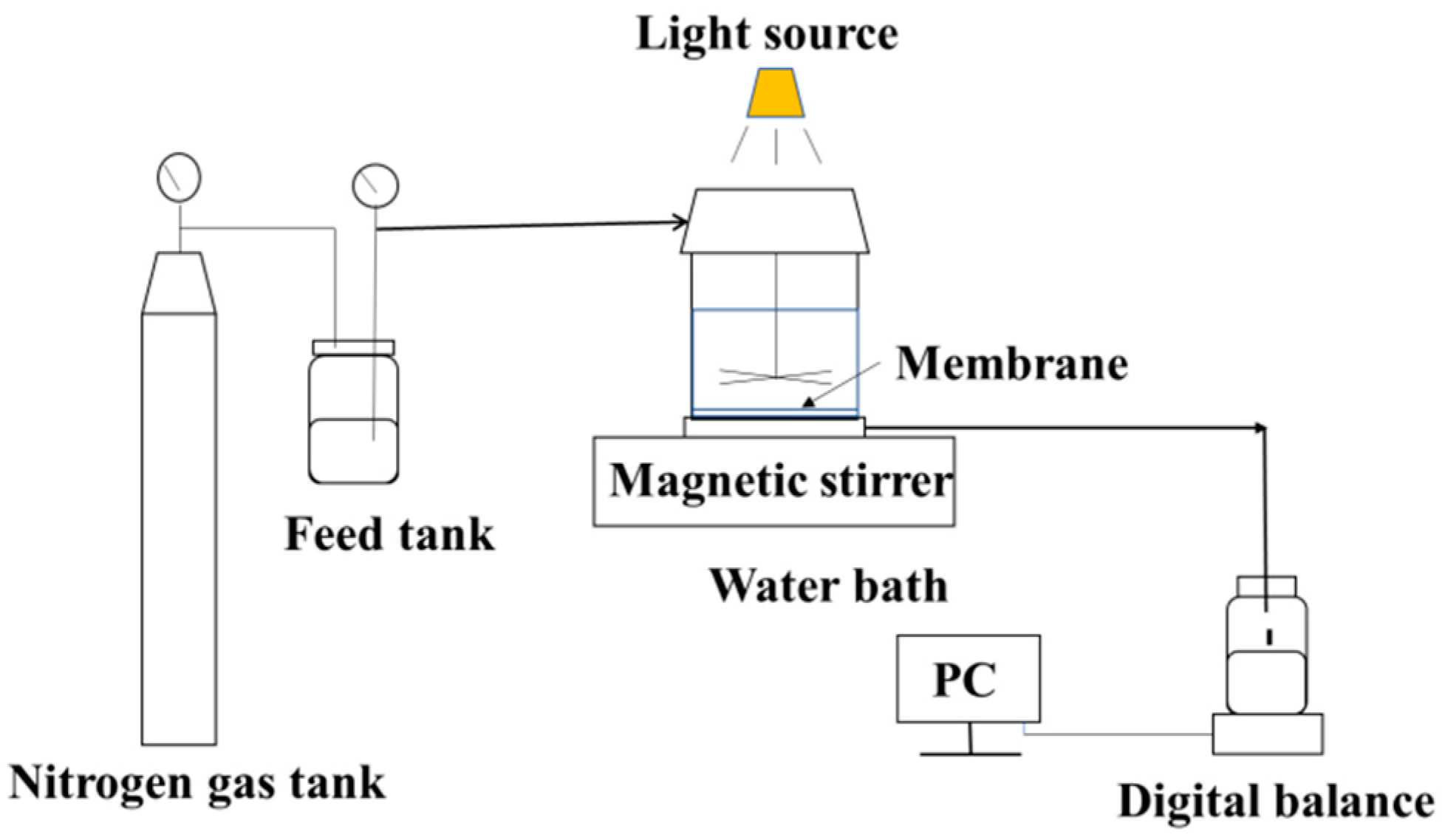
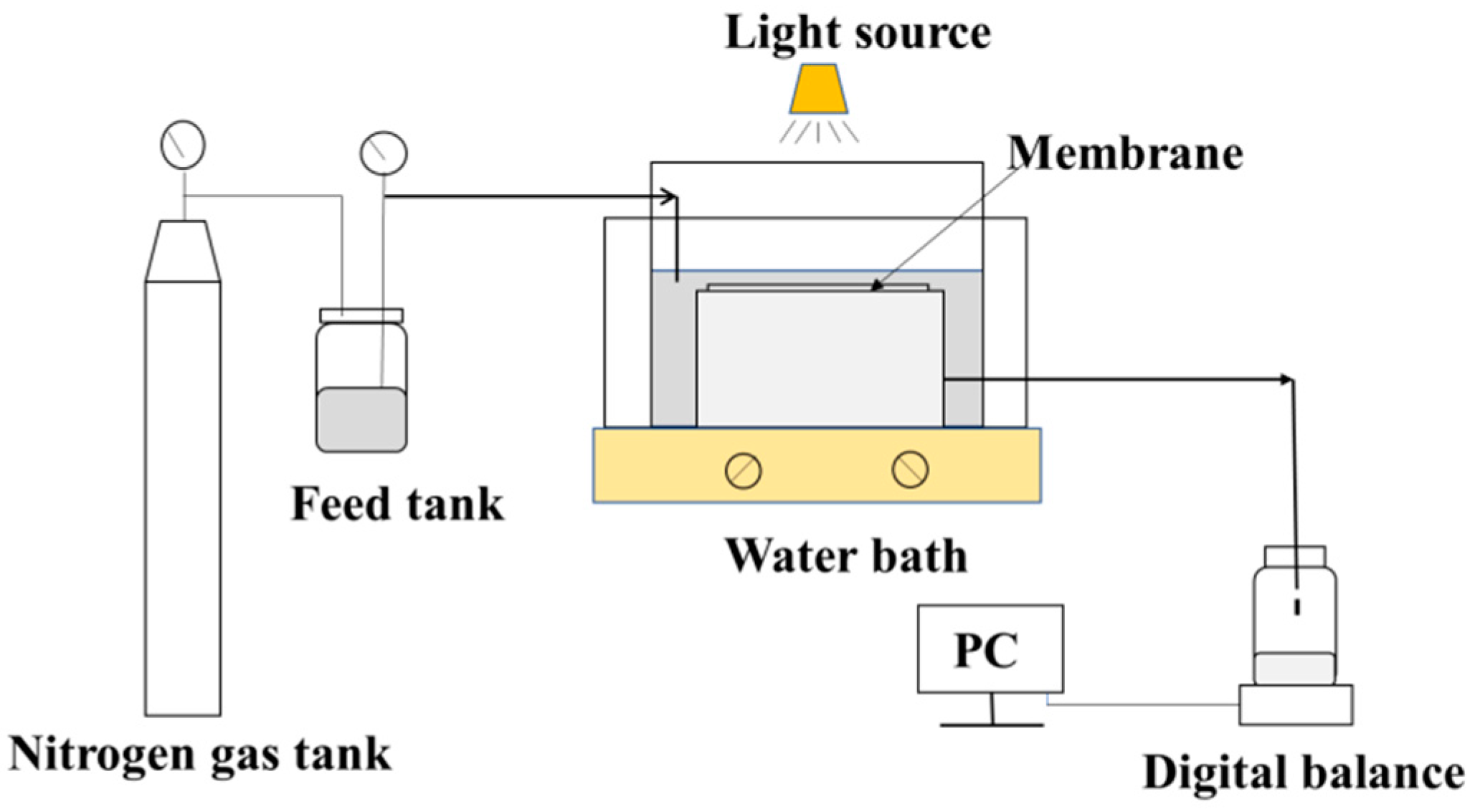
2.5. Modified Membrane Performance Tests
3. Results and Discussion
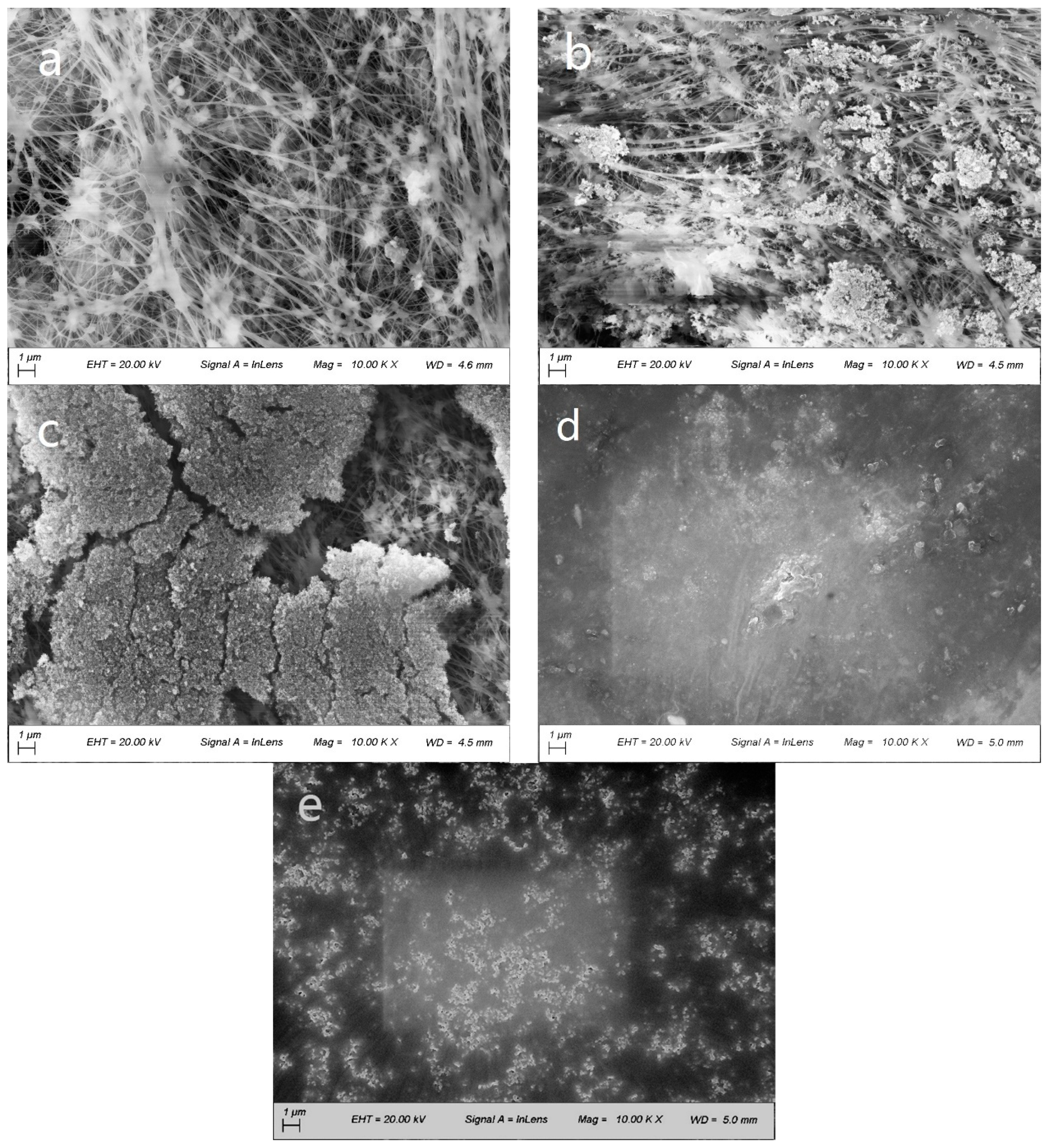
3.1. Modified Membrane Morphology
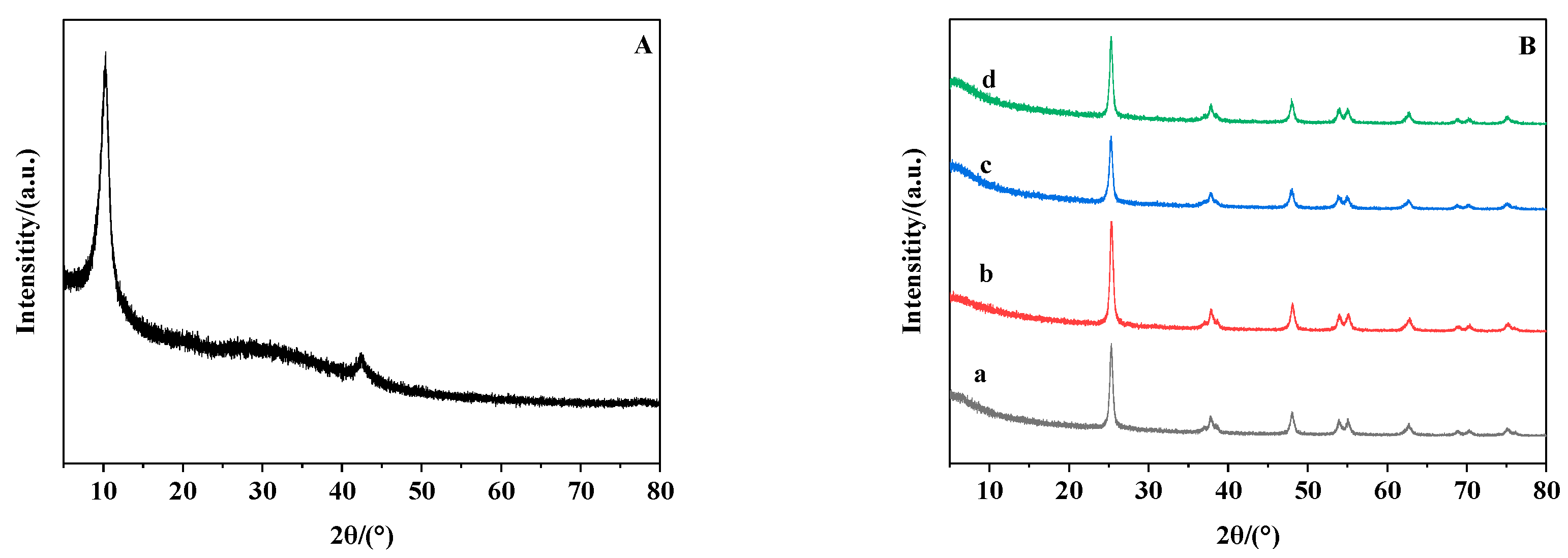
3.2. The X-Ray Diffraction Analysis of Modified Membrane
3.3. The Water Contact Angle of the Modified Membrane
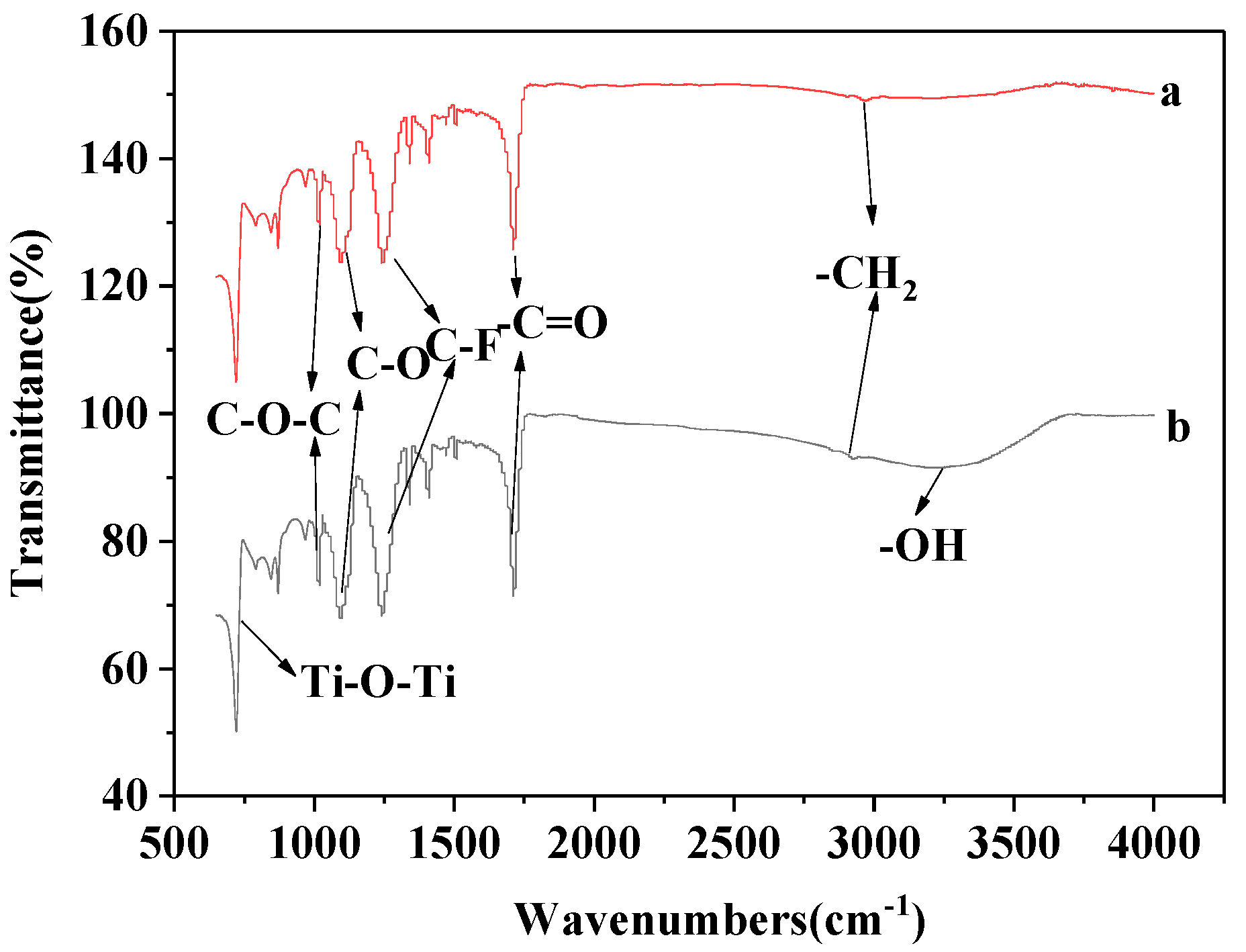
3.4. The Infrared Spectroscopy Analysis of the Modified Membranes
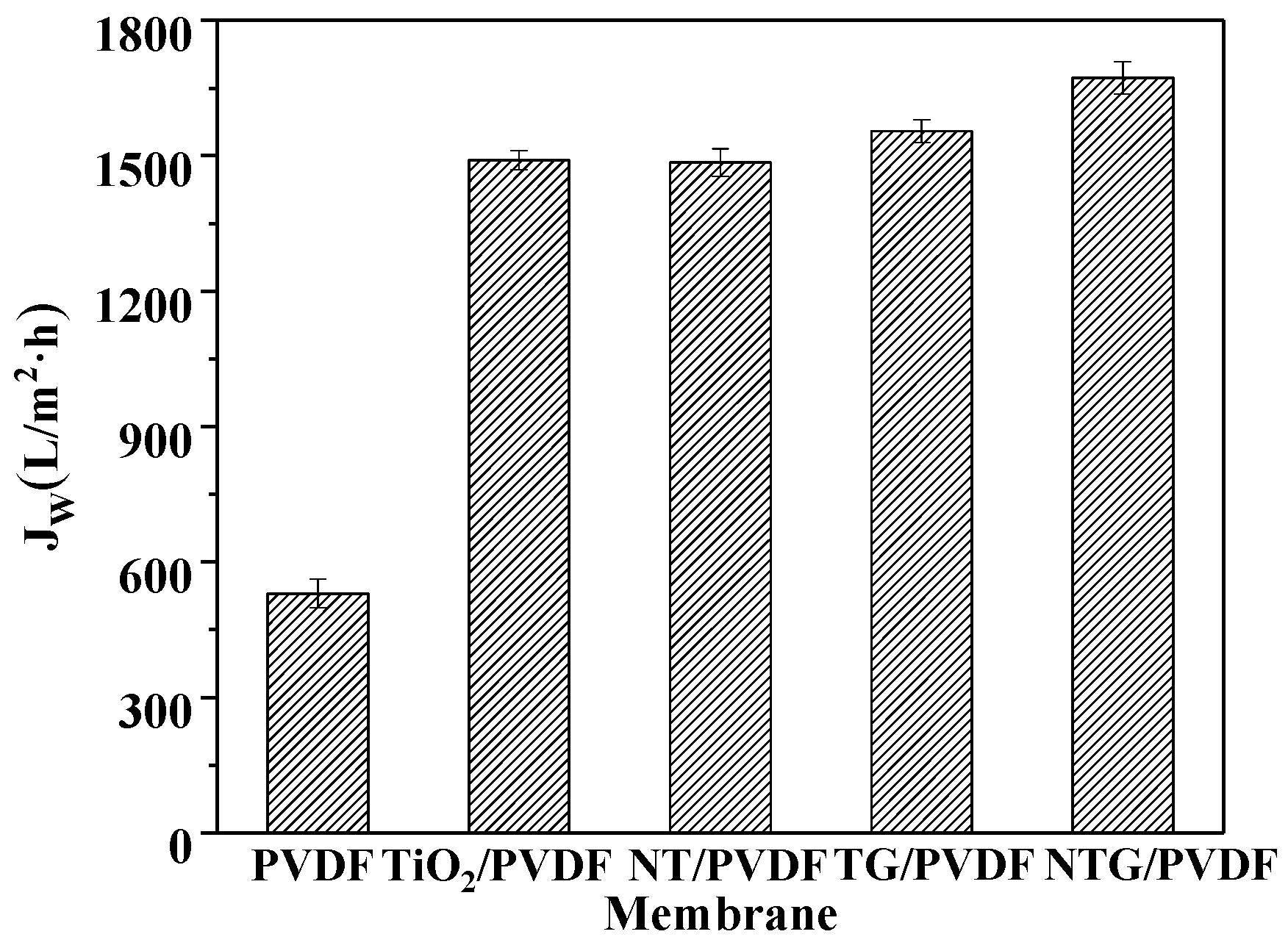
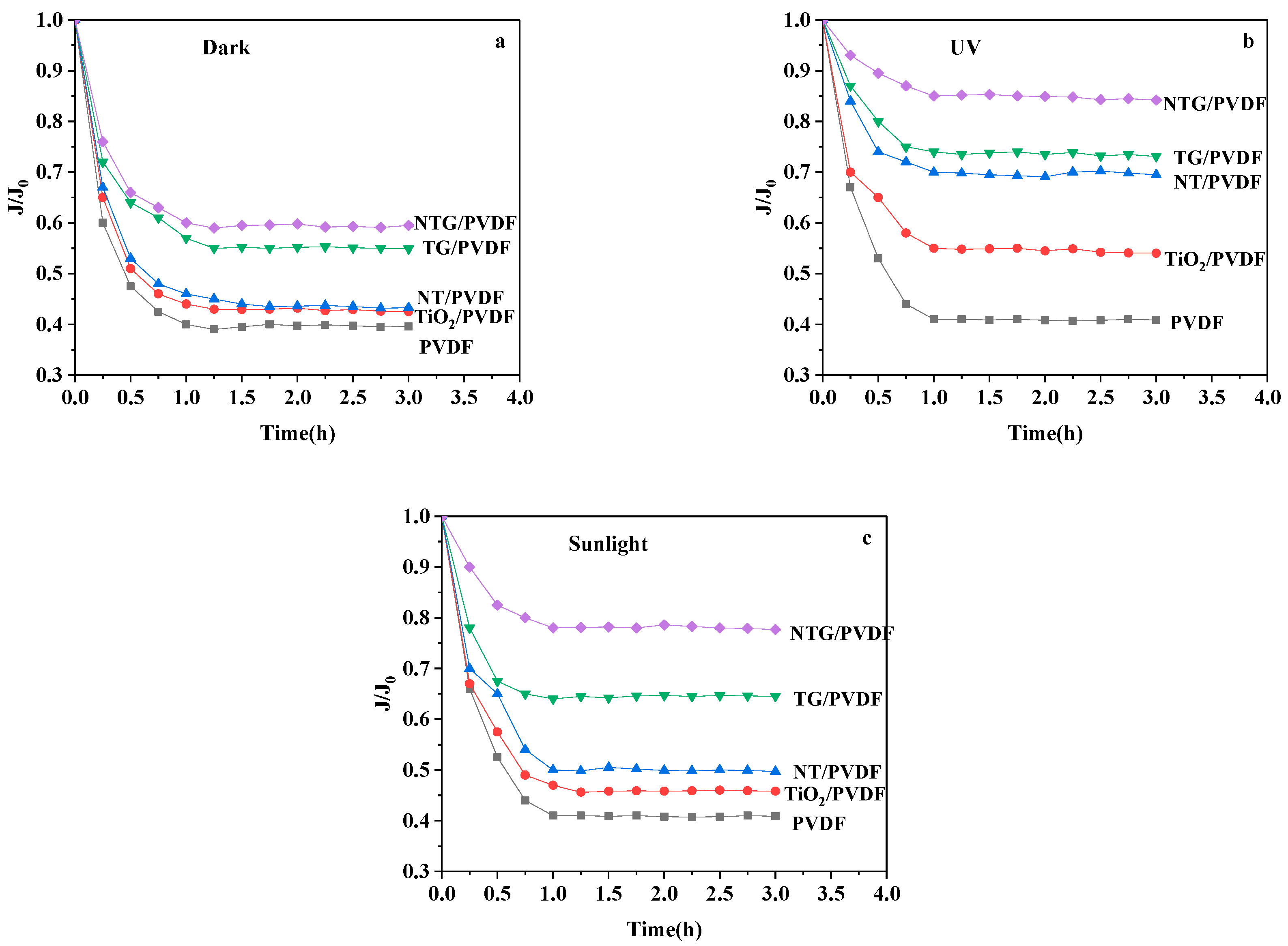
3.5. The Flux Change Analysis of the Modified Membrane
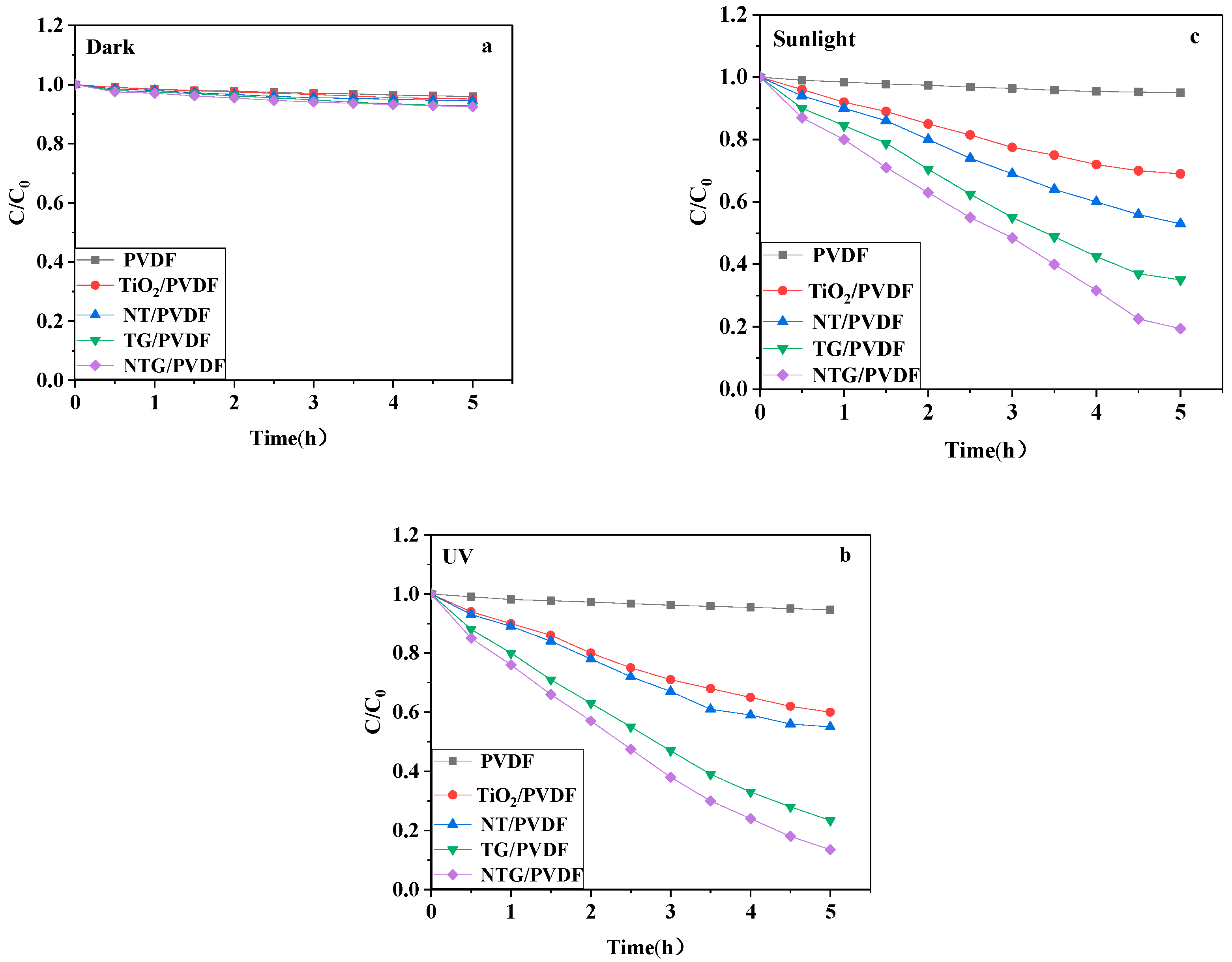
3.6. The Photocatalytic Degradation Properties of the Modified Membranes
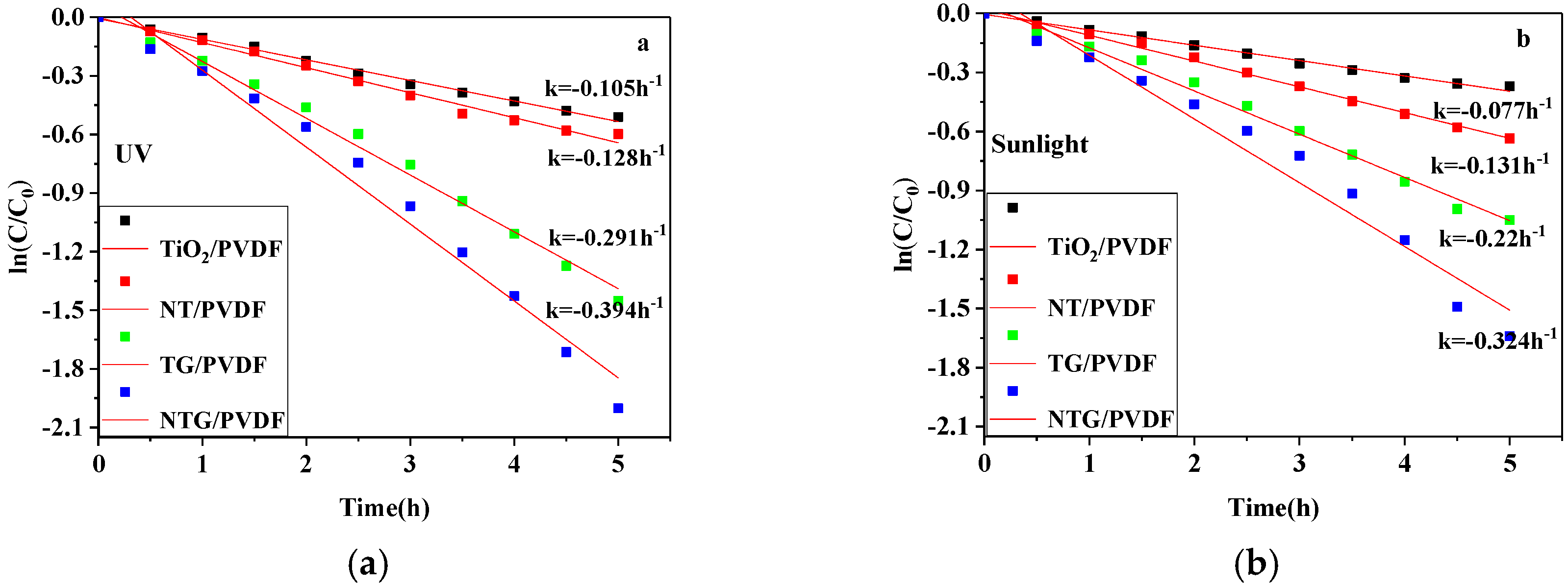
3.7. The Kinetic Analysis of the Photocatalytic Degradation of the Modified Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buonomenna, M.G. Membrane processes for a sustainable industrial growth. RSC Adv. 2013, 3, 5694–5740. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wang, J.Y. Catalytic Separation Membrane in Water Treatment. Bull. Chin. Ceram. Soc. 2016, 35, 1130–1136. [Google Scholar]
- Gao, Y.; Hu, M.; Mi, B. Membrane surface modification with TiO2 –graphene oxide for enhanced photocatalytic performance. J. Membrane Sci. 2014, 455, 49–356. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: a review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Han, C.; Pelaez, M.; Zu, D. Enhanced visible light photocatalytic activity of C N-codoped TiO2 films for the degradation of microcystin-LR. J. Mol. Catal. A: Chem. 2013, 372, 58–65. [Google Scholar] [CrossRef]
- Liu, G.; Han, C.; Pelaez, M.; Zhu, D.; Liao, S.; Likodimos, V.; Ioannidis, N.; Kontos, A.G.; Falaras, P.; Dunlop, P.S. Synthesis, characterization and photocatalytic evaluation of visible light activated C-doped TiO2 nanoparticles. Nanotechnology 2012, 23, 294003. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Athanasekou, C.P.; Katsaros, F.K.; Kanellopoulos, N.K.; Dionysiou, D.D.; Likodimos, V.; Falaras, P. Double-side active TiO2-modified nanofiltration membranes in continuous flow photocatalytic reactors for effective water purification. J. Hazard. Mater. 2003, 99, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Almeida, N.A.; Martins, P.M.; Teixeira, S.; Lopes da Silva, H.A.; Sencadas, V.; Kühn, K.; Cuniberti, G.; Lanceros-Mendez, S.; Marqueset, P.A.A.P. TiO2/graphene oxide immobilized in (PVDF-TrFE) electrospun membranes with enhanced visible-light-induced photocatalytic performance. J. Mater. Sci. 2016, 51, 6974–6986. [Google Scholar] [CrossRef]
- Sher Shah, M.S.; Kim, W.J.; Park, J.; Rhee, D.K.; Jang, I.-H.; Park, N.-G.; Lee, J.Y.; Yoo, P.J. Highly Efficient and Recyclable Nanocomplexed Photocatalysts of AgBr/N-Doped and Amine-Functionalized Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2014, 6, 20819–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zou, L.; Li, S.; Zheng, F. Nanospherical like reduced graphene oxide decorated TiO2 nanoparticles: an advanced catalyst for the hydrogen evolution reaction. Sci. Rep. 2016, 6, 20335. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.; Wu, Y.; Sun, J.; Yang, P.; Du, Y.; Lu, C. TiO₂ nanoparticles-functionalized N-doped graphene with superior interfacial contact and enhanced charge separation for photocatalytic hydrogen generation. ACS Appl. Mater. Interfaces 2014, 6, 13798–13806. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Karthikeyan, D.; Lee, Y.R. Effective photocatalytic degradation of anthropogenic dyes using graphene oxide grafting titanium dioxide nanoparticles under UV-light irradiation. J. Photochem. Photobiol. A: Chem. 2017, 333, 92–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Tang, Z.R.; Xu, Y.J. Improving the photocatalytic performance of graphene-TiO2 nanocomposites via a combined strategy of decreasing defects of graphene and increasing interfacial contact. Phys. Chem. Chem. Phys. 2012, 14, 9167–9175. [Google Scholar] [CrossRef] [PubMed]
- Kibechu, R.W.; Ndinteh, D.T.; Msagati, T.A.M.; Mamba, B.B.; Sampath, S. Effect of incorporating graphene oxide and surface imprinting on polysulfone membranes on flux, hydrophilicity and rejection of salt and polycyclic aromatic hydrocarbons from water. Phys. Chem. Earth 2017, 100, 126–134. [Google Scholar] [CrossRef]
- Cao, X.; Ma, J.; Shi, X.; Ren, Z. Effect of TiO2, nano particle size on the performance of PVDF membrane. Appl. Surf. Sci. 2006, 253, 2003–2010. [Google Scholar] [CrossRef]
- Ye, T.; Chen, W.; Xu, H.; Geng, N.; Cai, Y. Preparation of TiO2/graphene composite with appropriate N-doping ratio for humic acid removal. J. Mater. Sci. 2018, 53, 613–625. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Thin film nanocomposite reverse osmosis membrane modified by reduced graphene oxide/TiO2 with improved desalination performance. J. Membrane Sci. 2015, 489, 43–54. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemant, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.R.; Tyler, J.L.; Stretz, H.A.; Wells, M.J.M. Effects of a dual nanofiller, nano-TiO2 and MWCNT, for polysulfone-based nanocomposite membranes for water purification. Desalination 2015, 372, 47–56. [Google Scholar] [CrossRef]
- Ganesh, B.M.; Isloor, A.M.; Ismail, A.F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 2013, 313, 199–207. [Google Scholar] [CrossRef]
- Rabiee, H.; Vatanpour, V. Preparation and characterization of emulsion poly (vinyl chloride) (EPVC)/TiO2 nanocomposite ultrafiltration membrane. J. Membrane Sci. 2014, 472, 185–193. [Google Scholar] [CrossRef]
- Nie, C.; Ma, L.; Xia, Y.; He, C.; Deng, J.; Wang, L.; Cheng, C.; Sun, S.; Zhao, C. Novel heparin-mimicking polymer brush grafted carbon nanotube/PES composite membranes for safe and efficient blood purification. J. Membrane Sci. 2015, 475, 455–468. [Google Scholar] [CrossRef]
- Li, D.M.; Jiang, P.; Ye, T.J.; Liang, J.L.; Li, S.X.; Jiang, S.X. Study on preparation conditions and antifouling properties of GO-TiO2 modified PVDF hollow fiber membrane. Acta Sci. Circum. 2017, 37, 3746–3754. [Google Scholar]
- Xu, H.; Ding, M.; Liu, S.; Li, Y.; Shen, Z.; Wang, K. Preparation and characterization of novel polysulphone hybrid ultrafiltration membranes blended with N-doped GO/TiO2 nanocomposites. Polymer 2017, 117, 198–207. [Google Scholar] [CrossRef]
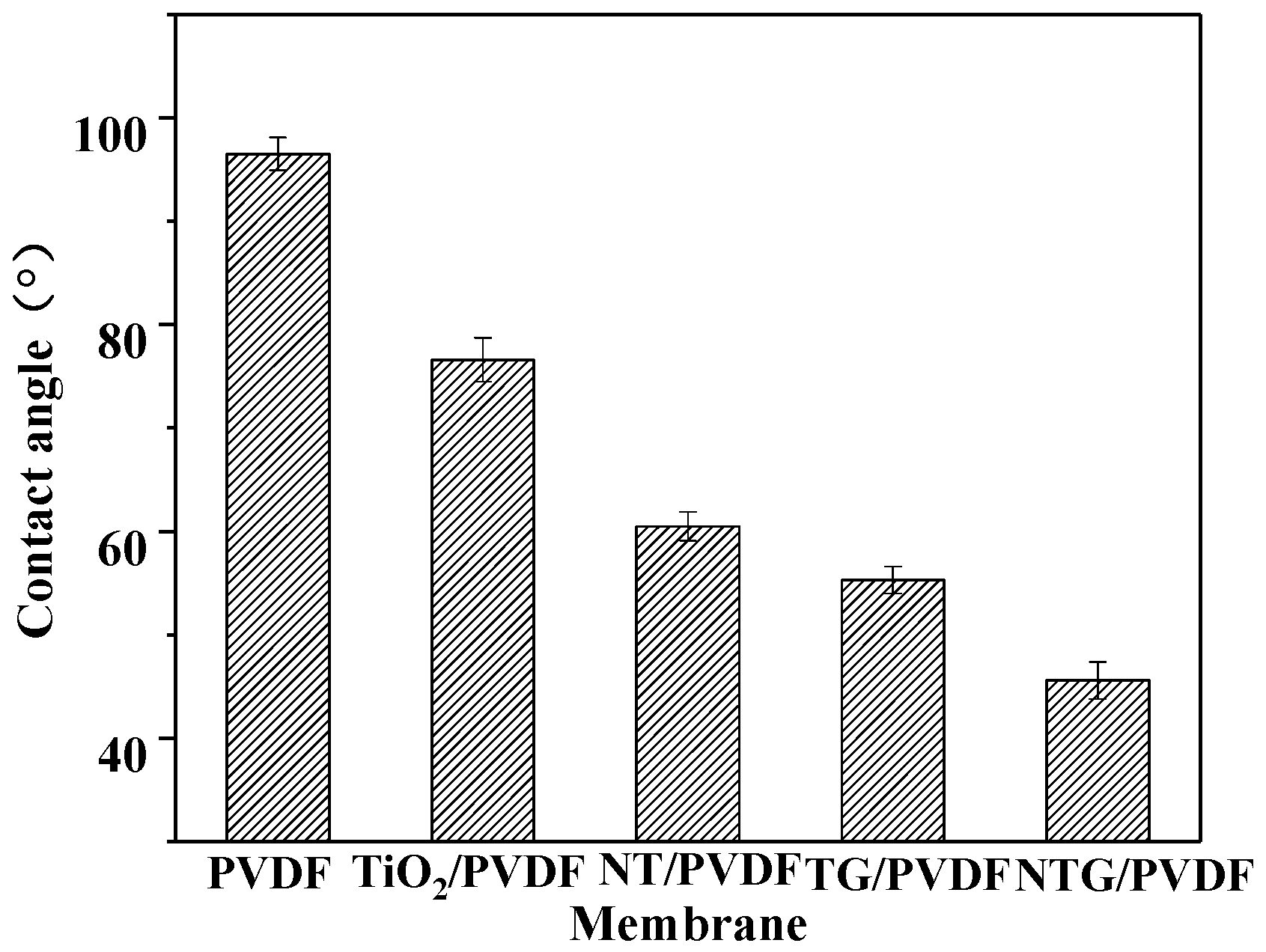
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Gao, Y.; Zhou, J.; Zhang, M.; Fu, X.; Liu, F. A Membrane Modified with Nitrogen-Doped TiO2/Graphene Oxide for Improved Photocatalytic Performance. Appl. Sci. 2019, 9, 855. https://doi.org/10.3390/app9050855
Li T, Gao Y, Zhou J, Zhang M, Fu X, Liu F. A Membrane Modified with Nitrogen-Doped TiO2/Graphene Oxide for Improved Photocatalytic Performance. Applied Sciences. 2019; 9(5):855. https://doi.org/10.3390/app9050855
Chicago/Turabian StyleLi, Tingting, Yong Gao, Junwo Zhou, Manying Zhang, Xiaofei Fu, and Fang Liu. 2019. "A Membrane Modified with Nitrogen-Doped TiO2/Graphene Oxide for Improved Photocatalytic Performance" Applied Sciences 9, no. 5: 855. https://doi.org/10.3390/app9050855
APA StyleLi, T., Gao, Y., Zhou, J., Zhang, M., Fu, X., & Liu, F. (2019). A Membrane Modified with Nitrogen-Doped TiO2/Graphene Oxide for Improved Photocatalytic Performance. Applied Sciences, 9(5), 855. https://doi.org/10.3390/app9050855




