Yttria Ceria Co-Stabilized Zirconia Reinforced with Alumina and Strontium Hexaaluminate
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results
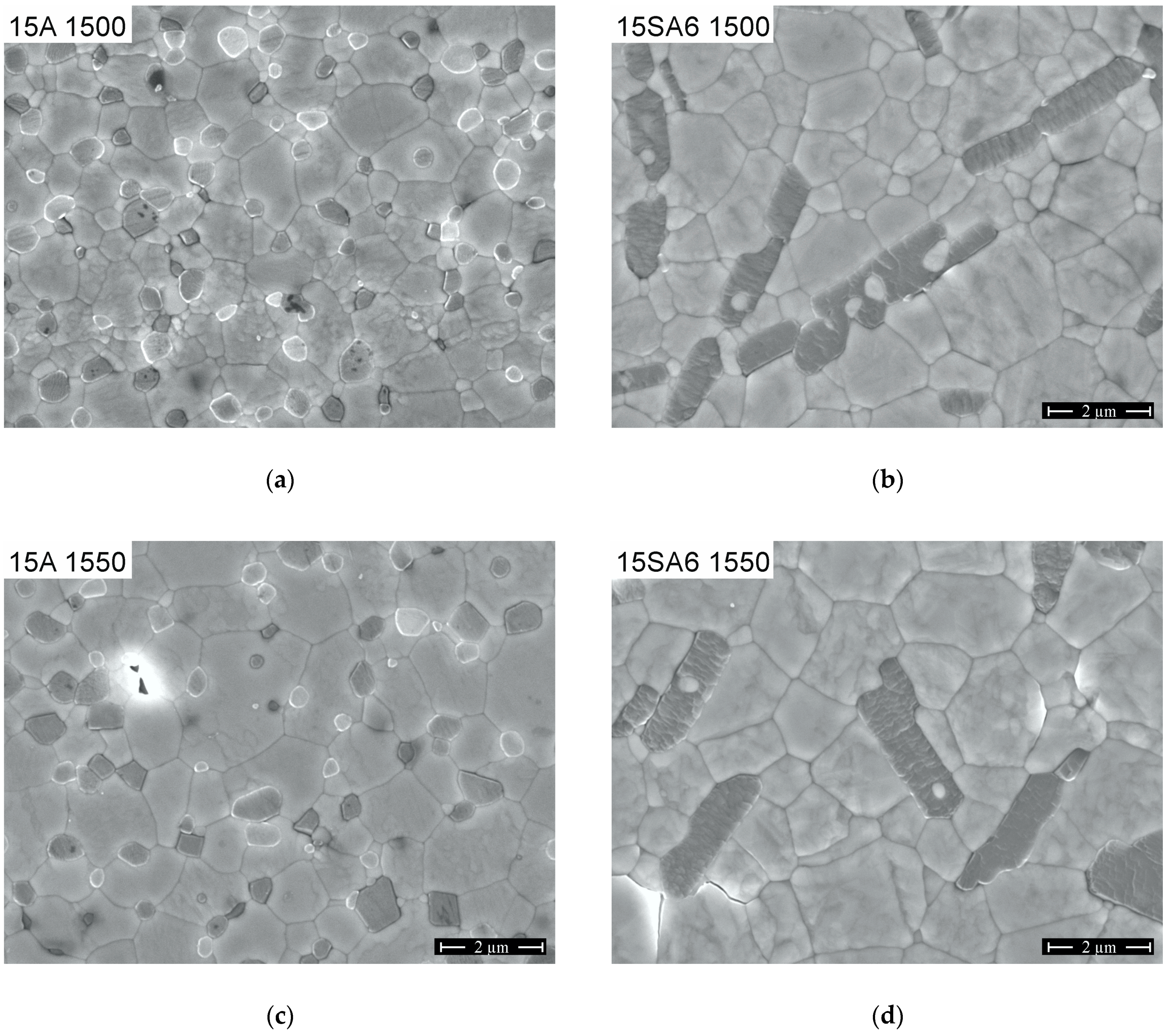
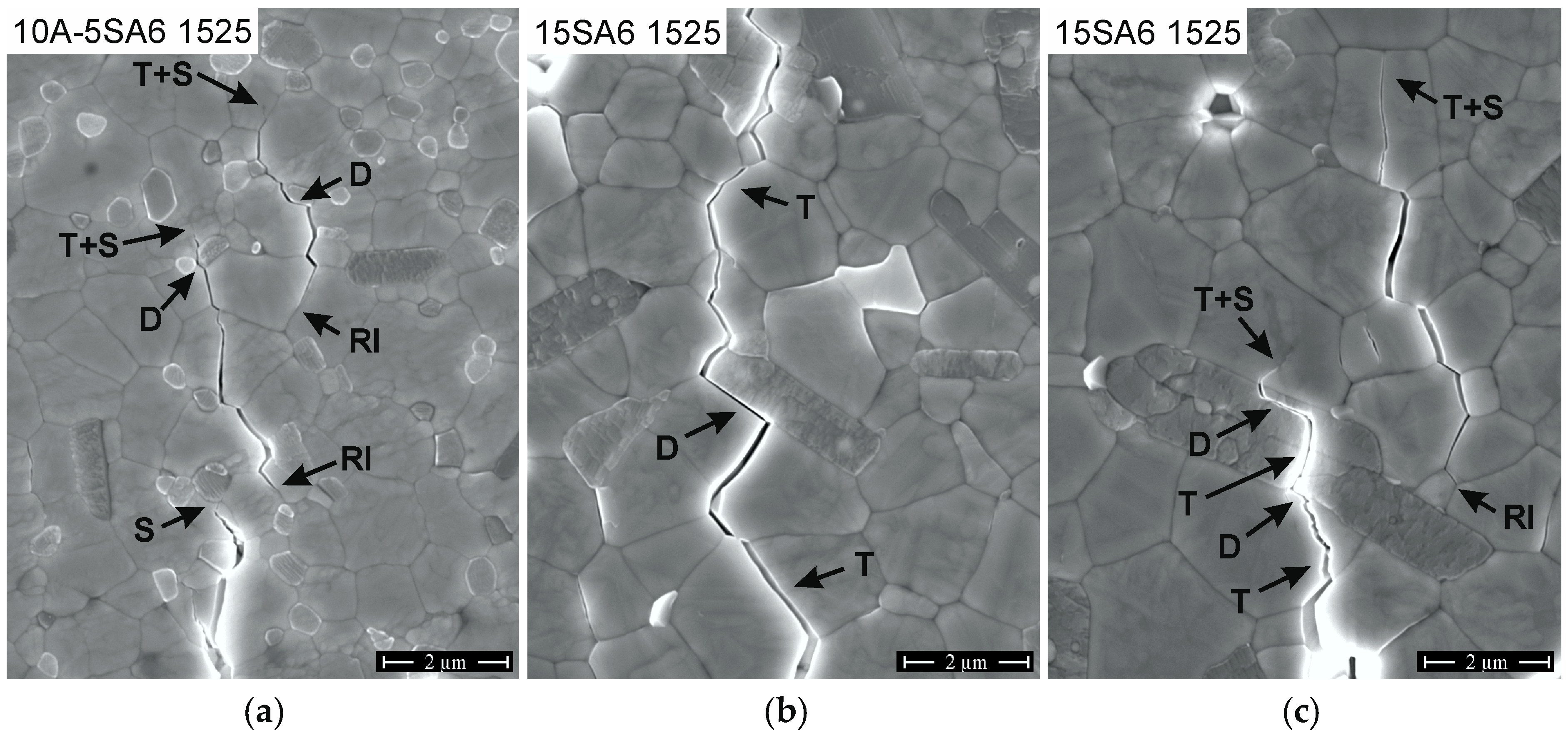
3.1. Microstructure and Crack Deflection
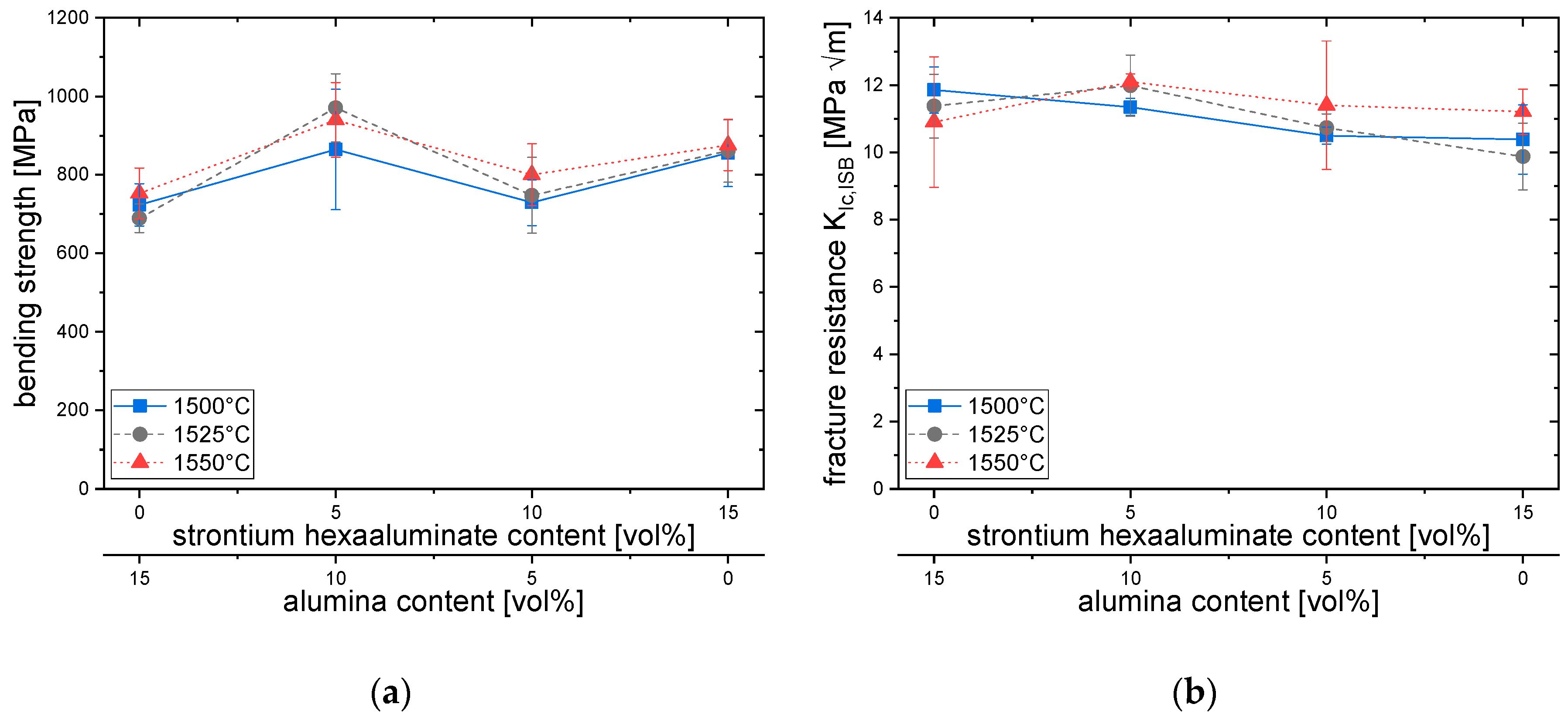
3.2. Density and Mechanical Properties
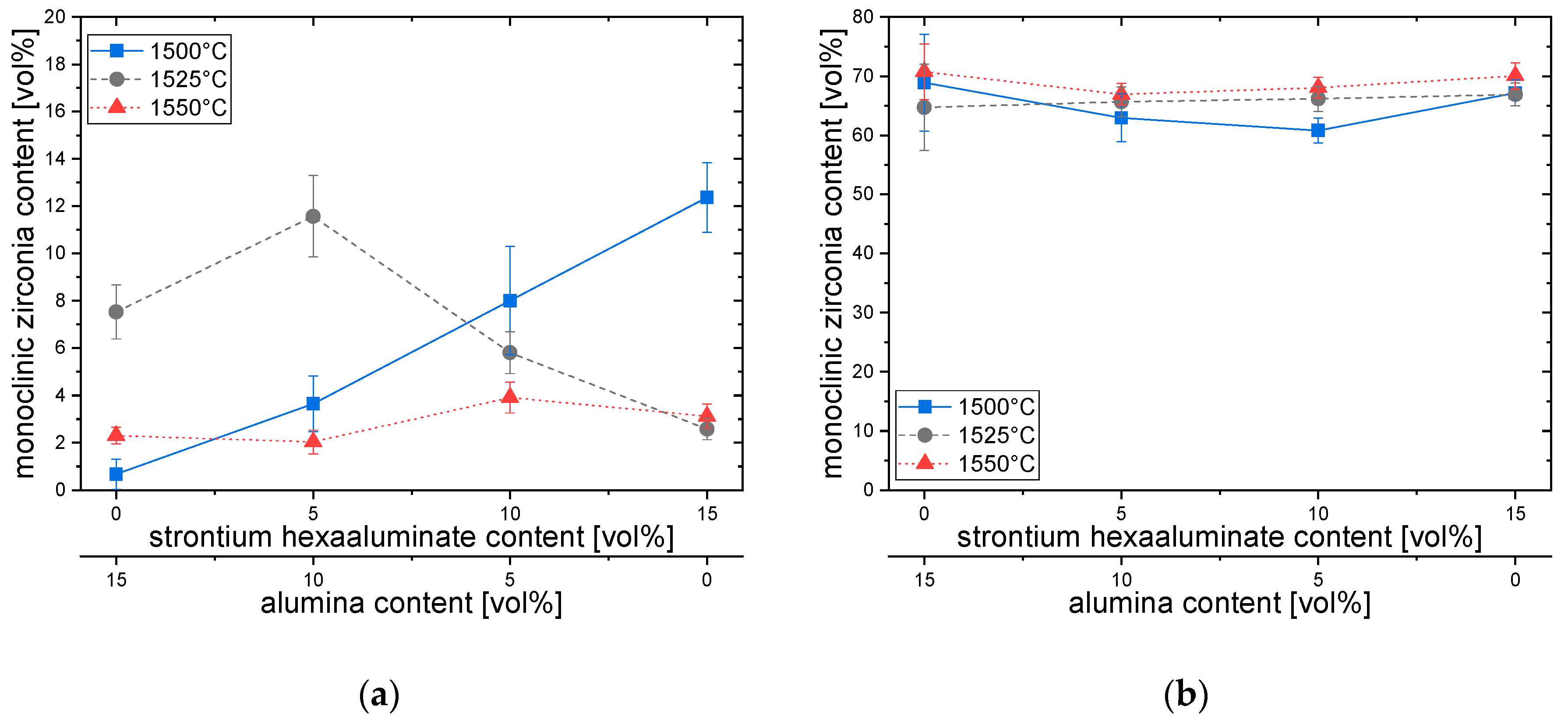
3.3. Phase Composition and Transformation Toughness
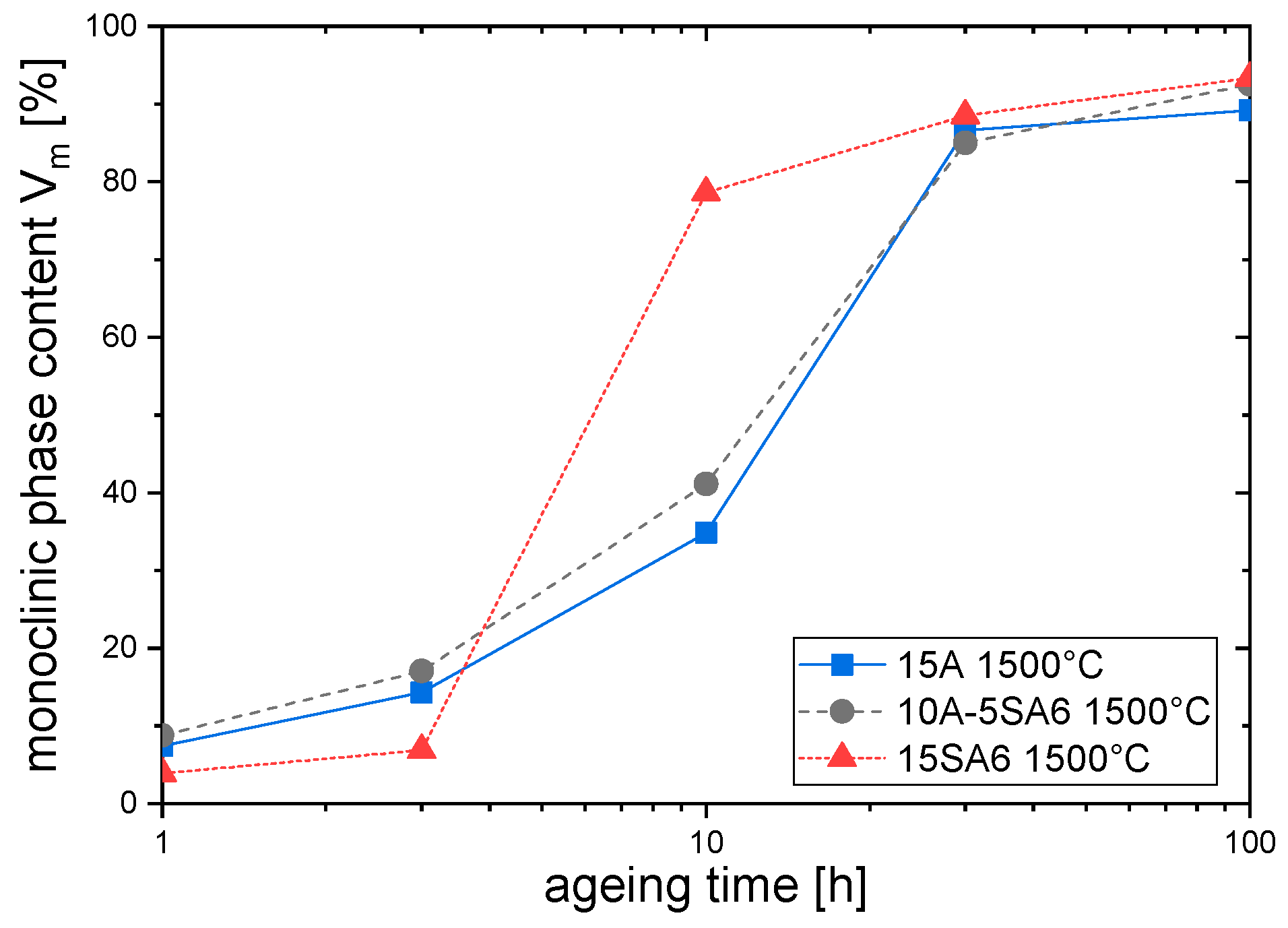
3.4. Low Temperature Degradation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hannink, R.H.J.; Kelly, P.M.; Muddle, B.C. Transformation Toughening in Zirconia-Containing Ceramics. J. Am. Ceram. Soc. 2000, 83, 461–487. [Google Scholar] [CrossRef]
- Li, P.; Chen, I.-W.; Penner-Hahn, J.E. Effect of Dopants on Zirconia Stabilization—An X-ray Absorption Study: I, Trivalent Dopants. J. Am. Ceram. Soc. 1994, 77, 118–128. [Google Scholar] [CrossRef]
- Li, P.; Chen, I.-W.; Penner-Hahn, J.E. Effect of Dopants on Zirconia Stabilization—An X-ray Absorption Study: II, Tetravalent Dopants. J. Am. Ceram. Soc. 1994, 77, 1281–1288. [Google Scholar] [CrossRef]
- Kelly, P.M.; Francis Rose, L.R. The martensitic transformation in ceramics—Its role in transformation toughening. Prog. Mater. Sci. 2002, 47, 463–557. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kuwajima, H.; Masaki, T. Phase change and mechanical properties of ZrO2-Y2O3 solid electrolyte after ageing. Solid State Ion. 1981, 3–4, 489–493. [Google Scholar] [CrossRef]
- Chevalier, J.; Cales, B.; Drouin, M. Low-Temperature Aging of Y-TZP Ceramics. J. Am. Ceram. Soc. 1999, 82, 2150–2154. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Deville, S. Low-Temperature Degradation of Zirconia and Implications for Biomedical Implants. Annu. Rev. Mater. Res. 2007, 37, 1–32. [Google Scholar] [CrossRef]
- Lin, J.-D.; Duh, J.-G. Correlation of mechanical properties and composition in tetragonal CeO2-Y2O3-ZrO2 ceramic system. Mater. Chem. Phys. 2002, 78, 246–252. [Google Scholar] [CrossRef]
- Lin, J.-D.; Duh, J.-G. Fracture toughness and hardness of ceria- and yttria-doped tetragonal zirconia ceramics. Mater. Chem. Phys. 2002, 78, 253–261. [Google Scholar] [CrossRef]
- Tsukuma, K.; Shimada, M. Strength, fracture toughness and Vickers hardness of CeO2-stabilized tetragonal ZrO2 polycrystals (Ce-TZP). J. Mater. Sci. 1985, 20, 1178–1184. [Google Scholar] [CrossRef]
- Tsukuma, K.; Ueda, K.; Shimada, M. Strength and Fracture Toughness of Isostatically Hot-Pressed Composites of Al2O3 and Y2O3-Partially-Stabilized ZrO2. J. Am. Ceram. Soc. 1985, 68, C4–C5. [Google Scholar] [CrossRef]
- Nawa, M.; Bamba, N.; Sekino, T.; Niihara, K. The effect of TiO2 addition on strengthening and toughening in intragranular type of 12Ce-TZP/Al2O3 nanocomposites. J. Eur. Ceram. Soc. 1998, 18, 209–219. [Google Scholar] [CrossRef]
- Benzaid, R.; Chevalier, J.; Saadaoui, M.; Fantozzi, G.; Nawa, M.; Diaz, L.A.; Torrecillas, R. Fracture toughness, strength and slow crack growth in a ceria stabilized zirconia-alumina nanocomposite for medical applications. Biomaterials 2008, 29, 3636–3641. [Google Scholar] [CrossRef] [PubMed]
- Touaiher, I.; Saâdaoui, M.; Chevalier, J.; Preiss, L.; Reveron, H. Fracture behavior of Ce-TZP/alumina/aluminate composites with different amounts of transformation toughening. Influence of the testing methods. J. Eur. Ceram. Soc. 2018, 38, 1778–1789. [Google Scholar] [CrossRef]
- Ross, I.; Rainforth, W.; McComb, D.; Scott, A.; Brydson, R. The role of trace additions of alumina to yttria–tetragonal zirconia polycrystals (Y–TZP). Scr. Mater. 2001, 45, 653–660. [Google Scholar] [CrossRef]
- Nogiwa-Valdez, A.A.; Rainforth, W.M.; Zeng, P.; Ross, I.M. Deceleration of hydrothermal degradation of 3Y-TZP by alumina and lanthana co-doping. Acta Biomater. 2013, 9, 6226–6235. [Google Scholar] [CrossRef] [PubMed]
- Kern, F.; Gadow, R. Alumina toughened zirconia from yttria coated powders. J. Eur. Ceram. Soc. 2012, 32, 3911–3918. [Google Scholar] [CrossRef]
- Swain, M.V.; Rose, L.R.F. Strength Limitations of Transformation-Toughened Zirconia Alloys. J. Am. Ceram. Soc. 1986, 69, 511–518. [Google Scholar] [CrossRef]
- Cutler, R.A.; Mayhew, R.J.; Prettyman, K.M.; Virkar, A.V. High-Toughness Ce-TZP/Al2O3 Ceramics with Improved Hardness and Strength. J. Am. Ceram. Soc. 1991, 74, 179–186. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sakamoto, W.; Yogo, T.; Fujii, T.; Hirano, S.-I. In Situ Formation of Ce-TZP/Ba Hexaaluminate Composites. J. Ceram. Soc. Jpn. 1999, 107, 814–819. [Google Scholar] [CrossRef]
- Tsai, J.-F.; Chon, U.; Ramachandran, N.; Shetty, D.K. Transformation Plasticity and Toughening in CeO2-Partially-Stabilized Zirconia-Alumina (Ce-TZP/Al2O3) Composites Doped with MnO. J. Am. Ceram. Soc. 1992, 75, 1229–1238. [Google Scholar] [CrossRef]
- Miura, M.; Hongoh, H.; Yogo, T.; Hirano, S.; Fujii, T. Formation of plate-like lanthanum-betha-Aluminate crystal in Ce-TZP matrix. J. Mater. Sci. 1994, 29, 262–268. [Google Scholar] [CrossRef]
- Tsukuma, K. Conversion from β-Ce2O3·11Al2O3 to α-Al2O3 in Tetragonal ZrO2 Matrix. J. Am. Ceram. Soc. 2000, 83, 3219–3221. [Google Scholar] [CrossRef]
- Heussner, K.-H.; Claussen, N. Yttria- and ceria-stabilized tetragonal zirconia polycrystals (Y-TZP, Ce-TZP) reinforced with Al2O3 platelets. J. Eur. Ceram. Soc. 1989, 5, 193–200. [Google Scholar] [CrossRef]
- Kern, F.; Gommeringer, A. Reinforcement Mechanisms in Yttria-Ceria-Co-Stabilized Zirconia-Alumina-Strontium Hexaaluminate Composite Ceramics. J. Ceram. Sci. Technol. 2017, 9, 93–98. [Google Scholar] [CrossRef]
- Yuan, Z.X.; Vleugels, J.; van der Biest, O. Preparation of Y2O3-coated ZrO2 powder by suspension drying. J. Mater. Sci. Lett. 2000, 19, 359–361. [Google Scholar] [CrossRef]
- Kern, F.; Gadow, R. Tough to brittle transition with increasing grain size in 3Yb-TZP ceramics manufactured from stabilizer coated nanopowder. J. Ceram. Soc. Jpn. 2016, 124, 1083–1089. [Google Scholar] [CrossRef]
- Chantikul, P.; Anstis, G.R.; Lawn, B.R.; Marshall, D.B. A Critical Evaluation of Indentation Techniques for Measuring Fracture Toughness: II, Strength Method. J. Am. Ceram. Soc. 1981, 64, 539–543. [Google Scholar] [CrossRef]
- Toraya, H.; Yoshimura, M.; Somiya, S. Calibration Curve for Quantitative Analysis of the Monoclinic-Tetragonal ZrO2 System by X-Ray Diffraction. J. Am. Ceram. Soc. 1984, 67, C119–C121. [Google Scholar] [CrossRef]
- Kosmać, T.; Wagner, R.; Claussen, N. X-Ray Determination of Transformation Depths in Ceramics Containing Tetragonal ZrO2. J. Am. Ceram. Soc. 1981, 64, C-72–C-73. [Google Scholar] [CrossRef]
- McMeeking, R.M.; Evans, A.G. Mechanics of Transformation-Toughening in Brittle Materials. J. Am. Ceram. Soc. 1982, 65, 242–246. [Google Scholar] [CrossRef]
- Evans, A.G. Perspective on the Development of High-Toughness Ceramics. J. Am. Ceram. Soc. 1990, 73, 187–206. [Google Scholar] [CrossRef]
- Wei, C.; Gremillard, L. Towards the prediction of hydrothermal ageing of 3Y-TZP bioceramics from processing parameters. Acta Mater. 2018, 144, 245–256. [Google Scholar] [CrossRef]
- Swain, M.V. Limitation of Maximum Strength of Zirconia-Toughened Ceramics by Transformation Toughening Increment. J. Am. Ceram. Soc. 1985, 68, C-97–C-99. [Google Scholar] [CrossRef]
- DeAza, A.H.; Chevalier, J.; Fantozzi, G.; Schehl, M.; Torrecillas, R. Slow-Crack-Growth Behavior of Zirconia-Toughened Alumina Ceramics Processed by Different Methods. J. Am. Ceram. Soc. 2003, 86, 115–120. [Google Scholar] [CrossRef]
- Lange, F.F. Transformation toughening: Part 1 Size effects associated with the thermodynamics of constrained transformations. J. Mater. Sci. 1982, 17, 225–234. [Google Scholar] [CrossRef]
- Evans, A.G.; He, M.Y.; Hutchinson, J.W. Interface Debonding and Fiber Cracking in Brittle Matrix Composites. J. Am. Ceram. Soc. 1989, 72, 2300–2303. [Google Scholar] [CrossRef]
- Chen, P.-L.; Chen, I.-W. In-Situ Alumina/Aluminate Platelet Composites. J. Am. Ceram. Soc. 1992, 75, 2610–2612. [Google Scholar] [CrossRef]


| Composition | Abbreviation |
|---|---|
| 1 mol% Y2O3-6 mol% CeO2-TZP - 15 vol% Al2O3-0 vol%SA6 | 15A |
| 1 mol% Y2O3-6 mol% CeO2-TZP - 10 vol% Al2O3-5 vol%SA6 | 10A-5SA6 |
| 1 mol% Y2O3-6 mol% CeO2-TZP - 5 vol% Al2O3-10 vol%SA6 | 5A-10SA6 |
| 1 mol% Y2O3-6 mol% CeO2-TZP - 0 vol% Al2O3-15 vol%SA6 | 15SA6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gommeringer, A.; Nölle, L.; Kern, F.; Gadow, R. Yttria Ceria Co-Stabilized Zirconia Reinforced with Alumina and Strontium Hexaaluminate. Appl. Sci. 2019, 9, 729. https://doi.org/10.3390/app9040729
Gommeringer A, Nölle L, Kern F, Gadow R. Yttria Ceria Co-Stabilized Zirconia Reinforced with Alumina and Strontium Hexaaluminate. Applied Sciences. 2019; 9(4):729. https://doi.org/10.3390/app9040729
Chicago/Turabian StyleGommeringer, Andrea, Lennart Nölle, Frank Kern, and Rainer Gadow. 2019. "Yttria Ceria Co-Stabilized Zirconia Reinforced with Alumina and Strontium Hexaaluminate" Applied Sciences 9, no. 4: 729. https://doi.org/10.3390/app9040729
APA StyleGommeringer, A., Nölle, L., Kern, F., & Gadow, R. (2019). Yttria Ceria Co-Stabilized Zirconia Reinforced with Alumina and Strontium Hexaaluminate. Applied Sciences, 9(4), 729. https://doi.org/10.3390/app9040729






