Ultrafiltration/Granulated Active Carbon-Biofilter: Efficient Removal of a Broad Range of Micropollutants
Abstract
1. Introduction
2. Materials and Methods
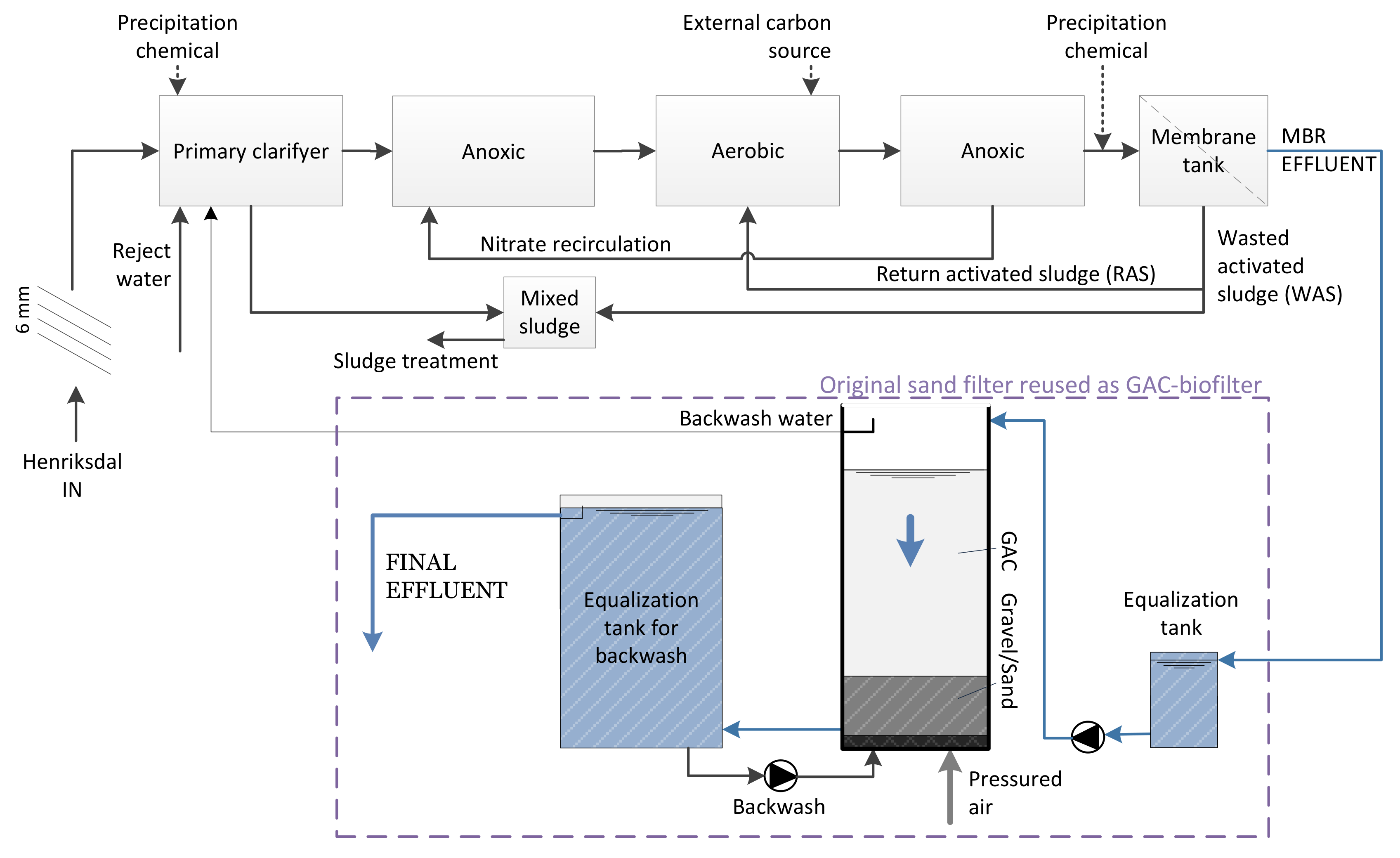
2.1. Pilot Characteristics
2.2. Sampling and Analysing
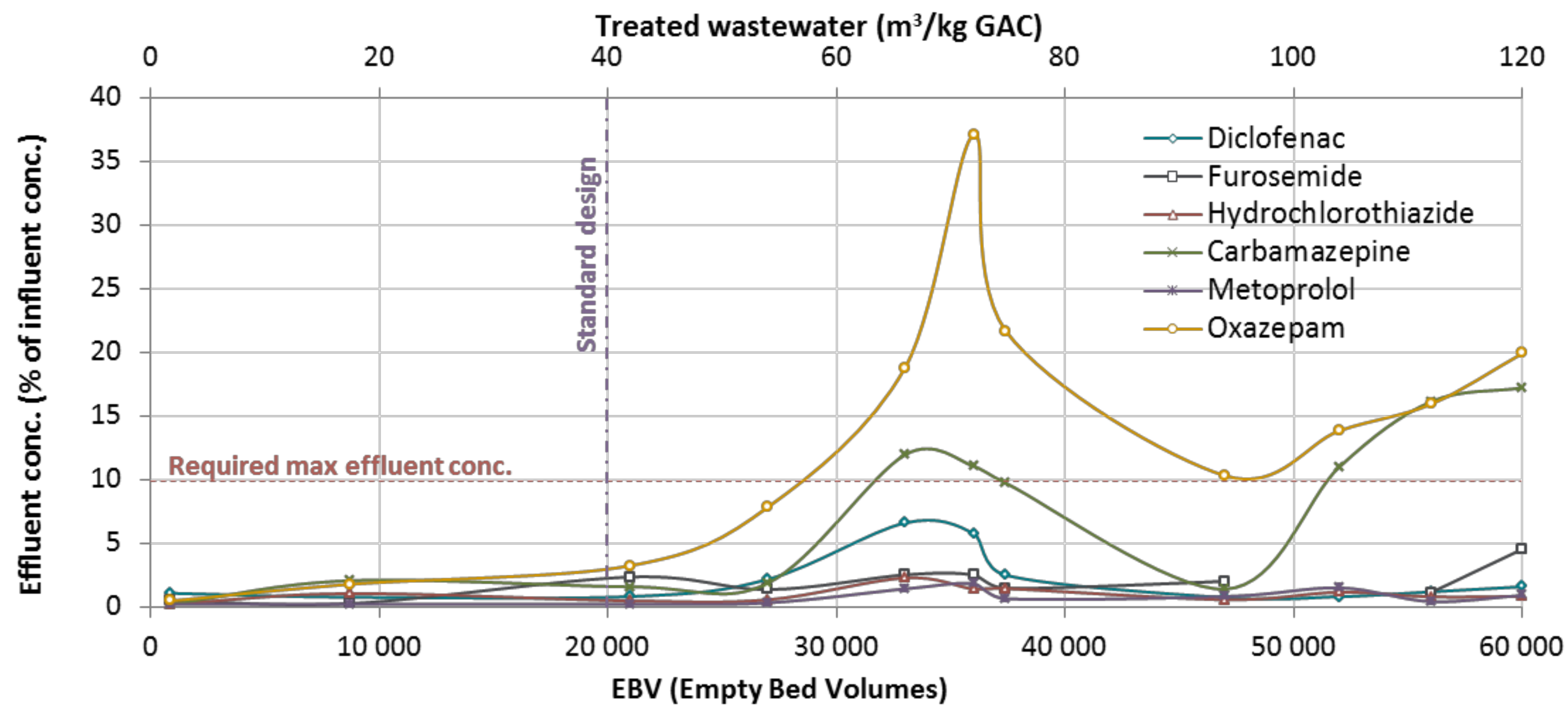
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brodin, T.; Fick, J.; Jonsson, M.; Klaminder, J. Dilute Concentrations of a Psychiatric Drug Alter Behavior of Fish from Natural Populations. Science 2013, 339, 814–815. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Fick, J.; Lindberg, R.H.; Kaj, L.; Brorström-Lundén, E. Results from the Swedish National Screening Programme 2010, Subreport 3, Pharmaceuticals; B2014; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2011. [Google Scholar]
- Fick, J.; Lindberg, R.H.; Schwesig, D.; Gawlik, B.M. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.I.; Lambrianides, A.; Schneider, M.; Kümmerer, K.; Fatta-Kassinos, D. Environmental side effects of pharmaceutical cocktails: What we know and what we should know. J. Hazard. Mater. 2014, 279, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Rempharmawater. Ecotoxicological Assessments and Removal Technologies for Pharmaceuticals in Wastewaters. Project Reference: EVK1-CT-2000-00048. 2003. Available online: http://www.unina.it (accessed on 22 August 2018).
- POSEIDON. Assessment of Technologies for the Removal of Pharmaceuticals and Personal Care Products in Sewage and Drinking Water Facilities to Improve the Indirect Potable Water Reuse. Project Reference: EVK1-CT-2000-00047. 2004. Available online: http://www.eu-poseidon.com (accessed on 22 August 2018).
- RiSKWa. Risk Management of Emerging Compounds and Pathogens in the Water Cycle; Bundesministerium für Bildung und Forschung (BMBF): Bonn, Germany, 2013; Available online: http://www.bmbf.riskwa.de (accessed on 22 August 2018).
- Abegglen, C.; Siegrist, H. Mikroverunreinigungen aus Kommunalem Abwasser. Verfahren zur Weitergehenden Elimination auf Kläranlagen (Micropollutants from Municipal Wastewater. Method for Further Elimination on Sewage Treatment Plants); Umwelt-Wissen Nr. 1214: 210; Bundesamt für Umwelt: Bern, Germany, 2012. [Google Scholar]
- Arge Spurenstoffe NRW. Teilprojekt 6. Elimination von Arzneimitteln und Organischen Spurenstoffen: Entwicklung von Konzeptionen und Innovativen, Kostengünstigen Reinigungsverfahren (Elimination of Pharmaceuticals and Organic Trace Substances: Development of Concepts and Innovative, Cost-Effective Cleaning Methods)–Abschlussbericht zur Phase 2; Arge Spurenstoffe NRW: Bochum, Germany, 2013. [Google Scholar]
- Baresel, C.; Ek, M.; Harding, M.; Bergström, R. Treatment of Biologically Treated Wastewater with Ozone or Activated Carbon; B2203; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2014. [Google Scholar]
- Baresel, C.; Dahlgren, L.; Nikolic, A.; de Kerchove, A.; Almemark, M.; Ek, M.; Harding, M.; Ottosson, E.; Karlsson, J.; Yang, J. Reuse of Treated Wastewater for Nonpotable Use (ReUse)–Final Report; B2219; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2015. [Google Scholar]
- Baresel, C.; Malmborg, J.; Ek, M.; Sehlén, R. Removal of pharmaceutical residues using ozonation as intermediate process step at Linköping WWTP, Sweden. Water Sci. Technol. 2016, 73, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Baresel, C.; Ek, M.; Ejhed, H.; Allard, A.S.; Magnér, J.; Dahlgren, L.; Westling, K.; Wahlberg, C.; Fortkamp, U.; Søhr, S. Handbook for the Treatment of Micropollutants at Sewage Treatment Plant–Planning and Installing Treatment Techniques for Pharmaceutical residues and Other Micropollutants; Final Report SystemLäk Project; B2288; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2017. [Google Scholar]
- Ek, M.; Baresel, C.; Magnér, J.; Bergström, R.; Harding, M. Activated carbon for the removal of pharmaceutical residues from treated wastewater. Water Sci. Technol. 2014, 69, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Wahlberg, C.; Björlenius, B.; Paxéus, N. Läkemedelsrester i Stockholms Vattenmiljö–Förekomst, Förebyggande Åtgärder och Rening av Avloppsvatten (Pharmaceutical Residues in Stockholm’s Aquatic Environment–Prevention, Prevention and Treatment of Sewage); Stockholm Vatten AB: Stockholm, Sweden, 2010; ISBN 978-91-633-6642-0. [Google Scholar]
- Altmann, J.; Ruhl, A.S.; Zietzschmann, F.; Jekel, M. Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment. Water Res. 2014, 55, 185–193. [Google Scholar] [CrossRef]
- Abegglen, C.; Escher, B.; Hollender, J.; Siegrist, H.; von Gunten, U.; Zimmermann, S.; Häner, A.; Ort, C.; Schärer, M. Ozonung von gereinigtem Abwasser zur Elimination von organischen Spurenstoffen. Grosstechnischer Pilotversuch Regensdorf (Schweiz) (Ozonation of purified wastewater to eliminate organic trace substances. Large-scale pilot test Regensdorf (Switzerland)). Korrespondenz Abwasser Abfall 2010, 57, 155–160. [Google Scholar]
- Gerrity, D.; Snyder, S. Review of Ozone for Water Reuse Applications: Toxicity, Regulations, and Trace Organic Contaminant Oxidation. Ozone Sci. Eng. 2011, 33, 253–266. [Google Scholar] [CrossRef]
- Magdeburg, A.; Stalter, D.; Oehlmann, J. Whole effluent toxicity assessment at a wastewater treatment plant upgraded with a full-scale post-ozonation using aquatic key species. Chemosphere 2012, 88. [Google Scholar] [CrossRef] [PubMed]
- Magdeburg, A.; Stalter, D.; Schlüsener, M.; Ternes, T.; Oehlmann, J. Evaluating the efficiency of advanced wastewater treatment: Target analysis of organic contaminants and (geno-)toxicity assessment tell a different story. Water Res. 2014, 50, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Maus, C.; Herbst, H.; Ante, S.; Becker, H.-P.; Glathe, W.; Bärgers, A.; Türk, J. Guidance on the interpretation and design of ozonation plants for micropollutants elimination. Korrespondenz Abwasser Abfall 2014, 61, 998–1006. [Google Scholar]
- Reungoat, J.; Escher, B.I.; Macova, M.; Keller, J. Biofiltration of wastewater treatment plant effluent: Effective removal of pharmaceuticals and personal care products and reduction of toxicity. Water Res. 2011, 45, 2751–2762. [Google Scholar] [CrossRef] [PubMed]
- Stalter, D.; Magdeburg, A.; Weil, M.; Knacker, T.; Oehlmann, J. Toxication or detoxication? In vivo toxicity assessment of ozonation as advanced wastewater treatment with the rainbow trout. Water Res. 2010, 44, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Stalter, D.; Magdeburg, A.; Oehlmann, J. Comparative toxicity assessment of ozone and activated carbon treated sewage effluents using an in vivo test battery. Water Res. 2010, 44, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Stalter, D.; Magdeburg, A.; Wagner, M.; Oehlmann, J. Ozonation and activated carbon treatment of sewage effluents: Removal of endocrine activity and cytotoxicity. Water Res. 2011, 45, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Wert, E.C.; Rosario-Ortiz, F.L.; Drury, D.D.; Snyder, S.A. Formation of oxidation byproducts from ozonation of wastewater. Water Res. 2007, 41, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Alt, K.; Mauritz, A. Projekt zur Teilstrombehandlung mit Pulveraktivkohle im Klärwerk Mannheim (Project for sidestream treatment with powdered activated carbon in the sewage treatment plant Mannheim). Korrespondenz Abwasser Abfall 2010, 57, 161. [Google Scholar]
- Boehler, M.; Zwickenpflug, B.; Hollender, J.; Ternes, T.; Joss, A.; Siegrist, H. Removal of micropollutants in municipal wastewater treatment plants by powderactivated carbon. Water Sci. Technol. 2012, 66, 2115. [Google Scholar] [CrossRef] [PubMed]
- Clausen, K.; Lübken, M.; Pehl, B.; Bendt, T.; Wichern, M. Einsatz reaktivierter Aktivkohle von Wasserwerken zur Spurenstoffelimination in kommunalen Kläranlagen am Beispiel Düsseldorf (Use of reactivated activated carbon from water works for the elimination of trace substances in municipal sewage treatment plants using the example of Düsseldorf). Korrespondenz Abwasser Abfall 2014, 61, 1007–1012. [Google Scholar]
- Grover, D.P.; Zhou, J.L.; Frickers, P.E.; Readman, J.W. Improved removal of estrogenic and pharmaceutical compounds in sewage effluent by full scale granular activated carbon: Impact on receiving river water. J. Hazard. Mater. 2011, 185, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Kovalova, L.; Siegrist, H.; von Gunten, U.; Eugster, J.; Hagenbuch, M.; Wittmer, A.; Moser, R.; McArdell, C.S. Elimination of Micropollutants during Post-Treatment of Hospital Wastewater with Powdered Activated Carbon, Ozone, and UV. Environ. Sci. Technol. 2013, 47, 7899–7908. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Metzger, S.; Tjoeng, I.O.; Rößler, A.; Schwentner, G.; Rölle, R. Kosten der Pulveraktivkohleanwendung zur Spurenstoffelimination am Beispiel ausgeführter und in Bau befindlicher Anlagen (Cost of powdered activated carbon application for the elimination of trace substances using the example of selected plants under construction.). Korrespondenz Abwasser Abfall 2014, 61, 1029–1037. [Google Scholar]
- Baresel, C.; Ek, M.; Harding, M.; Magnér, J.; Allard, A.S.; Karlsson, J. Complementary Tests for a Resource Efficient Advanced Sewage Treatment; Subreport SystemLäk Project; B2287; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2017. [Google Scholar]
- Barillon, B.; Ruel, S.M.; Langlais, C.; Lazarova, V. Energy efficiency in membrane bioreactors. Water Sci Technol. 2013, 67, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Ioannou-Ttofa, L.; Foteinis, S.; Chatzisymeon, E.; Fatta-Kassinos, D. The environmental footprint of a membrane bioreactor treatment process through Life Cycle Analysis. Sci. Total Environ. 2016, 568, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Larrea, A.; Rambor, A.; Fabiyi, M. Ten years of industrial and municipal membrane bioreactor (MBR) systems–Lessons from the field. Water Sci. Technol. 2014, 70, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Chae, S.-R.; Shin, H.-S.; Yang, F.; Zhou, Z. Recent Advances in Membrane Bioreactors: Configuration Development, Pollutant Elimination, and Sludge Reduction. Environ. Eng. Sci. 2012, 29, 139–160. [Google Scholar] [CrossRef]
- Pinnekamp, J. Membrantechnik für die Abwasserreinigung (Membrane Technology for Wastewater Treatment). In Siedlungswasser- und Siedlungsabfallwirtschaft Nordrhein-Westfalen; Aktual, A., Ed.; FiW-Verl: Aachen, Germany, 2006. [Google Scholar]
- Baresel, C.; Westling, K.; Samuelsson, O.; Andersson, S.; Royen, H.; Andersson, S.; Dahlén, N. Membrane Bioreactor Processes to Meet Todays and Future Municipal Sewage Treatment Requirements? Int. J. Water Wastewater Treat. 2017, 3. [Google Scholar] [CrossRef]
- Lipp, P.; Kreißel, K.; Meuler, S.; Bischof, F.; Tiehm, A. Influencing parameters for the operation of an MBR with respect to the removal of persistent organic pollutants. Desalin. Water Treat. 2009, 6, 102–107. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Sipma, J.; Osuna, B.; Collado, N.; Monclús, H.; Ferrero, G.; Comas, J.; Rodriguez-Roda, I. Comparison of removal of pharmaceuticals in MBR and activated sludge systems. Desalination 2010, 250, 653–659. [Google Scholar] [CrossRef]
- Ek, M.; Bergström, R.; Magnér, J.; Harding, M.; Baresel, C. Aktivt kol för Avlägsnande av Läkemedelsrester ur Behandlat Avloppsvatten (Activated Carbon for Removal of Pharmaceutical Residues from Treated Wastewater); B2089; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2013. [Google Scholar]
- Ek, M.; Bergström, R.; Baresel, C. Avskiljning av Läkemedelsrester Med Granulerat Aktivt kol–Försök vid Himmerfjärdsverket (Removal of Pharmaceutical Residues with Granulated Activated Carbon); U4492; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2013. [Google Scholar]
- Magnusson, K.; Jörundsdóttir, H.; Norén, F.; Lloyd, H.; Talvitie, J. Microlitter in Sewage Treatment Systems: A Nordic Perspective on Waste Water Treatment Plants as Pathways for Microscopic Anthropogenic Particles to Marine Systems; TemaNord, Nordic Council of Ministers: Copenhagen, Denmark, 2016.
- Park, J.; Yamashita, N.; Park, C.; Shimono, T.; Takeuchi, D.M.; Tanaka, H. Removal characteristics of pharmaceuticals and personal care products: Comparison between membrane bioreactor and various biological treatment processes. Chemosphere 2017, 179, 347–358. [Google Scholar] [CrossRef]
- El Gamal, M.; Mousa, H.A.; El-Naas, M.H.; Zacharia, R.; Judd, S. Bio-regeneration of activated carbon: A comprehensive review. Sep. Purif. Technol. 2018, 197, 345–359. [Google Scholar] [CrossRef]
- Sbardella, L.; Comas, J.; Fenu, A.; Rodriguez-Roda, I.; Weemaes, M. Advanced biological activated carbon filter for removing pharmaceutically active compounds from treated wastewater. Sci. Total Environ. 2018, 636, 519–529. [Google Scholar] [CrossRef]
- Baresel, C.; Cousins, A.P.; Hörsing, M.; Ek, M.; Ejhed, H.; Allard, A.S.; Magnér, J.; Westling, K.; Wahlberg, C.; Fortkamp, U.; et al. Pharmaceutical Residues and Other Emerging Substances in the Effluent of Sewage Treatment Plants–Review on Concentrations, Quantification, Behaviour, and Removal Options; B2226; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2015. [Google Scholar]
| Total Removed | Analysed in GAC | Adsorbed | |
|---|---|---|---|
| mg/kg GAC | mg/kg GAC | % | |
| Citalopram | 29.2 | 1.09 | 3.7 |
| Diclofenac | 67.9 | 0.13 | 0.2 |
| Furosemide | 49.2 | 0.57 | 1.2 |
| Hydrochlorothiazide | 143.4 | 3.97 | 2.8 |
| Ibuprofen | 8.1 | 0.01 | 0.1 |
| Carbamazepine | 41.2 | 13.1 | 31.8 |
| Metoprolol | 82.5 | 3.15 | 3.8 |
| Oxazepam | 54.3 | 7.03 | 12.9 |
| Propranolol | 6.7 | 0.87 | 12.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baresel, C.; Harding, M.; Fång, J. Ultrafiltration/Granulated Active Carbon-Biofilter: Efficient Removal of a Broad Range of Micropollutants. Appl. Sci. 2019, 9, 710. https://doi.org/10.3390/app9040710
Baresel C, Harding M, Fång J. Ultrafiltration/Granulated Active Carbon-Biofilter: Efficient Removal of a Broad Range of Micropollutants. Applied Sciences. 2019; 9(4):710. https://doi.org/10.3390/app9040710
Chicago/Turabian StyleBaresel, Christian, Mila Harding, and Johan Fång. 2019. "Ultrafiltration/Granulated Active Carbon-Biofilter: Efficient Removal of a Broad Range of Micropollutants" Applied Sciences 9, no. 4: 710. https://doi.org/10.3390/app9040710
APA StyleBaresel, C., Harding, M., & Fång, J. (2019). Ultrafiltration/Granulated Active Carbon-Biofilter: Efficient Removal of a Broad Range of Micropollutants. Applied Sciences, 9(4), 710. https://doi.org/10.3390/app9040710





