Quantitative Occurrence of Antibiotic Resistance Genes among Bacterial Populations from Wastewater Treatment Plants Using Activated Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Physicochemical Parameters and Number of Antibiotic-Resistant Bacteria
2.3. Bacterial Inoculation and DNA Extraction
2.4. Quantification Analysis of ARGs
2.4.1. Preparation of Standard Curves
2.4.2. qPCR Reaction Conditions
2.5. Data Analyses
3. Results
3.1. Concentration of Physicochemical Parameters and Number of Antibiotic-Resistant Bacteria
3.2. Quantitative Analysis of Antibiotic Resistance Genes and Taxonomic Genes
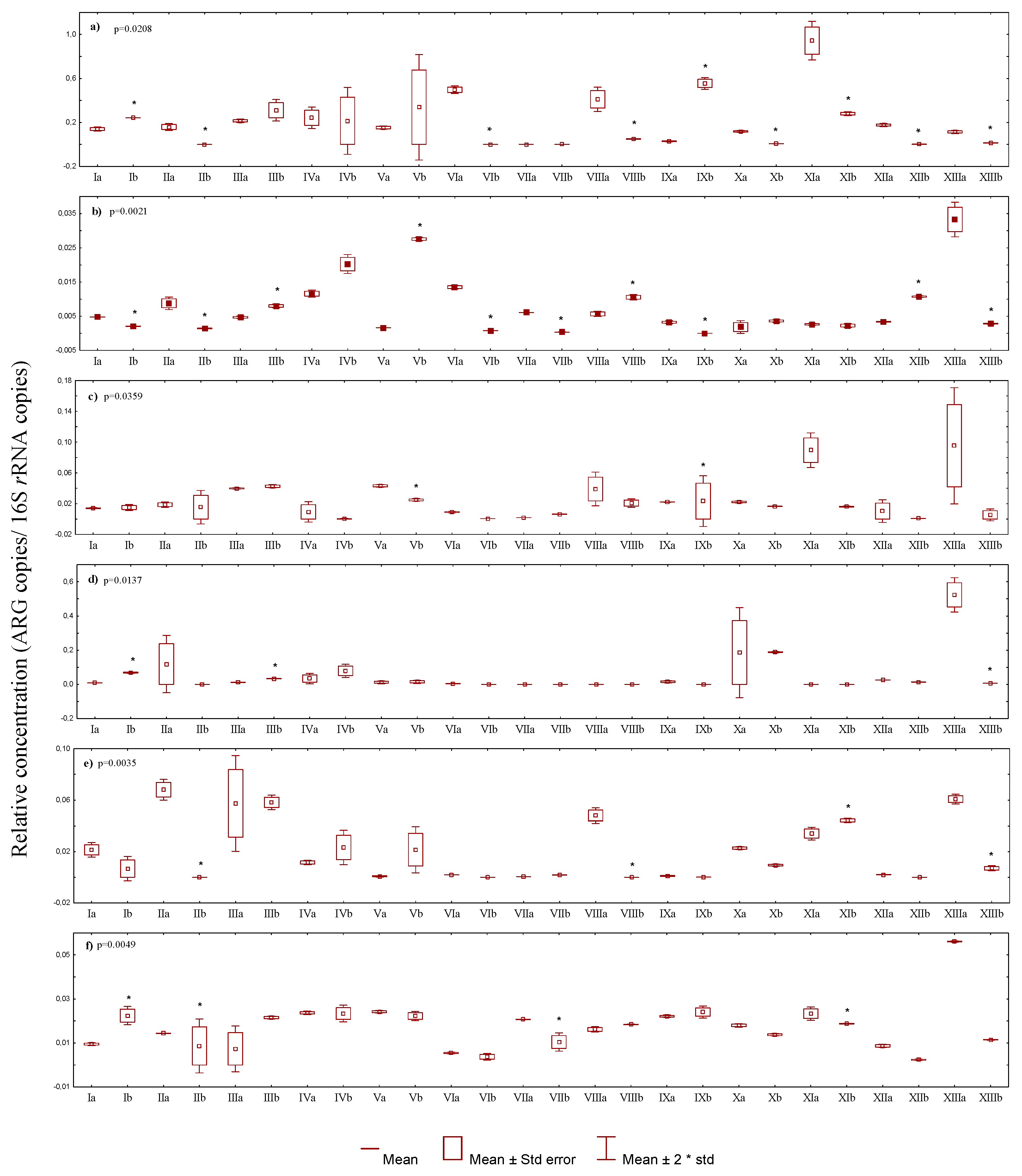
3.2.1. Quantitative Pccurrence of Antibiotic Resistance Genes among the Total Number of Antibiotic-Resistant Bacteria
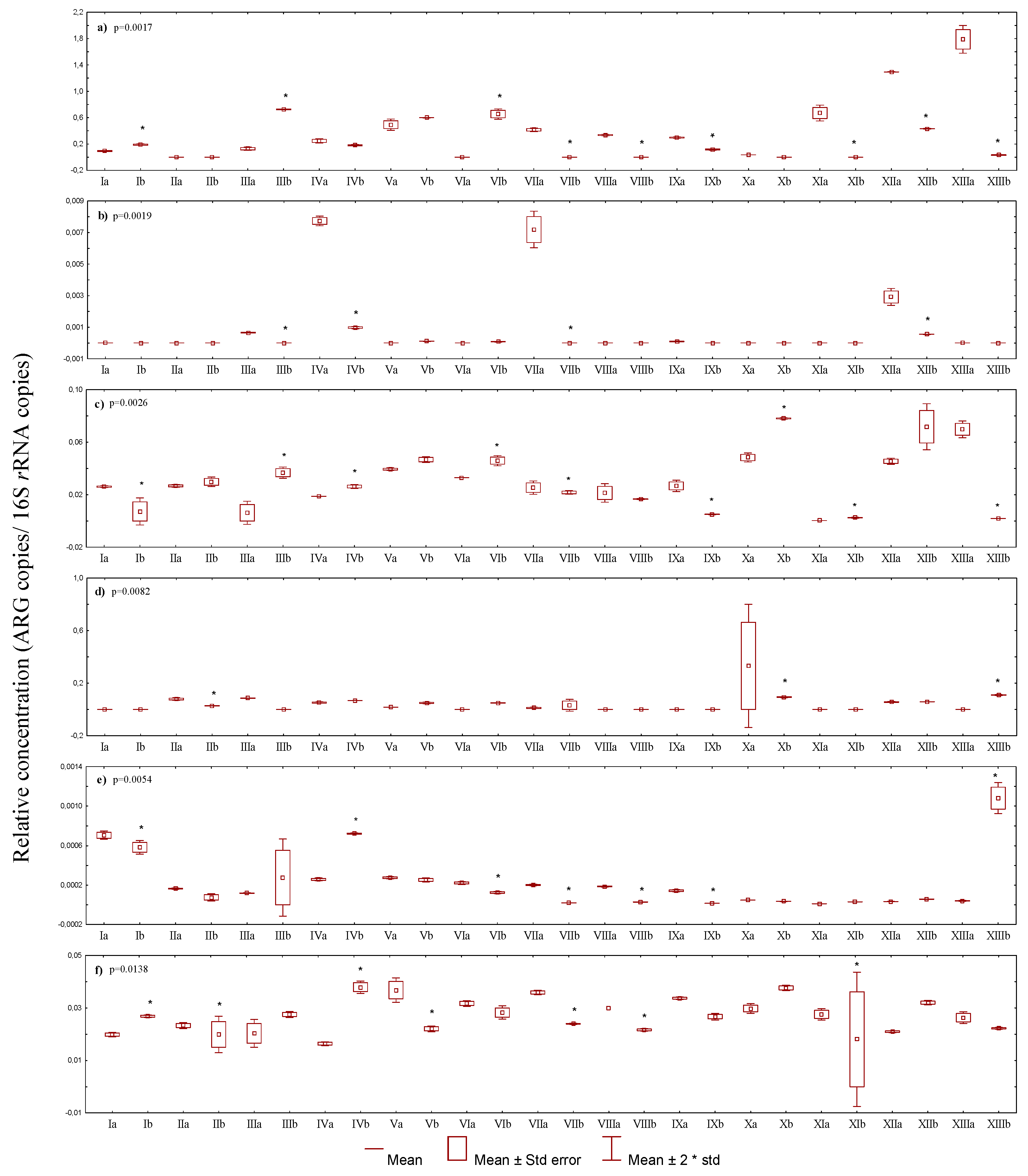
3.2.2. Quantitative Occurrence of Antibiotic Resistance Genes among Antibiotic-Resistant E. coli
3.2.3. Quantitative Occurrence of uidA Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leonard, A.F.C.; Zhang, L.H.; Balfour, A.; Garside, R.; Gaze, W.H. Human recreational exposure to antibiotic resistant bacteria in coastal bathing waters. Environ. Int. 2015, 82, 92–100. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance 2014. Available online: http://www.who.int/en/ (accessed on 6 February 2017).
- Martinez, J.L.; Coque, T.M.; Baquero, F. What is a resistance gene? Ranking risk in resistomes. Nat. Rev. Microbiol. 2015, 13, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Hembach, N.; Schmid, F.; Alexander, J.; Hiller, C.; Rogall, E.T.; Schwartz, T. Occurrence of the mcr-1 Colistin Resistance Gene and other Clinically Relevant Antibiotic Resistance Genes in Microbial Populations at Different Municipal Wastewater Treatment Plants in Germany. Front. Microbiol. 2017, 8, 1282. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Bollmann, A.; Seitz, W.; Schwartz, T. Microbiological characterization of aquatic microbiomes targeting taxonomical marker genes and antibiotic resistance genes of opportunistic bacteria. Sci. Total Environ. 2015, 512, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Czekalski, N.; Berthold, T.; Caucci, S.; Egli, A.; Burgmann, H. Increased levels of multiresistant bacteria and resistance genes after wastewater treatment and their dissemination into Lake Geneva, Switzerland. Front. Microbiol. 2012, 3, 106. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Hsu, B.M.; Ji, W.T.; Chen, J.S.; Hsu, T.K.; Ji, D.D.; Tseng, S.F.; Chiu, Y.C.; Kao, P.M.; Huang, Y.L. Antibiotic resistance pattern and gene expression of non-typhoid Salmonella in riversheds. Environ. Sci. Pollut. Res. 2015, 22, 7843–7850. [Google Scholar] [CrossRef]
- Gillings, M.R.; Gaze, W.H.; Pruden, A.; Smalla, K.; Tiedje, J.M.; Zhu, Y.G. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J. 2015, 9, 1269–1279. [Google Scholar] [CrossRef]
- Martinez, J.L. Antibiotics and antibiotic resistance genes in natural environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef]
- Salyers, A.; Shoemaker, N.B. Reservoirs of antibiotic resistance genes. Anim. Biotechnol. 2006, 17, 137–146. [Google Scholar] [CrossRef]
- Harnisz, M.; Korzeniewska, E.; Gołaś, I. The impact of a freshwater fish farm on the community of tetracycline-resistant bacteria and the structure of tetracycline resistance genes in river water. Chemosphere 2015, 128, 134–141. [Google Scholar] [CrossRef]
- Kotlarska, E.; Łuczkiewicz, A.; Pisowacka, M.; Burzyński, A. Antibiotic resistance and prevalence of class 1 and 2 integrons in Escherichia coli isolated from two wastewater treatment plants, and their receiving waters (Gulf of Gdansk, Baltic Sea, Poland). Environ. Sci. Pollut. Res. 2015, 22, 2018–2030. [Google Scholar] [CrossRef] [PubMed]
- Pruden, A. Balancing Water Sustainability and Public Health Goals in the Face of Growing Concerns about Antibiotic Resistance. Environ. Sci. Technol. 2014, 48, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Harnisz, M.; Korzeniewska, E. The prevalence of multidrug-resistant Aeromonas spp. in the municipal wastewater system and their dissemination in the environment. Sci. Total Environ. 2018, 626, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Berglund, B.; Fick, J.; Lindgren, P.E. Urban wastewater effluent increases antibiotic resistance gene concentrations in a receiving northern European river. Environ. Toxicol. Chem. 2015, 34, 192–196. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, B.; Zou, S.C.; Fang, H.H.P.; Zhang, T. Fate of antibiotic resistance genes in sewage treatment plant revealed by metagenomic approach. Water Res. 2014, 62, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Geng, J.J.; Ma, H.J.; Ren, H.Q.; Xu, K.; Ding, L.L. Characterization of microbial community and antibiotic resistance genes in activated sludge under tetracycline andsulfamethoxazole selection pressure. Sci. Total Environ. 2016, 571, 479–486. [Google Scholar] [CrossRef]
- Biswal, B.K.; Mazza, A.; Masson, L.; Gehr, R.; Frigon, D. Impact of wastewater treatment processes on antimicrobial resistance genes and their co-occurrence with virulence genes in Escherichia coli. Water Res. 2014, 50, 245–253. [Google Scholar] [CrossRef]
- Huang, K.L.; Tang, J.Y.; Zhang, X.X.; Xu, K.; Ren, H.Q. A Comprehensive Insight into Tetracycline Resistant Bacteria and Antibiotic Resistance Genes in Activated Sludge Using Next-Generation Sequencing. Int. J. Mol. Sci. 2014, 15, 10083–10100. [Google Scholar] [CrossRef]
- Davies, J. Are antibiotics naturally antibiotics? J. Ind. Microbiol. Biotechnol. 2006, 33, 496–499. [Google Scholar] [CrossRef]
- Warnes, S.L.; Highmore, C.J.; Keevil, C.W. Horizontal Transfer of Antibiotic Resistance Genes on Abiotic Touch Surfaces: Implications for Public Health. mBio 2012, 3, e00489-12. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sanchez-Melsio, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, A.; Eckert, E.M.; D’Urso, S.; Bertoni, R.; Gillan, D.C.; Wattiez, R.; Corno, G. Co-occurrence of integrase 1, antibiotic and heavy metal resistance genes in municipal wastewater treatment plants. Water Res. 2016, 94, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.X.; Yang, L.; Duan, R.; Chen, Z.Q. Monitoring and evaluation of antibiotic resistance genes in four municipal wastewater treatment plants in Harbin, Northeast China. Environ. Pollut. 2016, 212, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Hammarén, R.; Pal, C.; Östman, M.; Björlenius, B.; Flach, C.F.; Fick, J.; Kristiansson, E.; Tysklind, M.; Larsson, D.G.J. Elucidating selection processes for antibiotic resistance in sewage treatment plants using metagenomics. Sci. Total Environ. 2016, 572, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Ben, W.W.; Wang, J.; Cao, R.K.; Yang, M.; Zhang, Y.; Qiang, Z.M. Distribution of antibiotic resistance in the effluents of ten municipal wastewater treatment plants in China and the effect of treatment processes. Chemosphere 2017, 172, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Niestępski, S. The prevalence and characterization of antibiotic-resistant and virulent Escherichia coli strains in the municipal wastewater system and their environmental fate. Sci. Total Environ. 2017, 577, 367–375. [Google Scholar] [CrossRef]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Niestępski, S. Impact of type of wastewater treatment process on the antybiotic resistance of bacterial populations. E3S Web Conf. 2017, 17, 00070. [Google Scholar] [CrossRef]
- Korzeniewska, E.; Harnisz, M. Extended-spectrum beta-lactamase (ESBL)-positive Enterobacteriaceae in municipal sewage and their emission to the environment. J. Environ. Manag. 2013, 128, 904–911. [Google Scholar] [CrossRef]
- Rafraf, I.D.; Lekunberri, I.; Sanchez-Melsio, A.; Aouni, M.; Borrego, C.M.; Balcazar, J.L. Abundance of antibiotic resistance genes in five municipal wastewater treatment plants in the Monastir Governorate, Tunisia. Environ. Pollut. 2016, 219, 353–358. [Google Scholar] [CrossRef]
- Hendricks, R.; Pool, E.J. The effectiveness of sewage treatment processes to remove faecal pathogens and antibiotic residues. J. Environ. Sci. Health A 2012, 47, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, J.; Siitonen, A.; Heinonen-Tanski, H. Elimination of enteric bacteria in biological-chemical wastewater treatment and tertiary filtration units. Water Res. 2003, 37, 690–698. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Munoz-Price, L.S. The new beta-lactamases. N. Engl. J. Med. 2005, 352, 380–391. [Google Scholar] [CrossRef]
- Ojer-Usoz, E.; Gonzalez, D.; Garcia-Jalon, I.; Vitas, A.I. High dissemination of extended-spectrum beta-lactamase-producing Enterobacteriaceae in effluents from wastewater treatment plants. Water Res. 2014, 56, 37–47. [Google Scholar] [CrossRef]
- Guo, X.; Yan, Z.; Zhang, Y.; Xu, W.; Kong, D.; Shan, Z.; Wang, N. Behavior of antibiotic resistance genes under extremely high-level antibiotic selection pressures in pharmaceutical wastewater treatment plants. Sci. Total Environ. 2018, 612, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.N.; Zhou, Z.C.; Chen, T.; Wei, Y.Y.; Zheng, J.; Gao, R.X. Biomarkers of antibiotic of resistance genes during seasonal changes in wastewater treatment systems. Environ. Pollut. 2018, 234, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.B.; Guo, M.T.; Yang, J. Monitoring and assessing the impact of wastewater treatment on release of both antibiotic-resistant bacteria and their typical genes in a Chinese municipal wastewater treatment plant. Environ. Sci. 2014, 16, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, C.; Bissa, M.; Illiano, E.; Mezzanotte, V.; Marazzi, F.; Turolla, A.; Antonelli, M.; De Giuli Morghen, C.; Radaelli, A. Identification of antibiotic-resistant Escherichia coli isolated from a municipal wastewater treatment plant. Chemosphere 2016, 164, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Makowska, N.; Koczura, R.; Mokracka, J. Class 1 integrase, sulfonamide and tetracycline resistance genes in wastewater treatment plant and surface water. Chemosphere 2016, 144, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.; Adriana, A.; Jessica, S.; Carles, B.; Marinella, F.; Marta, L.; Pierre, S. Antibiotic resistance in urban and hospital wastewaters and their impact on a receiving freshwater ecosystem. Chemosphere 2018, 206, 70–82. [Google Scholar] [CrossRef]
| Spearman’s Rank Coefficient | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSA/1 mL | TSA-AMO/1 mL | TSA-CTX/1 mL | TSA-OX/1 mL | TSA-DOX/1 mL | tetA | tetM | blaTEM | blaOXA | blaSHV | uidA | HRT | TSS | BOD | |
| TSA-AMO/1 mL | 0.95 | |||||||||||||
| TSA-CTX/1 mL | 0.94 | 0.94 | ||||||||||||
| TSA-OX/1 mL | 0.90 | 0.92 | 0.89 | |||||||||||
| TSA-DOX/1 mL | 0.85 | 0.92 | 0.90 | 0.87 | ||||||||||
| tetA | 0.22 | 0.30 | 0.28 | 0.26 | 0.38 | |||||||||
| tetM | 0.20 | 0.21 | 0.28 | 0.20 | 0.29 | 0.13 | ||||||||
| blaTEM | 0.42 | 0.34 | 0.36 | 0.27 | 0.30 | 0.47 | 0.03 | |||||||
| blaOXA | 0.17 | 0.16 | 0.20 | 0.19 | 0.21 | 0.02 | 0.44 | 0.15 | ||||||
| blaSHV | 0.41 | 0.37 | 0.42 | 0.32 | 0.46 | 0.47 | 0.33 | 0.42 | 0.42 | |||||
| uidA | −0.01 | 0.00 | 0.03 | −0.04 | 0.03 | 0.39 | 0.11 | 0.68 | 0.22 | 0.26 | ||||
| HRT | −0.05 | 0.00 | −0.02 | −0.11 | 0.01 | 0.41 | 0.10 | 0.01 | −0.28 | 0.31 | 0.39 | |||
| TSS | 0.03 | −0.04 | −0.09 | 0.06 | −0.04 | −0.37 | −0.15 | 0.29 | 0.08 | 0.08 | −0.40 | −0.49 | ||
| BOD | −0.68 | −0.72 | −0.71 | −0.62 | −0.66 | −0.01 | −0.27 | −0.24 | −0.18 | −0.18 | 0.00 | 0.04 | 0.00 | |
| COD | −0.30 | −0.35 | −0.34 | −0.43 | −0.38 | −0.45 | 0.11 | −0.18 | −0.08 | −0.15 | 0.30 | 0.19 | −0.14 | 0.12 |
| Sewage Treatment Technology Used | Treatment Plant | tetA | tetM | blaTEM | blaOXA | blaSHV | uidA |
|---|---|---|---|---|---|---|---|
| A. WWTPs with A2O system | I | * | 56.66 | * | * | 36.97 | * |
| II | 99.88 | 83.58 | * | 100 | 100 | * | |
| B. WWTPs with mechanical-biological system | III | * | * | * | * | * | * |
| IV | * | * | 96.73 | * | * | 1.21 | |
| V | * | * | 42.45 | * | * | 7.97 | |
| VI | 99.87 | 94.27 | 93.90 | 100 | 97.05 | 31.93 | |
| VII | * | 93.87 | * | ** | * | 49.51 | |
| C. WWTPs with Sequencing Batch Reactors (SBR) | VIII | 87.89 | * | 46.94 | ** | 100 | * |
| IX | * | 99.98 | * | 100 | 90.66 | * | |
| X | 94.46 | * | 25.24 | ** | 58.49 | 23.50 | |
| D. WWTPs with mechanical-biological system with elevated removal of nutrients | XI | 70.34 | 13.17 | 81.96 | ** | * | 19.44 |
| XII | 98.68 | * | 95.82 | 47.98 | 100 | 72.85 | |
| XIII | 87.16 | 91.55 | 88.59 | 98.78 | 88.34 | 79.56 |
| Spearman’s Rank Coeffcient | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mFc/1 mL | mFc-AMO/1 mL | mFc-CTX/1 mL | mFc-OX/1 mL | mFc-DOX/1 mL | tetA | tetM | blaTEM | blaOXA | blaSHV | uidA | HRT | TSS | BOD | |
| mFc-AMO/1 mL | 0.93 | |||||||||||||
| mFc-CTX/1 mL | 0.66 | 0.74 | ||||||||||||
| mFc-OX/1 mL | 0.81 | 0.83 | 0.59 | |||||||||||
| mFc-DOX/1 mL | 0.68 | 0.69 | 0.65 | 0.73 | ||||||||||
| tetA | 0.24 | 0.13 | 0.13 | 0.14 | 0.07 | |||||||||
| tetM | 0.17 | 0.14 | 0.17 | 0.05 | −0.02 | 0.56 | ||||||||
| blaTEM | 0.16 | 0.07 | −0.11 | 0.09 | 0.13 | 0.30 | 0.24 | |||||||
| blaOXA | −0.09 | −0.02 | −0.12 | 0.02 | −0.03 | −0.12 | 0.28 | 0.22 | ||||||
| blaSHV | 0.10 | 0.13 | 0.15 | 0.01 | 0.00 | 0.05 | 0.37 | −0.02 | 0.21 | |||||
| uidA | −0.04 | −0.12 | −0.32 | −0.15 | −0.06 | 0.11 | 0.06 | 0.32 | −0.04 | 0.02 | ||||
| HRT | −0.05 | −0.08 | 0.08 | −0.11 | −0.23 | 0.03 | 0.14 | −0.61 | −0.38 | −0.10 | −0.12 | |||
| TSS | −0.01 | 0.06 | 0.46 | 0.14 | 0.23 | −0.03 | −0.31 | −0.05 | −0.12 | −0.19 | −0.01 | −0.07 | ||
| BOD | −0.63 | −0.63 | −0.69 | −0.54 | −0.43 | −0.31 | −0.35 | −0.11 | −0.13 | −0.23 | 0.05 | 0.04 | −0.15 | |
| COD | −0.29 | −0.38 | −0.28 | −0.41 | −0.34 | 0.20 | 0.27 | 0.17 | −0.21 | −0.09 | 0.25 | 0.19 | −0.28 | 0.12 |
| Sewage Treatment Technology Used | Treatment Plant | tetA | tetM | blaTEM | blaOXA | blaSHV | uidA |
|---|---|---|---|---|---|---|---|
| A. WWTPs with A2O system | I | * | 93.66 | 44.30 | ** | 17.50 | * |
| II | ** | ** | * | 64.30 | 53.95 | 14.67 | |
| B. WWTPs with mechanical-biological system | III | * | 99.66 | * | 100 | * | * |
| IV | * | * | * | * | * | * | |
| V | * | * | * | * | 7.63 | 40.10 | |
| VI | ** | * | * | ** | 43.52 | 10.82 | |
| VII | 100 | 99.997 | 14.50 | * | 90.47 | 33.22 | |
| C. WWTPs with Sequencing Batch Reactors (SBR) | VIII | 100 | 86.13 | 21.88 | ** | 84.69 | 27.77 |
| IX | 60.98 | 99.76 | 80.98 | ** | 88.40 | 20.75 | |
| X | 100 | 68.71 | * | 85.93 | 26.06 | * | |
| D. WWTPs with mechanical-biological system with elevated removal of nutrients | XI | 100 | * | * | ** | * | * |
| XII | 66.62 | 80.96 | * | * | * | * | |
| XIII | 98.21 | 19.55 | 97.30 | ** | * | 15.52 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osińska, A.; Korzeniewska, E.; Harnisz, M.; Niestępski, S. Quantitative Occurrence of Antibiotic Resistance Genes among Bacterial Populations from Wastewater Treatment Plants Using Activated Sludge. Appl. Sci. 2019, 9, 387. https://doi.org/10.3390/app9030387
Osińska A, Korzeniewska E, Harnisz M, Niestępski S. Quantitative Occurrence of Antibiotic Resistance Genes among Bacterial Populations from Wastewater Treatment Plants Using Activated Sludge. Applied Sciences. 2019; 9(3):387. https://doi.org/10.3390/app9030387
Chicago/Turabian StyleOsińska, Adriana, Ewa Korzeniewska, Monika Harnisz, and Sebastian Niestępski. 2019. "Quantitative Occurrence of Antibiotic Resistance Genes among Bacterial Populations from Wastewater Treatment Plants Using Activated Sludge" Applied Sciences 9, no. 3: 387. https://doi.org/10.3390/app9030387
APA StyleOsińska, A., Korzeniewska, E., Harnisz, M., & Niestępski, S. (2019). Quantitative Occurrence of Antibiotic Resistance Genes among Bacterial Populations from Wastewater Treatment Plants Using Activated Sludge. Applied Sciences, 9(3), 387. https://doi.org/10.3390/app9030387






