Multi-Imaging Investigation to Evaluate the Relationship between Serum Cystatin C and Features of Atherosclerosis in Non-ST-Segment Elevation Acute Coronary Syndrome
Abstract
:1. Introduction
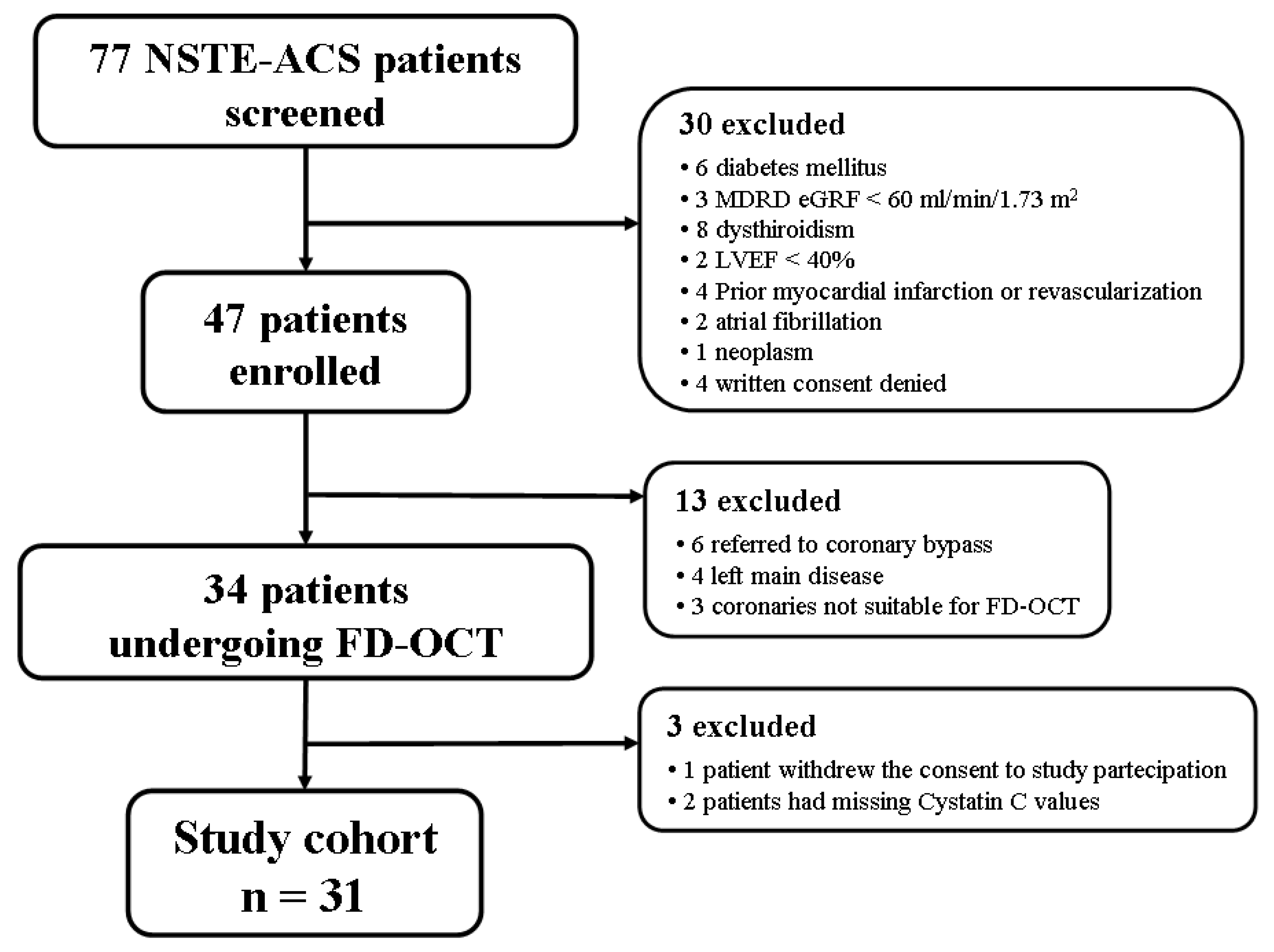
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Abrahamson, M.; Dalboge, H.; Olafsson, I.; Carlsen, S.; Grubb, A. Efficient production of native, biologically active human cystatin C by Escherichia coli. FEBS Lett. 1988, 236, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Dharnidharka, V.R.; Kwon, C.; Stevens, G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am. J. Kidney Dis. 2002, 40, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Grubb, A.; Nyman, U.; Björk, J.; Lindström, V.; Rippe, B.; Sterner, G.; Christensson, A. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin. Chem. 2005, 51, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Saver, J.L.; Huang, W.H.; Chow, J.; Chang, K.H.; Ovbiagele, B. Impact of elevated cystatin C level on cardiovascular disease risk in predominantly high cardiovascular risk populations: A meta-analysis. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 675–683. [Google Scholar] [CrossRef]
- Taglieri, N.; Koenig, W.; Kaski, J.C. Cystatin C and cardiovascular risk. Clin. Chem. 2009, 55, 1932–1943. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M.G.; Katz, R.; Sarnak, M.J.; Fried, L.F.; Newman, A.B.; Stehman-Breen, C.; Seliger, S.L.; Kestenbaum, B.; Psaty, B.; Tracy, R.P.; et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann. Intern. Med. 2006, 145, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M.G.; Matsushita, K.; Ärnlöv, J.; Inker, L.A.; Katz, R.; Polkinghorne, K.R.; Rothenbacher, D.; Sarnak, M.J.; Astor, B.C.; Coresh, J.; et al. Cystatin C versus creatinine in determining risk based on kidney function. N. Engl. J. Med. 2013, 369, 932–943. [Google Scholar] [CrossRef]
- Liu, J.; Sukhova, G.K.; Sun, J.S.; Xu, W.H.; Libby, P.; Shi, G.P. Lysosomal cysteine proteases in atherosclerosis. Arter. Thromb. Vasc. Biol. 2004, 24, 1359–1366. [Google Scholar] [CrossRef]
- Shi, G.P.; Sukhova, G.K.; Grubb, A.; Ducharme, A.; Rhode, L.H.; Lee, R.T.; Ridker, P.M.; Libby, P.; Chapman, H.A. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J. Clin. Investig. 1999, 104, 1191–1197. [Google Scholar] [CrossRef] [Green Version]
- Svensson-Färbom, P.; Almgren, P.; Hedblad, B.; Engström, G.; Persson, M.; Christensson, A.; Melander, O. Cystatin C Is Not Causally Related to Coronary Artery Disease. PLoS ONE 2015, 10, e0129269. [Google Scholar] [CrossRef]
- Van Der Laan, S.W.; Fall, T.; Soumaré, A.; Teumer, A.; Sedaghat, S.; Baumert, J.; Zabaneh, D.; van Setten, J.; Isgum, I.; Galesloot, T.E.; et al. Cystatin C and Cardiovascular Disease: A Mendelian Randomization Study. J. Am. Coll. Cardiol. 2016, 68, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Taglieri, N.; Nanni, C.; Ghetti, G.; Bonfiglioli, R.; Saia, F.; Bacchi Reggiani, M.L.; Lima, G.M.; Marco, V.; Prati, F.; Fanti, S.; et al. Relation between thoracic aortic inflammation and features of plaque vulnerability in the coronary tree in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention. An FDG-positron emission tomography and optical coherence tomography study. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1878–1887. [Google Scholar]
- Bucerius, J.; Mani, V.; Moncrieff, C.; Machac, J.; Fuster, V.; Farkouh, M.E.; Tawakol, A.; Rudd, J.H.; Fayad, Z.A. Optimizing 18F-FDG PET/CT imaging of vessel wall inflammation: The impact of 18F-FDG circulation time, injected dose, uptake parameters, and fasting blood glucose levels. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 369–383. [Google Scholar] [CrossRef]
- Borque, L.; Bellod, L.; Rus, A.; Seco, M.L.; Galisteo-Gonzalez, F. Development and validation of an automated and ultrasensitive immunoturbidimetric assay for C-reactive protein. Clin. Chem. 2000, 46, 1839–1842. [Google Scholar] [PubMed]
- Mussap, M.; Ruzzante, N.; Varagnolo, M.; Plebani, M. Quantitative automated particle-enhanced immunonephelometric assay for the routinary measurement of human cystatin C. Clin. Chem. Lab. Med. 1998, 36, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Bashore, T.M.; Bates, E.R.; Berger, P.B.; Clark, D.A.; Cusma, J.T.; Dehmer, G.J.; Kern, M.J.; Laskey, W.K.; O’Laughlin, M.P.; Oesterle, S.; et al. American College of Cardiology/Society for Cardiac Angiography and Interventions Clinical Expert Consensus Document on cardiac catheterization laboratory standards. A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 2001, 37, 2170–2214. [Google Scholar] [PubMed]
- Di Vito, L.; Agozzino, M.; Marco, V.; Ricciardi, A.; Concardi, M.; Romagnoli, E.; Gatto, L.; Calogero, G.; Tavazzi, L.; Arbustini, E.; et al. Identification and quantification of macrophage presence in coronary atherosclerotic plaques by optical coherence tomography. Eur. Heart. J. Cardiovasc. Imaging 2015, 16, 807–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, F.; Joner, M.; Prati, F.; Virmani, R.; Narula, J. Clinical classification of plaque morphology in coronary disease. Nat. Rev. Cardiol. 2014, 11, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Bucerius, J.; Hyafil, F.; Verberne, H.J.; Slart, R.H.; Lindner, O.; Sciagra, R.; Agostini, D.; Ubleis, C.; Gimelli, A.; Hacker, M. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 780–792. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Einstein, A.J.; Johnson, L.L.; Bokhari, S.; Son, J.; Thompson, R.C.; Bateman, T.M.; Hayes, S.W.; Berman, D.S. Agreement of visual estimation of coronary artery calcium from low-dose CT attenuation correction scans in hybrid PET/CT and SPECT/CT with standard Agatston score. J. Am. Coll. Cardiol. 2010, 56, 1914–1921. [Google Scholar] [CrossRef]
- Chambers, J.C.; Zhang, W.; Lord, G.M.; van der Harst, P.; Lawlor, D.A.; Sehmi, J.S.; Gale, D.P.; Wass, M.N.; Ahmadi, K.R.; Bakker, S.J.; et al. Genetic loci influencing kidney function and chronic kidney disease. Nat. Genet. 2010, 42, 373–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottgen, A.; Pattaro, C.; Boger, C.A.; Fuchsberger, C.; Olden, M.; Glazer, N.L.; Parsa, A.; Gao, X.; Yang, Q.; Smith, A.V.; et al. New loci associated with kidney function and chronic kidney disease. Nat. Genet. 2010, 42, 376–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akerblom, A.; Eriksson, N.; Wallentin, L.; Siegbahn, A.; Barratt, B.J.; Becker, R.C.; Budaj, A.; Himmelmann, A.; Husted, S.; Storey, R.F.; et al. Polymorphism of the cystatin C gene in patients with acute coronary syndromes: Results from the PLATelet inhibition and patient Outcomes study. Am. Heart J. 2014, 168, 96–102.e2. [Google Scholar] [CrossRef]
- Loew, M.; Hoffmann, M.M.; Koenig, W.; Brenner, H.; Rothenbacher, D. Genotype and plasma concentration of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events. Arter. Thromb. Vasc. Biol. 2005, 25, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Conte, M.; Della Bona, R.; Altamura, L.; Siviglia, M.; Dato, I.; Ferrante, G.; Leone, A.M.; Porto, I.; Burzotta, F.; et al. Cystatin C is associated with an increased coronary atherosclerotic burden and a stable plaque phenotype in patients with ischemic heart disease and normal glomerular filtration rate. Atherosclerosis 2008, 198, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Doganer, Y.C.; Aydogan, U.; Aydogdu, A.; Aparci, M.; Akbulut, H.; Nerkiz, P.; Turker, T.; Cayci, T.; Barcin, C.; Saglam, K. Relationship of cystatin C with coronary artery disease and its severity. Coronary Artery Dis. 2013, 24, 119–126. [Google Scholar] [CrossRef]
- Prati, F.; Regar, E.; Mintz, G.S.; Arbustini, E.; Di Mario, C.; Jang, I.K.; Akasaka, T.; Costa, M.; Guagliumi, G.; Grube, E.; et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: Physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur. Heart J. 2010, 31, 401–415. [Google Scholar] [CrossRef]
- Huang, H.; Virmani, R.; Younis, H.; Burke, A.P.; Kamm, R.D.; Lee, R.T. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation 2001, 103, 1051–1056. [Google Scholar] [CrossRef]
- Sangiorgi, G.; Rumberger, J.A.; Severson, A.; Edwards, W.D.; Gregoire, J.; Fitzpatrick, L.A.; Schwartz, R.S. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: A histologic study of 723 coronary artery segments using nondecalcifying methodology. J. Am. Coll. Cardiol. 1998, 31, 126–133. [Google Scholar] [CrossRef]
- Greenland, P.; Alpert, J.S.; Beller, G.A.; Benjamin, E.J.; Budoff, M.J.; Fayad, Z.A.; Foster, E.; Hlatky, M.A.; Hodgson, J.M.; Kushner, F.G.; et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2010, 122, e584–e636. [Google Scholar] [PubMed]
- Imai, A.; Komatsu, S.; Ohara, T.; Kamata, T.; Yoshida, J.; Miyaji, K.; Shimizu, Y.; Takewa, M.; Hirayama, A.; Deshpande, G.A.; et al. Serum cystatin C is associated with early stage coronary atherosclerotic plaque morphology on multidetector computed tomography. Atherosclerosis 2011, 218, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Tawakol, A. Imaging atherosclerosis with positron emission tomography. Eur. Heart J. 2016, 37, 2974–2980. [Google Scholar] [CrossRef] [PubMed]
- Tawakol, A.; Singh, P.; Mojena, M.; Pimentel-Santillana, M.; Emami, H.; MacNabb, M.; Rudd, J.H.; Narula, J.; Enriquez, J.A.; Traves, P.G.; et al. HIF-1alpha and PFKFB3 Mediate a Tight Relationship Between Proinflammatory Activation and Anerobic Metabolism in Atherosclerotic Macrophages. Arter. Thromb. Vasc. Biol. 2015, 35, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.; Berger, K.; Claycombe, K.; Huang, R.; Patel, R.; Abela, G.S. Noninvasive detection and localization of vulnerable plaque and arterial thrombosis with computed tomography angiography/positron emission tomography. Circulation 2008, 117, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Rudd, J.H.; Myers, K.S.; Bansilal, S.S.; Machac, J.; Woodward, M.; Fuster, V.; Farkouh, M.E.; Fayad, Z.A. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: A prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ. Cardiovasc. Imaging 2009, 2, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, M.; Cohade, C.; Nakamoto, Y.; Wahl, R.L. Fluorodeoxyglucose uptake in the aortic wall at PET/CT: Possible finding for active atherosclerosis. Radiology 2003, 229, 831–837. [Google Scholar] [CrossRef]
- Tawakol, A.; Migrino, R.Q.; Bashian, G.G.; Bedri, S.; Vermylen, D.; Cury, R.C.; Yates, D.; LaMuraglia, G.M.; Furie, K.; Houser, S.; et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J. Am. Coll. Cardiol. 2006, 48, 1818–1824. [Google Scholar] [CrossRef]
- Stone, J.R.; Bruneval, P.; Angelini, A.; Bartoloni, G.; Basso, C.; Batoroeva, L.; Buja, L.M.; Butany, J.; d’Amati, G.; Fallon, J.T.; et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: I. Inflammatory diseases. Cardiovasc. Pathol. 2015, 24, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Oorni, K.; Sneck, M.; Bromme, D.; Pentikainen, M.O.; Lindstedt, K.A.; Mayranpaa, M.; Aitio, H.; Kovanen, P.T. Cysteine protease cathepsin F is expressed in human atherosclerotic lesions, is secreted by cultured macrophages, and modifies low density lipoprotein particles in vitro. J. Biol. Chem. 2004, 279, 34776–34784. [Google Scholar] [CrossRef]
- Schulte, S.; Sun, J.; Libby, P.; Macfarlane, L.; Sun, C.; Lopez-Ilasaca, M.; Shi, G.P.; Sukhova, G.K. Cystatin C deficiency promotes inflammation in angiotensin II-induced abdominal aortic aneurisms in atherosclerotic mice. Am. J. Pathol. 2010, 177, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Burkhoff, D.; Fried, L.P.; Gottdiener, J.; King, D.L.; Kitzman, D.W. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction: The Cardiovascular Health Study. J. Am. Coll. Cardiol. 2007, 49, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Risch, L.; Herklotz, R.; Blumberg, A.; Huber, A.R. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin. Chem. 2001, 47, 2055–2059. [Google Scholar] [PubMed]
- Manetti, L.; Pardini, E.; Genovesi, M.; Campomori, A.; Grasso, L.; Morselli, L.L.; Lupi, I.; Pellegrini, G.; Bartalena, L.; Bogazzi, F.; et al. Thyroid function differently affects serum cystatin C and creatinine concentrations. J. Endocrinol. Investig. 2005, 28, 346–349. [Google Scholar] [CrossRef]
- Joshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.; Calvert, P.A.; Craighead, F.H.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014, 383, 705–713. [Google Scholar] [CrossRef]
- Figueroa, A.L.; Subramanian, S.S.; Cury, R.C.; Truong, Q.A.; Gardecki, J.A.; Tearney, G.J.; Hoffmann, U.; Brady, T.J.; Tawakol, A. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: A comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ. Cardiovasc. Imaging 2012, 5, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Silvera, S.S.; Aidi, H.E.; Rudd, J.H.; Mani, V.; Yang, L.; Farkouh, M.; Fuster, V.; Fayad, Z.A. Multimodality imaging of atherosclerotic plaque activity and composition using FDG-PET/CT and MRI in carotid and femoral arteries. Atherosclerosis 2009, 207, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Rudd, J.H.; Myers, K.S.; Bansilal, S.; Machac, J.; Rafique, A.; Farkouh, M.; Fuster, V.; Fayad, Z.A. 18 Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: Implications for atherosclerosis therapy trials. J. Am. Coll. Cardiol. 2007, 50, 892–896. [Google Scholar] [CrossRef]
- Figueroa, A.L.; Abdelbaky, A.; Truong, Q.A.; Corsini, E.; MacNabb, M.H.; Lavender, Z.R.; Lawler, M.A.; Grinspoon, S.K.; Brady, T.J.; Nasir, K.; et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc. Imaging 2013, 6, 1250–1259. [Google Scholar] [CrossRef]
- Tahara, N.; Kai, H.; Ishibashi, M.; Nakaura, H.; Kaida, H.; Baba, K.; Hayabuchi, N.; Imaizumi, T. Simvastatin attenuates plaque inflammation: Evaluation by fluorodeoxyglucose positron emission tomography. J. Am. Coll. Cardiol. 2006, 48, 1825–1831. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Maldonado, N.; Aikawa, E. Small entities with large impact: Microcalcifications and atherosclerotic plaque vulnerability. Curr. Opin. Lipidol. 2014, 25, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, N.; Trivieri MDweck, M.; Philip Robson, P.; Abgral, R.; Soler, R.; Calcagno, C.; Mani, V.; Tsoumpas, C.; Kovasic, J.; et al. Simultaneous assessment of carotid plaque inflammation and micro-calcification with dual-tracer 18F-FDG: 18F-NaF PET-MR imaging: A clinical feasibility study. J. Nucl. Med. 2017, 58, 446. [Google Scholar]
- Li, X.; Heber, D.; Wadsak, W.; Mitterhauser, M.; Hacker, M. Combined 18F-FDG PET/CT and 18F-NaF PET/CT imaging in assessing vascular inflammation and osteogenesis in calcified atherosclerotic lesions. J. Nucl. Med. 2016, 57, 68. [Google Scholar]

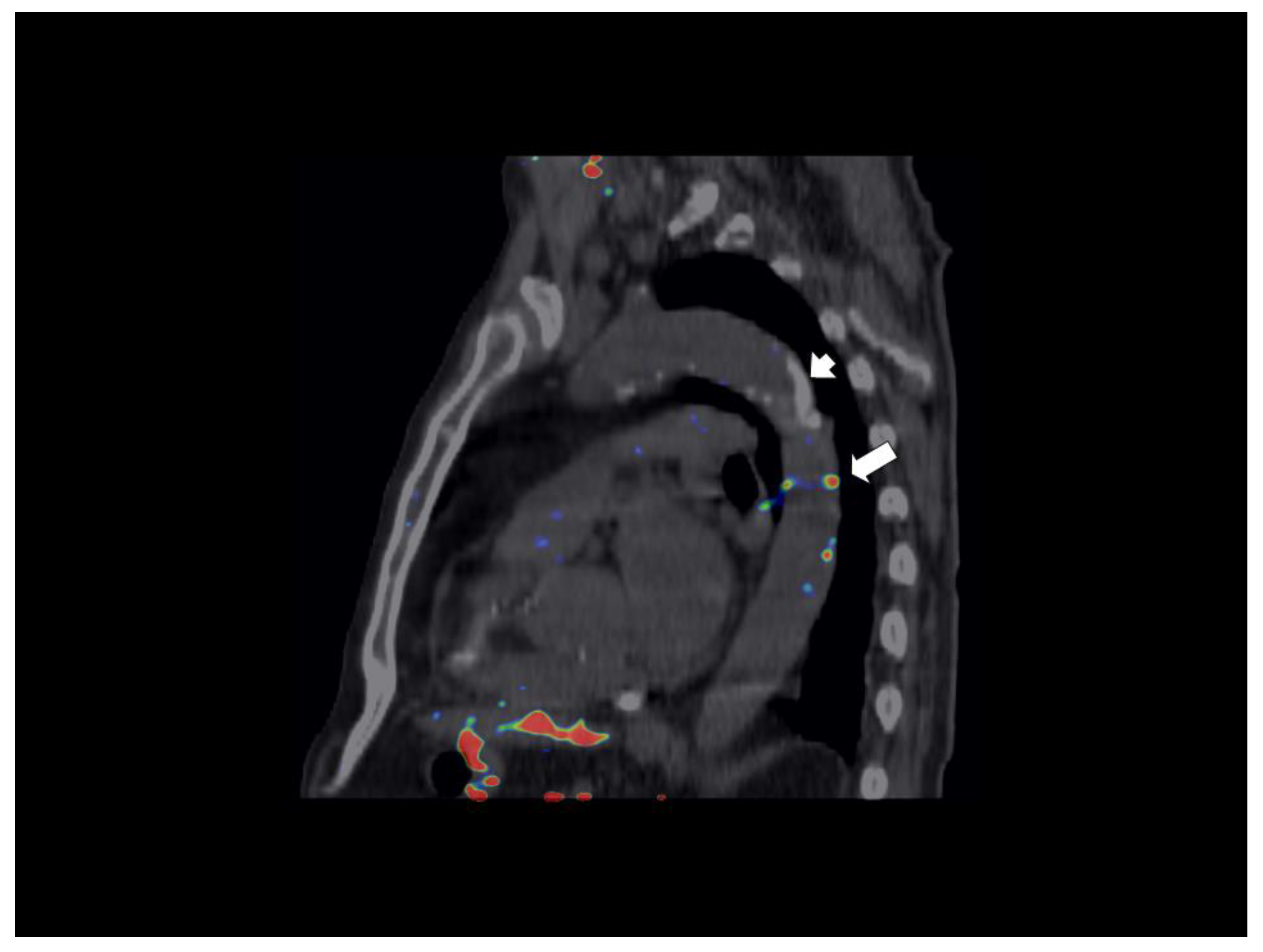
| Variable | All Patients | Cystatin C < 0.95 mg/mL | Cystatin C ≥ 0.95 mg/mL | P Value |
|---|---|---|---|---|
| No. of Patients | n = 31 | n = 14 | n = 17 | |
| Age, years, median (25th–75th) | 65 (55–72) | 66 (56–72) | 65 (51–72) | 0.84 |
| Male gender-no. (%) | 26 (84) | 11 (79) | 15 (88) | 0.63 |
| Risk Factors | ||||
| Hypercholesterolemia-no. (%) | 19 (61) | 8 (57) | 11 (64) | 0.72 |
| Hypertension-no. (%) | 19 (61) | 9 (64) | 10 (59) | 1.00 |
| Smokers-no. (%) | 26 (81) | 12 (86) | 13 (76) | 0.66 |
| Family-history of CAD-no. (%) | 7 (23) | 2 (14) | 5 (29) | 0.41 |
| Presenting Characteristics | ||||
| Systolic BP, mmHg, median (25th–75th) | 155 (140–170) | 152 (140–170) | 160 (150–160) | 0.89 |
| Heart rate, pulse/min, mean ± SD | 75 (64–88) | 84 (75–90) | 73 (64–83) | 0.06 |
| ECG changes-no. (%) | 21 (68) | 10 (71) | 11 (65) | 1.00 |
| Hb, g/dL, median (25th–75th) | 14.8 (13.9–15.6) | 14.5 (13.9–15.6) | 14.9 (14.3–15.6) | 0.56 |
| WBC, *103/mm3 median (25th–75th) | 8.42 (6.53–12.13) | 9.54 (7.57–12.13) | 7.62 (6.28–11:03) | 0.17 |
| Platelet count, *103/mm3 median (25th–75th) | 254 (209–291) | 273 (244–287) | 226 (192–291) | 0.17 |
| Mean platelet volume, fl median (25th–75th) | 7.4 (7.2–8.3) | 7.5 (7.2–10.7) | 7.4 (7.2–8.0) | 0.35 |
| Creatinine, mg/dL, median (25th–75th) | 0.94 (0.77–1.02) | 0.9 (0.76–0.96) | 0.94 (0.83–1.02) | 0.26 |
| MDRD eGRF, mL/min/1.73, median (25th–75th) | 87 (81–98) | 87 (81–100) | 97(76–96) | 0.49 |
| Cystatin C, mg/dL median (25th–75th) | 0.95 (0.88–1.08) | 0.88 (0.79–0.90) | 1.04 (0.98–1.22) | <0.001 |
| Low density liprotein, mg/dL median (25th–75th) | 136 (106–160) | 148 (127–155) | 132 (102–161) | 0.52 |
| High density liprotein, mg/dL median (25th–75th) | 37 (33–46) | 37 (35–39) | 38 (33–47) | 0.72 |
| CRP, ng/L median (25th–75th) | 0.40 (0.24–0.67) | 0.52 (0.29–0.95) | 0.34 (0.23–0.47) | 0.05 |
| LVEF, % median (25th–75th) | 57 (50–61) | 59 (48–65) | 57 (61–60) | 0.82 |
| Procedure | ||||
| Multivessel disease-no. (%) | 14 (45) | 7 (50) | 7 (41) | 0.73 |
| Multivessel treatment | 12 (38) | 7 (50) | 5 (29) | 0.28 |
| DES-no. (%) | 29 (94) | 14 (100) | 15 (88) | 0.48 |
| Variable | All Patients | Cystatin C <0.95 mg/mL | Cystatin C ≥0.95 mg/mL | P Value |
|---|---|---|---|---|
| No. of Patients | n = 31 | n = 14 * | n = 17 | |
| Optical coherence tomography | ||||
| Lipid rich plaques, n median (25th–75th) | 6 (4–8) | 6.5 (5–8) | 6 (4–7) | 0.55 |
| Lipid rich plaques with macrophages, n median (25th–75th) | 2 (4–5) | 4 (3–5) | 4 (2–5) | 0.78 |
| TCFA, n median (25th–75th) | 1 (1–2) | 1 (0–3) | 1 (1–3) | 0.32 |
| 18FDG-PET/CT | ||||
| Averaged mean TBR in AA, median (25th–75th) | 1.23 (1.12–1.30) | 1.24 (1.15–1.38) | 1.22 (1.09–1.24) | 0.23 |
| Averaged max TBR in AA, median (25th–75th) | 1.84 (1.71–2.04) | 1.95 (1.80–2.21) | 1.79 (1.61–1.93) | 0.05 |
| Averaged mean TBR in DA, median (25th–75th) | 1.21 (1.02–1.33) | 1.29 (1.11–1.41) | 1.15 (0.99–1.29) | 0.07 |
| Averaged max TBR in DA, median (25th–75th) | 1.74 (1.54–1.92) | 1.82 (1.62–2.11) | 1.61 (1.50–1.84) | 0.06 |
| Number of active slices in AA, median (25th –75th) | 11.5 (9–13) | 13 (11–14) | 11 (9–12) | 0.08 |
| Number of active slices in DA, median (25th–75th) | 22.5 (6.5–29) | 25 (12–37) | 18 (1–24) | 0.03 |
| CT scan | ||||
| Coronary calcium score, median (25th–75th) | 955 (224–1300) | 1298 (547–1339) | 466 (57–1183) | 0.07 |
| Variable | Odds Ratio | 95% CI | P Value | Adjusted Odds Ratio * | 95% CI | P Value |
|---|---|---|---|---|---|---|
| Optical coherence tomography | ||||||
| Lipid rich plaques, n | 0.87 | 0.63–1.20 | 0.40 | 0.85 | 0.58–1.24 | 0.41 |
| Lipid rich plaques with macrophages, n | 1.00 | 0.69–1.47 | 0.98 | 0.82 | 0.64–1.42 | 0.81 |
| TCFA, n | 1.07 | 0.65–1.77 | 0.77 | 0.98 | 0.58–1.64 | 0.94 |
| 18FDG-PET/CT | ||||||
| Averaged mean TBR in A, | 0.31 | 0.03–0.62 | 0.35 | 0.07 | 0.0003–14.8 | 0.33 |
| Averaged max TBR in AA, | 0.19 | 0.002–13.7 | 0.45 | 0.19 | 0.01–3.4 | 0.26 |
| Averaged mean TBR in DA, | 0.017 | 0.0002–1.49 | 0.07 | 0.02 | 0.00002–1.06 | 0.053 |
| Averaged max TBR in DA, | 0.07 | 0.004–1.50 | 0.09 | 0.02 | 0.0004–0.89 | 0.044 |
| Number of active slices in AA, | 0.77 | 0.57–1.06 | 0.11 | 0.69 | 0.47–1.02 | 0.069 |
| Number of active slices in DA, | 0.93 | 0.87–1.002 | 0.060 | 0.89 | 0.82–0.98 | 0.025 |
| CT scan | ||||||
| Coronary calcium score, | 0.99 | 0.99–1.00 | 0.50 | 0.99 | 0.99–1.00 | 0.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taglieri, N.; Nanni, C.; Ghetti, G.; Bonfiglioli, R.; Saia, F.; Buia, F.; Lima, G.M.; Marco, V.; Bruno, A.G.; Prati, F.; et al. Multi-Imaging Investigation to Evaluate the Relationship between Serum Cystatin C and Features of Atherosclerosis in Non-ST-Segment Elevation Acute Coronary Syndrome. Appl. Sci. 2019, 9, 657. https://doi.org/10.3390/app9040657
Taglieri N, Nanni C, Ghetti G, Bonfiglioli R, Saia F, Buia F, Lima GM, Marco V, Bruno AG, Prati F, et al. Multi-Imaging Investigation to Evaluate the Relationship between Serum Cystatin C and Features of Atherosclerosis in Non-ST-Segment Elevation Acute Coronary Syndrome. Applied Sciences. 2019; 9(4):657. https://doi.org/10.3390/app9040657
Chicago/Turabian StyleTaglieri, Nevio, Cristina Nanni, Gabriele Ghetti, Rachele Bonfiglioli, Francesco Saia, Francesco Buia, Giacomo Maria Lima, Valeria Marco, Antonio Giulio Bruno, Francesco Prati, and et al. 2019. "Multi-Imaging Investigation to Evaluate the Relationship between Serum Cystatin C and Features of Atherosclerosis in Non-ST-Segment Elevation Acute Coronary Syndrome" Applied Sciences 9, no. 4: 657. https://doi.org/10.3390/app9040657
APA StyleTaglieri, N., Nanni, C., Ghetti, G., Bonfiglioli, R., Saia, F., Buia, F., Lima, G. M., Marco, V., Bruno, A. G., Prati, F., Fanti, S., & Rapezzi, C. (2019). Multi-Imaging Investigation to Evaluate the Relationship between Serum Cystatin C and Features of Atherosclerosis in Non-ST-Segment Elevation Acute Coronary Syndrome. Applied Sciences, 9(4), 657. https://doi.org/10.3390/app9040657






