Featured Application
This paper investigated the effect of cell-free supernatant (CFS) from Aeromonas sobria on the spoilage of Shewanella putrefaciens in Pacific white shrimp during transportation, retailing, and domestic storage in which shrimp will be exposure to different temperatures and temperature fluctuation. It is an innovative method to use the interaction between bacteria to inhibit microbial activity by extracting CFS as an edible soaking solution in cold chain logistics.
Abstract
The objective of this study was to evaluate the effect of cell-free supernatant (CFS) from Aeromonas sobria on the growth and spoilage potential of Shewanella putrefaciens in Pacific white shrimp (Litopenaeus vannamei) during cold chain logistics, including transportation, retailing, and domestic storage. It was shown that the quality of shrimps deteriorated in the cold chain logistics over time. The temperature fluctuation during the experimental period favored the growth of S. putrefaciens, increased the total volatile basic nitrogen (TVB-N) and biogenic amine value, and decreased the sensory quality of shrimps. The application of CFS resulted in the decline on the growth of S. putrefaciens after the early stationary phase stored at a cold condition. It is concluded that the application of CFS can inhibit microbial growth and the spoilage potential of S. putrefaciens and offset the quality deterioration of shrimp exposed to temperature fluctuation during cold chain logistics.
1. Introduction
Pacific white shrimp (Litopenaeus vannamei) has been of increasing demand all over the world, commercially viable of the globe with high yield and high nutrition [1,2,3]. In spite of their delicacy and popularity, this high value crustacean is highly perishable because of a limited shelf-life, mainly caused by the enzymatic autolysis, chemical oxidation and microbial deterioration [4,5]. The activity of microorganism is the main cause to affect the quality of fresh shrimp [4]. Gram-negative psychrotrophic organisms are the most important bacteria in cold storage under aerobic conditions [6], such as Shewanella spp., Aeromonas spp., Pseudomonas spp. and genera of the Enterobacteriaceae family [7]. Shewanella spp. and Aeromonas spp. are largely responsible for the specific spoilers in Pacific white shrimp, known as specific spoilage microorganism (SSO) [8,9]. The SSO of Shewanella spp. and Aeromonas spp. can produce amines, trimethylamine, protein degradation and off-odor in previous researches to decrease the quality of shrimp [10,11,12,13]. Hence, it is essential to control the activity of SSO in fresh shrimp during cold storage by innovative preservation techniques [14]. Our previous research found that cell-free supernatant (CFS) from Aeromonas sobria could inhibit the growth ability of Shewanella putrefaciens, the ability of producing TVB-N and biogenic amines, the microbial decarboxylation of amino acids and the degradation of sarcoplasmic proteins of shrimp during cold storage at 4 °C. Because the role of metabolites of A. sobria in CFS can influence the micro-environment and the regulate production of substance and controlling the activities of enzymes [15,16]. For example, the CFS from Pseudomonas fluorescens had an inhibited effect on the activity of spoilage phenotypes in Shewanella baltica [12]. In the presence of Carnobacterium maltaromaticum, S. baltica imposed its characteristic to produce more volatile compounds, such as sulfur compounds like H2S that can lead to typical cabbage/sulfur odors of spoiled samples [17]. Hence, it is an innovative method to use the interaction between bacteria to inhibit microbial activity by extracting cell-free supernatant as an edible soaking solution.
During processing, transportation, retailing, and domestic storage, the temperature of food storage was not constant. Pacific white shrimp will be exposure to different temperatures and temperature fluctuation which have a negative effect on shrimps [18], due to the high water and protein content [19]. There were some related researches had been done about seafood storage during temperature fluctuation. For example, Shi et al. found a perfect method to predict the freshness of fillets stored from 0 °C to 10 °C based on feature viable by electronic nose and tongue in the cold chain [19]. Zhang et al. studied the effect of frozen-then-chilled storage on free Ca2+, proteolytic enzyme activity of calpains and the proteasome, water-holding capacity and sheer force of porcine (longissimus thoracis) at lumborum muscle and found that frozen-then-chilled storage increased free Ca2+ concentration, followed by a faster decrease of calpain-1 activity and activation of around 50% of calpain-2 and proteasome activity was reduced by around 40% following freezing-thawing [20]. Therefore, the freshness and changes of quality on shrimp is a worthy subject to focus on stored during temperature fluctuation in the cold chain. Additionally, there is almost no research to study the effect of CFS from A. sobria on the spoilage of S. putrefaciens in Pacific white shrimp with temperature fluctuation.
The object of this work was to investigate the effect of CFS from A. sobria on the spoilage of S. putrefaciens in Pacific white shrimp during transportation, retailing, and domestic storage in which shrimp will be exposure to different temperatures and temperature fluctuation. The growth of S. putrefaciens was analyzed. Physical and chemical properties including sensory evaluation, total volatile basic nitrogen (TVB-N), amino acid and biogenic amines were analyzed. Myofibrillar proteins of shrimp were analyzed by poly-acrylamide gel electrophoresis (SDS-PAGE).
2. Materials and Methods
2.1. Bacterial Strains
Strains of S. putrefaciens QY38 and A. sobria QY32 isolated from spoiled Pacific white shrimp previously were identified by 16SrRNA and VITEK®2 CompactA system (BIOMÉRIEUX, Lyon, France). They were stored at −80 °C in sterilized Tryptone soy broth (TSB; Qingdao Hope Bio-Technology Co., Ltd., Qingdao, China) containing 25% glycerine. The isolates were pre-cultured individually in brain heart infusion broth (BHI; Qingdao Hope Bio-Technology Co., Ltd., Qingdao, China) for 18 h and then in TSB for 8 h at 27 °C, before use. The cell concentration after preculture was about 7~9 log CFU/mL.
2.2. Preparation of CFS
A. sobria was cultured overnight in BHI at 30 °C. Cultures were then centrifuged at 10,000 g for 15 min at 4 °C for removing the cells from the growth medium. For obtaining CFS of A. sobria, the supernatant was filtered through a 0.22-μm-pore-size filter (Whatman, Inc., Cilfton, NJ, USA) and then stored at −20 °C [21]. The initial pH was 7.19.
2.3. Sample Preparation
Pacific white shrimp about 6 ± 2 g for each one were transported to the laboratory alive from the local market (Shanghai, China). All the shrimps were sacrificed and washed with ice slurry. Then the shrimps were sterilized by dipping in 75% ethanol for 120 s and washed twice with sterilized distilled water [22]. Finally, the shrimps were sterilized by ultraviolet (UV) again for 20 min. The shrimps were randomly divided into 6 groups. Overnight cultures of S. putrefaciens were inoculated into sterilized distilled water and then three group shrimps were dipping in the solutions. These three groups were used as control samples (CK). The other three groups were dipping in the above prepared S. putrefaciens solution containing a final concentration of 100% (v/v) CFS of A. sobria for 1 min. These three groups were used as test samples (SA). After draining in the relatively sterile environment, six shrimp groups were stored separately according to the cold chain logistics process in Figure 1, recorded as CK1, SA1; CK2, SA2; CK3, SA3.
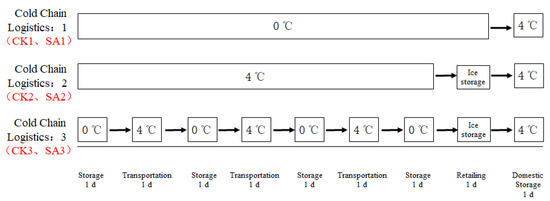
Figure 1.
Simulated situation of temperature changes in cold chain logistics process of Pacific white shrimp.
According to the basic requirements of food technology during cold chain logistics and food sold in supermarket, the temperature of food stored in cold storage is 0 °C, the temperature of the refrigerated transport box is 4 °C, the temperature of the ice station for retailing is 0 °C and the temperature of domestic storage is 4 °C. According to the temperature of the above simulated cold chain logistics process, the prepared shrimp samples were stored in a refrigerator with the corresponding temperature, respectively.
2.4. Microbiological Analysis
The bacteriological analysis in this study carried out including total H2S producer. Twenty-five grams of shrimps were homogenized with 225 mL sterilized saline water (NaCl, 0.85%, w/v, Qingdao Hope Biol-Technology Co., Ltd., Qingdao, China). Then, for bacteriological analysis [23], serial decimal dilutions of each group were carried out with sterilized saline water. 1.0 mL of the dilutions were spread plated into Iron Agar (IA, Qingdao Hope Biol-Technology Co., Ltd., Qingdao, China) for the enumeration of total H2S producer. The IA plates were inoculated at 30 °C for 72 h and black colonies were enumerated.
2.5. Sensory Evaluation
The whole shrimp samples for sensory analysis were displayed on a cleaned white porcelain plate by thirsty untrained panelists from the College of Food Science and Technology, Shanghai Ocean University, using a 9-point hedonic scales (9 = like extremely to 1 = dislike extremely). Sensory characteristics include odors, texture, color and other general appearance [24,25]. The mean of the scores represented the overall sensory quality of shrimps. The panelists should be familiar with the rating scales beforehand.
2.6. Determination of Total Volatile Basic Nitrogen (TVB-N)
TVB-N was determined according to the method of Dabadé et al. with the application of an Automatic Kjeldahl Apparatus (KjeltecTM8400; FOSS Quality Assurance Co., Ltd., Copenhagen, Denmark) [6]. TVB-N contents were expressed as mg N/100 g shrimp flesh.
2.7. Biogenic Amines Analysis
The extraction of biogenic amines was carried out according to the method of Ikonic et al. and Tasic et al. [26,27]. Then the accumulation of biogenic amines was analyzed by HPLC system (SHIMADZU, LC-2010C HT, Kyoto, Japan) after filtered through a 0.45-μm membrane filter. The parameters of HPLC analysis were settled according to the method of Qian et al. [28].
2.8. Amino Acid Analysis
The amino acid contents of shrimp samples were determined using an automatic amino acid analyzer (Hitachi Global Co., Ltd., Tokyo, Japan) [29]. Briefly, after 10min of nitrogen blowing, 100 mg shrimp samples were hydrolyzed in 6 M HCl solution (Sinopharm Chemical Reagent Co., Ltd., Beijing, China) including drops of phenol (Sinopharm Chemical Reagent Co., Ltd., Beijing, China) for 24 h at 110 °C. The residual was dissolved in 1.0 mL of citrate buffer (pH 2.2,Sinopharm Chemical Reagent Co., Ltd., Beijing, China) and then passed through a 0.22-μm membrane filter for injection into the analyzer.
2.9. Extraction of Muscle Proteins and Electrophoresis
The myofibrillar proteins were extracted using the method of Qian et al. [2]. The shrimp muscle (5 g) were m inced and stirred in phosphate buffer A (15.6 mmol/L Na2HPO4, 3.5 mmol/L KH2PO4, I = 0.05, pH 7.5, 4 °C; Sinopharm Chemical Reagent Co., Ltd., Beijing, China). The precipitate was collected after centrifugation at 1000 g and then centrifuged with phosphate buffer B (15.6 mmol/L Na2HPO4, 3.5 mmol/L KH2PO4, 0.45 mol/L KCl, I = 0.5, pH 7.5, 4 °C) at 1000 g, 4 °C. The Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, GenScript Co., Ltd., Nanjing, China) was conducted referring to the method of Qian et al. [28].
2.10. Statistical Analysis
All the experiments were repeated at least three times and the results are reported as averages. F test (LSD and Duncan’s test) was performed to evaluate the mean differences between measurements at the 5% confidence level (p < 0.05) using the SPSS software program (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Effect of the CFS on the Growth of S. putrefaciens in Pacific White Shrimp
Changes in the population of S. putrefaciens on Pacific while shrimp stored at different cold chain logistics are shown in Figure 2. The initial viable count of shrimp samples was about 4.0 log CFU/g and then an increase in growth of all the samples was detected. Group CK1 and SA1 increased slowly stored at 0 °C during transportation and retailing. Group CK1 and SA1 were significantly lower than group CK2, SA2, CK3 and SA3 at the storage of 4 days (Table 1). Group SA1 treated with CFS from A. sobria only reached 6.03 log CFU/g after domestic storage at 4 °C, which was significantly lower than that of group CK1 (Table 1). Group CK3 and SA3 exposure to temperature fluctuation during transportation and retailing increased significantly after six days storage, which was even higher than group CK2 and SA2 stored at 4 °C, respectively. At the storage of eight days, Table 1 shows that the initial viable count of group SA1 was lower than CK3, but there was not a significant difference among group CK1, CK2, SA2 and SA3. Lower counts were found in SA3 than CK3, indicating that CFS from A. sobria could inhibit the growth of S. putrefaciens effectively over time.
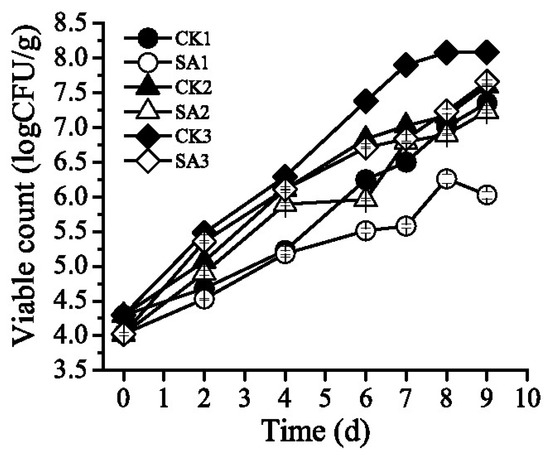
Figure 2.
Effect of cell-free supernatant (CFS) from Aeromonas sobria on the growth of Shewanella putrefaciens in Pacific white shrimp (Litopenaeus vannamei) during different cold chain logistics.

Table 1.
Effect of CFS from A. sobria on the growth of S.putrefaciens in Pacific white shrimp (L. vannamei) during different cold chain logistics by F test.
3.2. Effect of the CFS on Sensory Quality of Pacific White Shrimp
The sensory score of Pacific white shrimp (Figure 3) from 0 to 9 days with various cold chain logistics including transportation, retailing, and domestic storage decreased significantly during the storage time (p < 0.05). Sensorial qualities of shrimp include color, odor and firmness. In the experiment, fresh shrimp got a sensory score of 9 with the fresh sea-weedy odor and firm texture. As shown in Table 2, during storage of 0 to 4 days, there were no difference in sensory quality for all attributes among all the shrimp samples of refrigerated storage (p > 0.05). After 6 days of storage, group CK3 exposure to temperature fluctuation exhibited slight ammonia odor and slightly soft texture with a sensory score of 6.1, while the score of group CK2 is 7.1 and the sensory score of group CK3 was significantly lower than that of group CK1, CK2 and SA3 (Table 2). On the 8 days, compared to control samples, the shrimps treated by CFS from A. sobria retained its original color and firm texture. It was worth notice that soft texture, slime and sour off-flavors of group SA3 were better than group CK3 at the end of the storage.
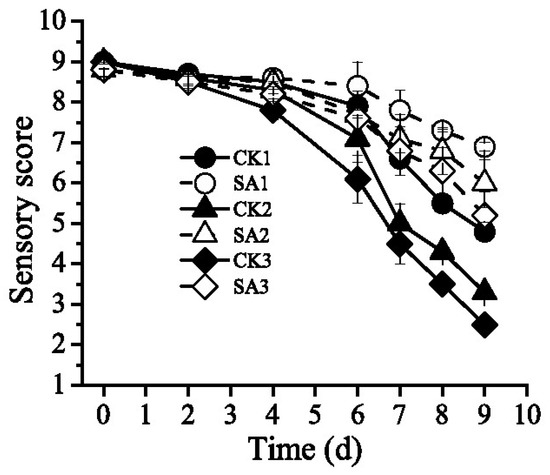
Figure 3.
Effect of CFS from A. sobria on sensory quality of Pacific white shrimp (L. vannamei) during different cold chain logistics.

Table 2.
Effect of CFS from A. sobria on sensory quality of Pacific white shrimp (L. vannamei) during different cold chain logistics by F test.
3.3. Effect of the CFS on TVB-N Content of Pacific White Shrimp
Figure 4 shows changes on the TVB-N content of Pacific white shrimp during transportation, retailing, and domestic storage. At the beginning of the storage, the TVB-N value of control samples was about 5.14 mg N/100 g and that of test samples was 4.86 mg N/100 g. After the storage of 6 days, the TVB-N value of shrimp samples was significantly increased (Figure 4). As shown in Table 3, group CK1 stored at 0 °C was obviously lower than group CK2 and CK3 at the storage of 6 days and group CK3 exposure to temperature fluctuation during transportation and retailing was slightly higher than group CK2 stored at 4 °C. After domestic storage, group SA1, group SA2 and group SA3 treated by CFS from A. sobria inhibited TVB-N production by 34.89%, 29.86% and 17.85%, respectively.
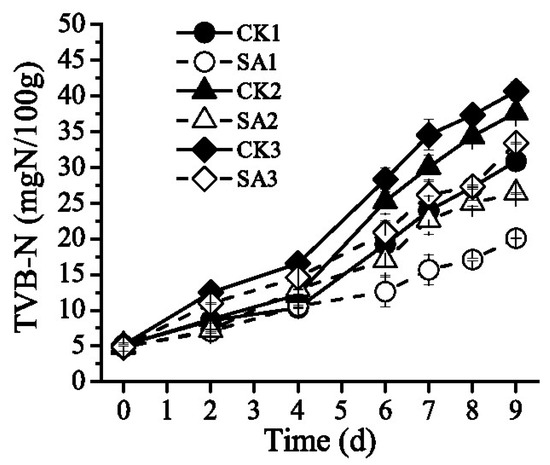
Figure 4.
Effect of CFS from A. sobria on TVB-N content of Pacific white shrimp (L. vannamei) during different cold chain logistics.

Table 3.
Effect of CFS from A. sobria on TVB-N content of Pacific white shrimp (L. vannamei) during different cold chain logistics by F test.
3.4. Effect of the CFS on Biogenic Amine Production of Pacific White Shrimp
Putrescine and cadaverine were the two major biogenic amines found in shrimp, and their changes were shown in Figure 5. During the cold chain logistics, the accumulations of biogenic amines were increased. Through the cold chain logistics, the contents of two biogenic amines in CK3 were higher than CK1 and CK2 (Figure 5). As shown in Table 4 and Table 5, the contents of two biogenic amines in group SA3 were lower than that of group CK3 at the end of the storage. At the storage of 6 days, Table 5 shows that the content of cadaverine in group CK3 was significantly higher than group CK1, CK2 and SA3 (p < 0.05), while there was not obviously difference among group CK1, CK2, SA1, SA2 and SA3 (p > 0.05). Additionally, at the end of the storage, for group SA1, SA2, and SA3 treated with CFS from A. sobria, the levels of putrescine in shrimp samples reduced by 18.65%, 18.21% and 16.05%, and the levels of cadaverine decreased by 22.81%, 22.80% and 18.96%, respectively.
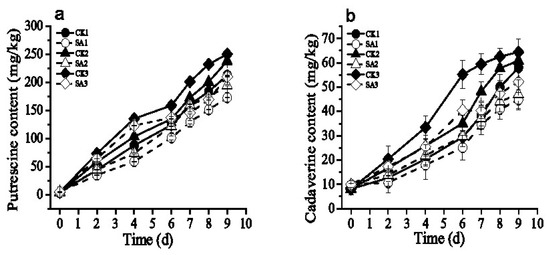
Figure 5.
Effect of CFS from A. sobria on (a) putrescine and (b) cadaverine production of Pacific white shrimp (L. vannamei) during different cold chain logistics.

Table 4.
Effect of CFS from A. sobria on putrescine production of Pacific white shrimp (L. vannamei) during different cold chain logistics.

Table 5.
Effect of CFS from A. sobria on cadaverine production of Pacific white shrimp (L. vannamei) during different cold chain logistics.
3.5. Effect of the CFS on Amino Acid Content of Pacific White Shrimp
The content of 15 amino acidsthat were changed obviously of all samples stored in various cold chain logistics are depicted in Table 6. During transportation, retailing, and domestic storage, the content of total amino acid decreased by levels of 14.96%, 24.30% and 26.23% in group CK1, CK2 and CK3, and the content of total amino acid reduced by levels of 3.09%, 7.57% and 11.82% in group SA1, SA2 and SA3 treated by CFS from A. sobria, respectively. In detail, compared with group CK3, group SA3 treated with CFS from A. sobria inhibited the decreasing of lysine and arginine.

Table 6.
Effect of CFS from A. sobria on amino acid content of Pacific white shrimp (L. vannamei).
3.6. Effect of the CFS on the Sodium Dodecyl Sulfate-polyacrylamide Gel Electrophoresis (SDS-PAGE) Profile of the Myofibrillar Proteins of Pacific White Shrimp
Figure 6 shows the SDS-PAGE profile of the myofibrillar proteins of shrimp during the cold chain logistics, including transportation, retailing, and domestic storage. In fresh shrimps, the bands of group SA treated by CFS from A. sobria were more intense compared with control samples. Additionally, there was almost no difference in band I among group SA1, SA2 and SA3, but the band II in group SA2 was little lighter than SA1 and SA3.
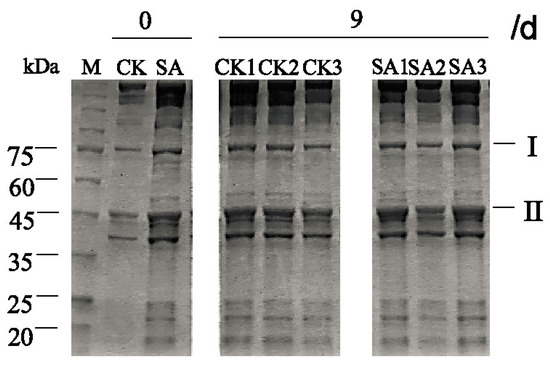
Figure 6.
Effect of cell-free culture supernatant (CFS) from A. sobria on the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) profile of the myofibrillar proteins of Pacific white shrimp (L. vannamei) during different cold chain logistics.
4. Discussions
This study obtained an insight into the effect of CFS from A. sobria on the spoilage of S. putrefaciens in Pacific white shrimp in different cold chain logistics during transportation, retailing, and domestic storage, which have a negative effect on shrimp [18,19]. Our previous research also indicated that Shewanella and Aeromonas species were identified the specific spoilage bacteria in the spoiled Pacific white shrimp and that CFS from A. sobria could inhibit the growth ability and the spoilage abilities of S. putrefaciens during the refrigerated condition. Therefore, the effect of CFS from A. sobria on the spoilage of S. putrefaciens in Pacific white shrimp in different cold chain logistics is a worthy subject to be studied. Until now, the related results have been largely unknown. Our data indicated that the initial bacterial counts of the fresh shrimp samples inoculated by S. putrefaciens were initially around 4.0 log CFU/g. The inhibitory effect on bacterial growth was temperature-related. S. putrefaciens counts in group CK1 and SA1 grew slowly, indicating that 0 °C is not suitable for S. putrefaciens, while CK2 and SA2 grew much faster. Similar studies were also reported that the total counts (TAC) in tilapia fillets increased faster at 10 °C than stored at 0, 4 and 7 °C [19]. Table 1 shows that S. putrefaciens counts in group CK3 and SA3 were higher than group CK2 and SA2, respectively (p < 0.05), which indicated that temperature fluctuation during transportation and retailing was beneficial for the growth of S. putrefaciens [18]. The CFS from A. sobria results in the decline on the growth of S. putrefaciens after the early stationary phase at refrigerated storage consistent with our previous research results. It was noticeable that bacteria counts were lower in group SA3 treated with CFS from A. sobria in comparison with that in group CK3, which showed that the CFS also has a role in the condition of temperature fluctuation during storage. Similar studies were also reported that the addition of CFS from P. fluorescens to S. baltica culture has caused cell decline during the stationary phase, while had no effect on the growth rate during the lag and exponential phase [12]. It could be hypothesized that plenty of metabolites secreted by A. sobria may influence the micro-environment and regulate the production of substance, which might inhibit the growth of S. putrefaciens.
The acceptability of shrimp to customer is closely related to their sensory evaluation, including color, odor, texture and elasticity of shrimp. The fresh shrimps were fresh sea-weedy odor and firm texture, while it became strong ammonia odor, sulfur odor and soft texture by the end of storage [30]. The sensory scores for the evaluated attributes of shrimp samples (Figure 3) all decreased with increasing storage time at different cold chain logistics. These results were consistent with Özogul et al. who found that the odor and texture of cooked fillets reduced significantly during refrigerated storage (p < 0.05) [31]. The differences between CK2 and CK3 were not significant, and CK1 seemed to be much better than them. The results indicated that the temperature fluctuation can decrease the sensory quality of shrimp. The sensory quality of group SA3 was significantly different from that of group CK3 (Table 2). Therefore, CFS from A. sobria could offset the quality deterioration of shrimp exposed to temperature fluctuation during cold chain logistics. TVB-N is composed of basic nitrogenous substances, such as ammonia and amines, due to the activity of spoilage bacteria and endogenous enzymes. The spoilage activity of S. putrefaciens can be quantified by determining the accumulation of TVB-N [22]. Highest TVB-N value after 6 days of storage were found in CK3 and CK2, and lowest value were found in SA1. Therefore, constant temperature and CFS from A. sobria could inhibit the accumulation of TVB-N. The differences of TVB-N between CK3 and SA3 were less significant than that between CK1 and SA1.
The spoilage potential of S. putrefaciens also relates to the activity of amino acid decarboxylase, which catalyzes the production of biogenic amine [11]. S. putrefaciens is chiefly involved in the production of putrescine and cadaverine. Application of CFS from A. sobria in culturing of S. putrefaciens produced a reduction in the formation of putrescine and cadaverine during the experimental period. In addition, there was not a significant difference in the contents of cadaverine in group CK2 and SA2 (Table 5), indicated that the CFS had few effects on the accumulation of cadaverine in shrimps stored at 4 °C. The contents of two biogenic amines in CK3 were higher than CK1 and CK2 and group CK3 was obviously increased on 6 days, due to temperature fluctuation stimulating the growth of bacteria and then accelerating the amino acid decarboxylase. The contents of two biogenic amines in group SA3 were lower than that of group CK3 at the end of the storage. It is concluded that the application of CFS could inhibit the accumulation of biogenic amines in shrimps exposed to temperature fluctuation during cold chain logistics. Arginine and ornithine are the main precursors of putrescine, and lysine is for cadaverine [13,32]. The changes between amino acids and biogenic amines showed a strong relationship, as the content of arginine and lysine also decreased significantly during the experimental period. It was assumed that the metabolites of A. sobria influenced the activities of enzymes in S. putrefaciens and related to the content of metabolites in cold environment [7]. The decreasing in the contents of serine, glutamic acid and glycine of Pacific white shrimp may also lead to the reduction of umami characteristics and then affecting its flavor and taste [33]. The results of the SDS-PAGE profile of myofibrillar proteins of shrimp were consistent with that of TVB-N and biogenic amine. It showed that application of CFS from A. sobria lead to less protein hydrolysis.
5. Conclusions
The effect of CFS from A. sobria on the spoilage of S. putrefaciens in Pacific white shrimp was observed in different cold chain logistics during transportation, retailing, and domestic storage. TVB-N and biogenic amine increased, while sensory scores decreased, with different cold chain logistics. Temperature fluctuation could accelerate the growth rate of S. putrefaciens. It was preliminarily confirmed that CFS from A. sobria could inhibit the activity of enzymes related to protein hydrolysis and production of TVB-N, putrescine and cadaverine. In addition, the CFS from A. sobria could offset the negative effect of temperature fluctuation on shrimp storage during the experimental period. Further studies are needed to research on the metabolites and regulatory mechanism of CFS against spoilage potential of S. putrefaciens in shrimp.
Author Contributions
J.-X.Y. conceived the idea, designed and performed the experiments, and wrote and revised the paper; Y.-F.Q. and J.X. analyzed the experimental results and revised the paper; S.-P.Y. performed the experiments and revised the paper.
Funding
This work was financially supported by National Natural Science Foundation of China [grant number: 31501551, 31571914], Shanghai Municipal Science and Technology Project to Enhance the Capabilities of the Platform [grant number: 16DZ2280300], and Shanghai Science and Technology Key Project on Agriculture from Shanghai Municipal Agricultural Commission [grant number: (2016) 1-1].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jescovitch, L.N.; Ullman, C.; Rhodes, M.; Davis, D.A. Effects of different feed management treatments on water quality for Pacific white shrimp Litopenaeus vannamei. Aquac. Res. 2018, 49, 526–531. [Google Scholar] [CrossRef]
- Qian, Y.-F.; Xie, J.; Yang, S.-P.; Huang, S.; Wu, W.-H.; Li, L. Inhibitory effect of a quercetin-based soaking formulation and modified atmospheric packaging (MAP) on muscle degradation of Pacific white shrimp (Litopenaeus vannamei). LWT-Food Sci. Technol. 2015, 63, 1339–1346. [Google Scholar] [CrossRef]
- Okpala, C.O.R. Investigation of quality attributes of ice-stored Pacific white shrimp (Litopenaeus vannamei) as affected by sequential minimal ozone treatment. LWT-Food Sci. Technol. 2014, 57, 538–547. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Fall, P.-A.; Leroi, F.; Cardinal, M.; Chevalier, F.; Pilet, M.-F. Inhibition of Brochothrix thermosphacta and sensory improvement of tropical peeled cooked shrimp by Lactococcus piscium CNCM I-4031. Lett. Appl. Microbiol. 2010, 50, 357–361. [Google Scholar] [CrossRef]
- Dabadé, D.S.; den Besten, H.M.; Azokpota, P.; Nout, M.R.; Hounhouigan, D.J.; Zwietering, M.H. Spoilage evaluation, shelf-life prediction, and potential spoilage organisms of tropical brackish water shrimp (Penaeus notialis) at different storage temperatures. Food Microbiol. 2015, 48, 8–16. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Nychas, G.-J.E. Quorum sensing in the context of food microbiology. Appl. Environ. Microbiol. 2012, 78, 5473–5482. [Google Scholar] [CrossRef]
- Ginson, J.; Panda, S.K.; Bindu, J.; Kamalakanth, C.K.; Gopal, T.K.S. Effect of high pressure treatment on microbiological quality of Indian white prawn (Fenneropenaeus indicus) during chilled storage. Food Microbiol. 2015, 46, 596–603. [Google Scholar] [CrossRef]
- Yang, S.-P.; Xie, J.; Qian, Y.-F. Determination of spoilage microbiota of Pacific white shrimp during ambient and cold storage using next-generation sequencing and culture-dependent method. J. Food Sci. 2017, 82, 1178–1183. [Google Scholar] [CrossRef]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria—Problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef]
- Fan, H.; Luo, Y.; Yin, X.; Bao, Y.; Feng, L. Biogenic amine and quality changes in lightly salt-and sugar-salted black carp (Mylopharyngodon piceus) fillets stored at 4 °C. Food Chem. 2014, 159, 20–28. [Google Scholar] [CrossRef]
- Zhao, A.; Zhu, J.; Ye, X.; Ge, Y.; Li, J. Inhibition of biofilm development and spoilage potential of Shewanella baltica by quorum sensing signal in cell-free supernatant from Pseudomonas fluorescens. Int. J. Food Microbiol. 2016, 230, 73–80. [Google Scholar] [CrossRef]
- Jorgensen, L.V.; Dalgaard, P.; Huss, H.H. Multiple compound quality index for cold-smoked salmon (Salmo salar) developed by multivariate regression of biogenic amines and pH. J. Agric. Food Chem. 2000, 48, 2448–2453. [Google Scholar] [CrossRef]
- Macé, S.; Cardinal, M.; Jaffrès, E.; Cornet, J.; Lalanne, V.; Chevalier, F.; Sérot, T.; Pilet, M.-F.; Dousset, X.; Joffraud, J.-J. Evaluation of the spoilage potential of bacteria isolated from spoiled cooked whole tropical shrimp (Penaeus vannamei) stored under modified atmosphere packaging. Food Microbiol. 2014, 40, 9–17. [Google Scholar] [CrossRef]
- Laursen, B.G.; Leisner, J.J.; Dalgaard, P. Carnobacterium species: Effect of metabolic activity and interaction with Brochothrix thermosphacta on sensory characteristics of modified atmosphere packed shrimp. J. Agric. Food Chem. 2006, 54, 3604–3611. [Google Scholar] [CrossRef]
- Gui, M.; Wu, R.; Liu, L.; Wang, S.; Zhang, L.; Li, P. Effects of quorum quenching by AHL lactonase on AHLs, protease, motility and proteome patterns in Aeromonas veronii LP-11. Int. J. Food Microbiol. 2017, 252, 61–68. [Google Scholar] [CrossRef]
- Jaffres, E.; Lalanne, V.; Mace, S.; Cornet, J.; Cardinal, M.; Serot, T.; Dousset, X.; Joffraud, J.-J. Sensory characteristics of spoilage and volatile compounds associated with bacteria isolated from cooked and peeled tropical shrimps using SPME-GC-MS analysis. Int. J. Food Microbiol. 2011, 147, 195–202. [Google Scholar] [CrossRef]
- Yue, J.; Liu, L.; Li, Z.; Li, D.; Fu, Z. Improved quality analytical models for aquatic products at the transportation in the cold chain. Math. Comp. Model. Dyn. 2013, 58, 474–479. [Google Scholar] [CrossRef]
- Shi, C.; Yang, X.; Han, S.; Fan, B.; Zhao, Z.; Wu, X.; Qian, J. Nondestructive prediction of tilapia fillet freshness during storage at different temperatures by integrating an electronic nose and tongue with radial basis function neural networks. Food Bioprocess. Technol. 2018, 11, 1840–1852. [Google Scholar] [CrossRef]
- Zhang, Y.; Ertbjerg, P. Effects of frozen-then-chilled storage on proteolytic enzyme activity and water-holding capacity of pork loin. Meat Sci. 2018, 145, 375–382. [Google Scholar] [CrossRef]
- Surette, M.G.; Bassler, B.L. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 1998, 95, 7046–7050. [Google Scholar] [CrossRef]
- Qian, Y.-F.; Xie, J.; Yang, S.-P.; Wu, W.-H.; Xiong, Q.; Gao, Z.-L. In vivo study of spoilage bacteria on polyphenoloxidase activity and melanosis of modified atmosphere packaged Pacific white shrimp. Food Chem. 2014, 155, 126–131. [Google Scholar] [CrossRef]
- Jeyasekaran, G.; Ganesan, P.; Anandaraj, R.; Shakila, R.J.; Sukumar, D. Quantitative and qualitative studies on the bacteriological quality of Indian white shrimp (Penaeus indicus) stored in dry ice. Food Microbiol. 2006, 23, 526–533. [Google Scholar] [CrossRef]
- Qian, Y.-F.; Xie, J.; Yang, S.-P.; Wu, W.-H. Study of the quality changes and myofibrillar proteins of white shrimp (Litopenaeus vannamei) under modified atmosphere packaging with varying CO2 levels. Eur. Food Res. Technol. 2013, 236, 629–635. [Google Scholar] [CrossRef]
- Luzuriaga, D.A.; Korel, F.; Balaban, M.O. Odor evaluation of shrimp treated with different chemicals using an electronic nose and a sensory panel. J. Aquat. Food Prod. Technol. 2007, 16, 57–75. [Google Scholar] [CrossRef]
- Ikonic, P.; Tasic, T.; Petrovic, L.; Skaljac, S.; Jokanovic, M.; Mandic, A.; Ikonic, B. Proteolysis and biogenic amines formation during the ripening of Petrovska klobasa, traditional dry-fermented sausage from Northern Serbia. Food Control 2013, 30, 69–75. [Google Scholar] [CrossRef]
- Tasic, T.; Ikonic, P.; Mandic, A.; Jokanovic, M.; Tomovic, V.; Savatic, S.; Petrovic, L. Biogenic amines content in traditional dry fermented sausage Petrovska klobasa as possible indicator of good manufacturing practice. Food Control 2012, 23, 107–112. [Google Scholar] [CrossRef]
- Qian, Y.-F.; Ye, J.-X.; Yang, S.-P.; Lin, Z.-Q.; Cao, W.; Xie, J. Evaluation of the spoilage potential of Shewanella putrefaciens, Aeromonas hydrophila, and Aeromonas sobria isolated from spoiled Pacific white shrimp (Litopenaeus vannamei) during cold storage. J. Food Saf. 2018, 38, e12550. [Google Scholar] [CrossRef]
- Deng, Y.; Luo, Y.; Wang, Y.; Zhao, Y. Effect of different drying methods on the myosin structure, amino acid composition, protein digestibility and volatile profile of squid fillets. Food Chem. 2015, 171, 168–176. [Google Scholar] [CrossRef]
- Manju, S.; Jose, L.; Gopal, T.K.S.; Ravishankar, C.N.; Lalitha, K.V. Effects of sodium acetate dip treatment and vacuum-packaging on chemical, microbiological, textural and sensory changes of Pearlspot (Etroplus suratensis) during chill storage. Food Chem. 2007, 102, 27–35. [Google Scholar] [CrossRef]
- Ozogul, Y.; Durmus, M.; Ucar, Y.; Ozogul, F.; Regenstein, J.M. Comparative study of nanoemulsions based on commercial oils (sunflower, canola, corn, olive, soybean, and hazelnut oils): Effect on microbial, sensory, and chemical qualities of refrigerated farmed sea bass. Innov. Food Sci. Emerg. Technol. 2016, 33, 422–430. [Google Scholar] [CrossRef]
- Coffino, P. Regulation of cellular polyamines by antizyme. Nat. Rev. Mol. Cell Biol. 2001, 2, 188–194. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Moral, A. Free amino acids in muscle of Norway lobster (Nephrops novergicus (L.)) in controlled and modified atmospheres during chilled storage. Food Chem. 2004, 86, 85–91. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).