A Review of Germanium-Antimony-Telluride Phase Change Materials for Non-Volatile Memories and Optical Modulators
Abstract
:1. Introduction
2. Material Properties
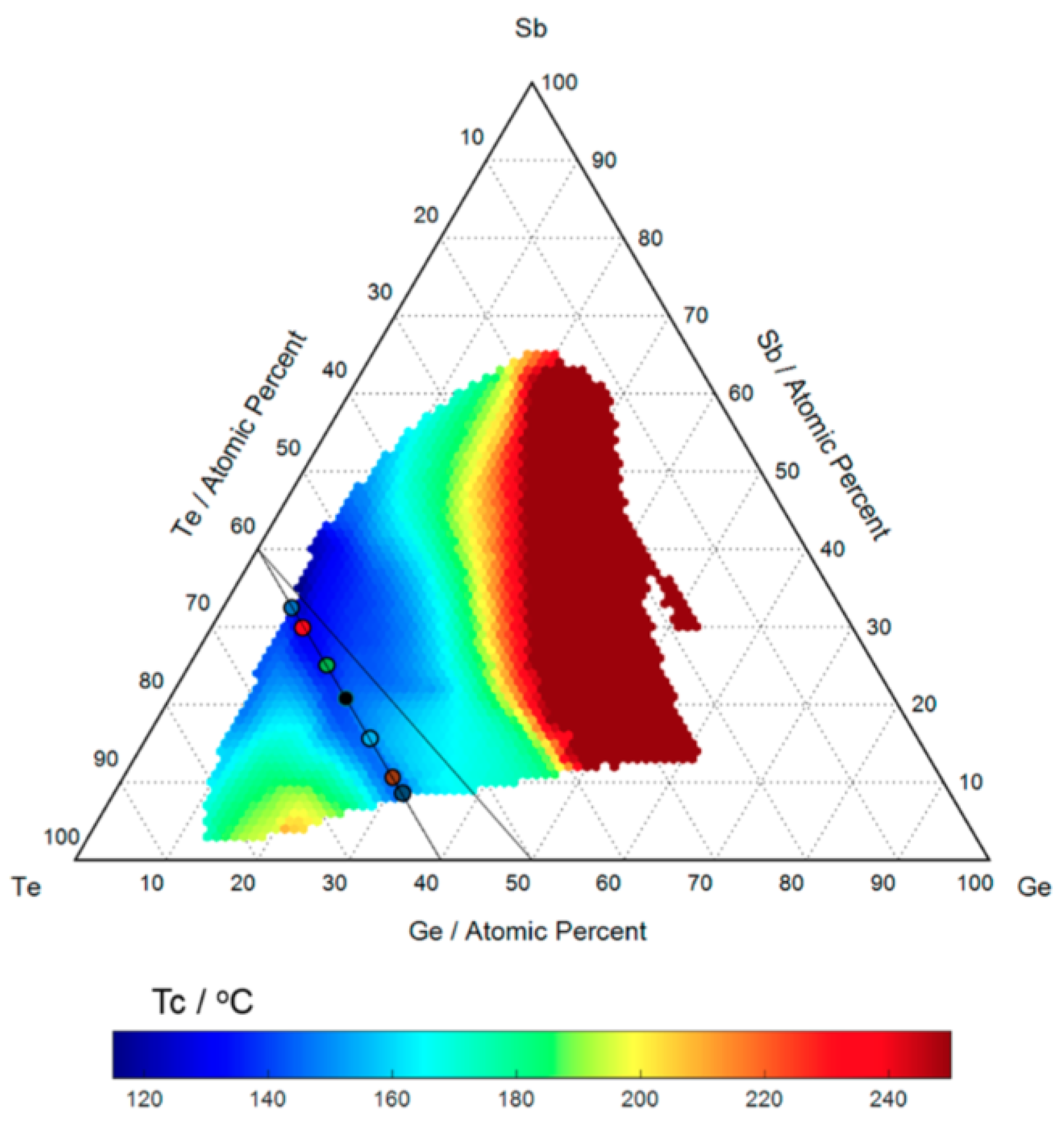
2.1. GeSbTe Alloys
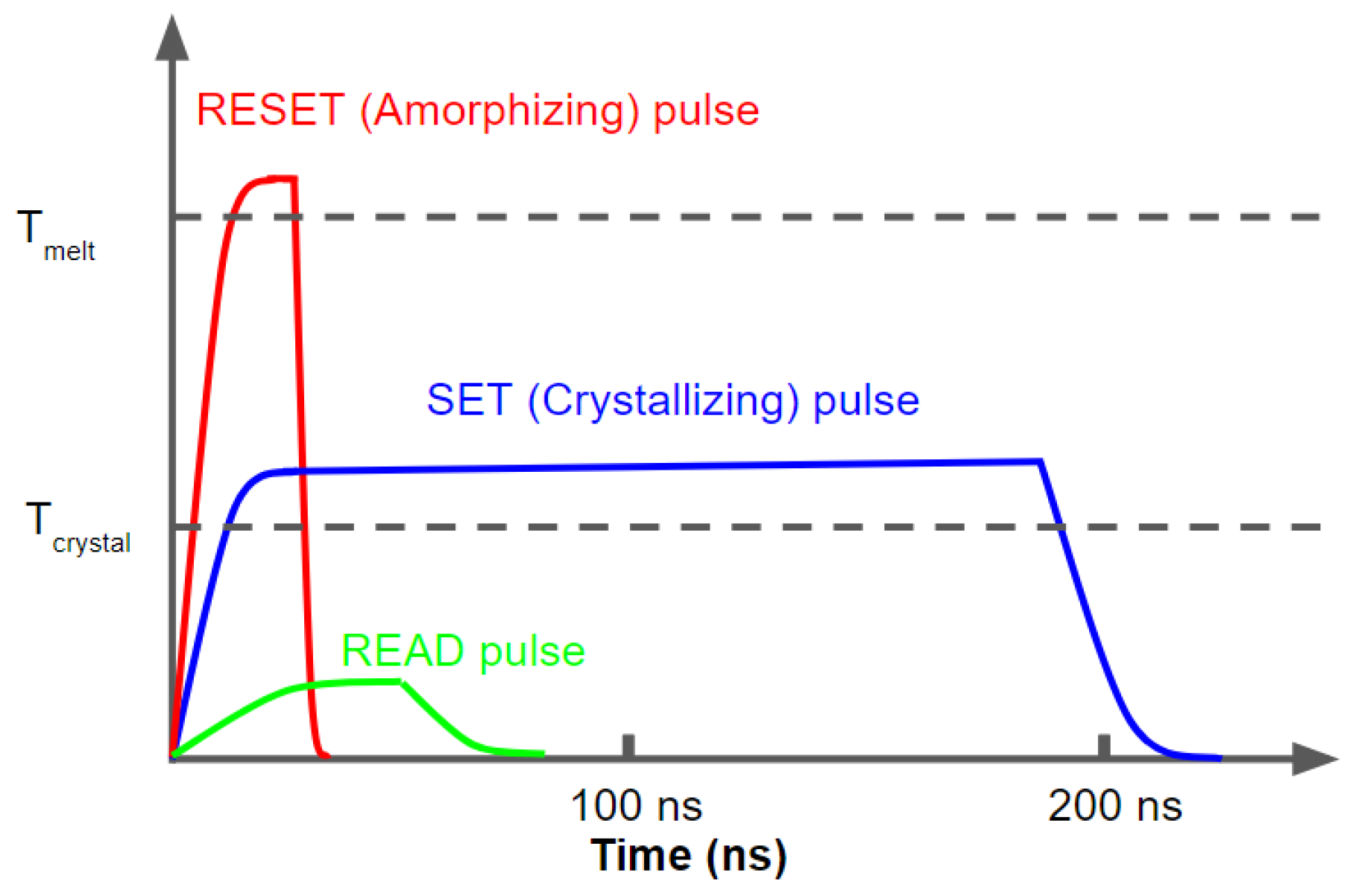
2.2. Switching Properties
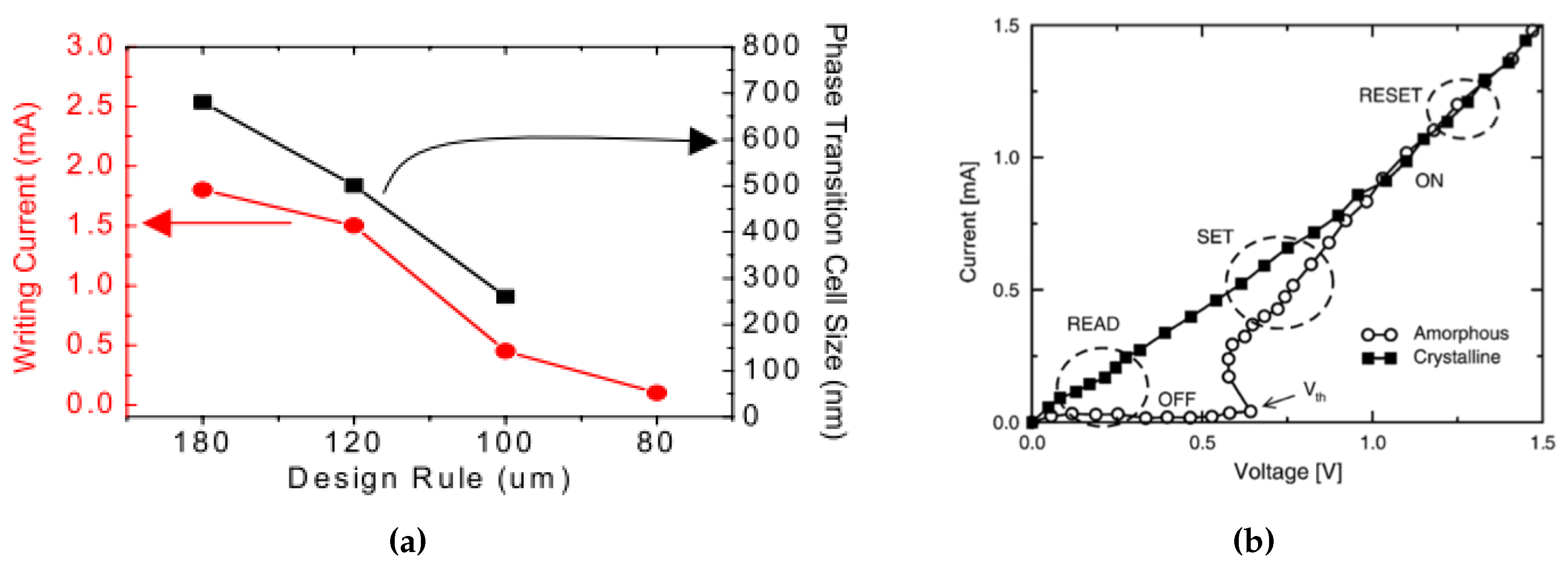
2.3. Electrical Properties
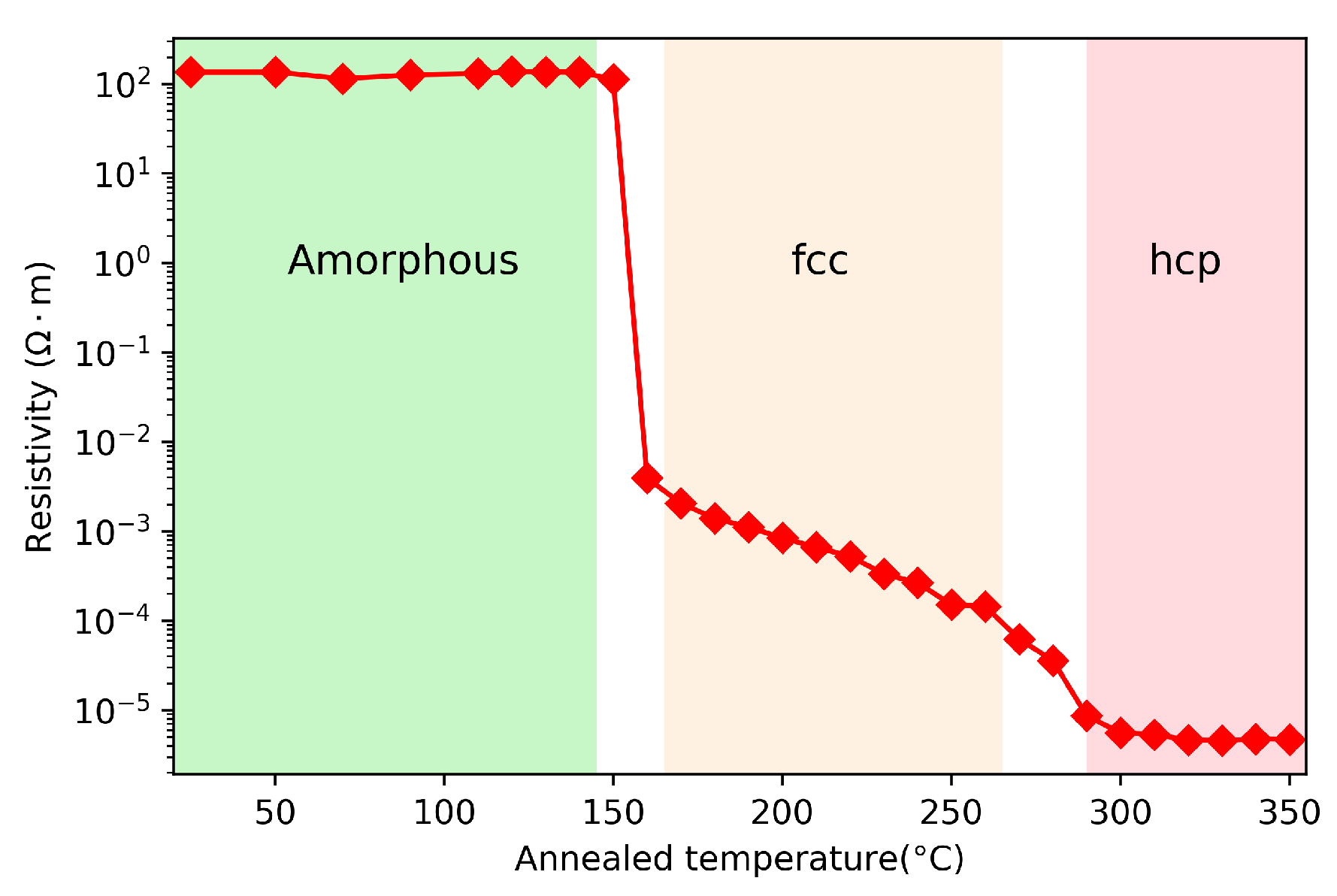
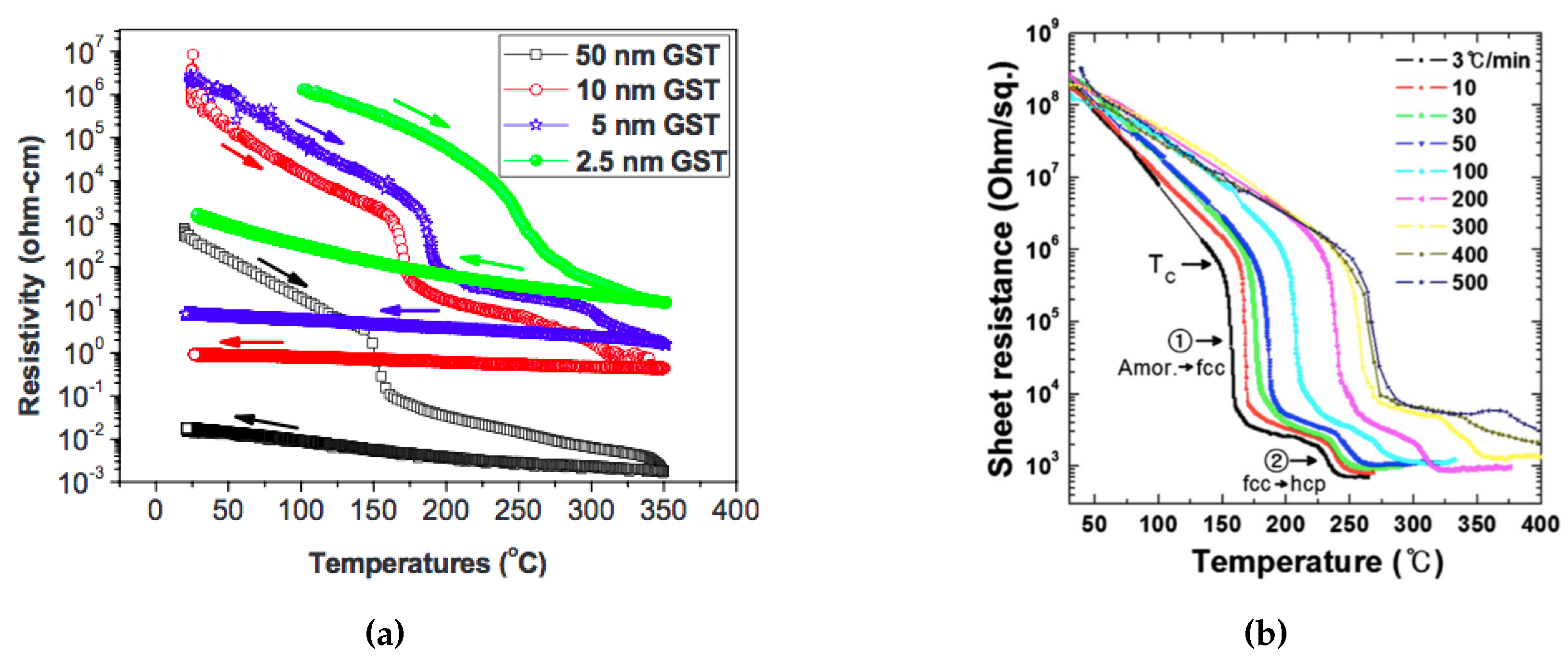
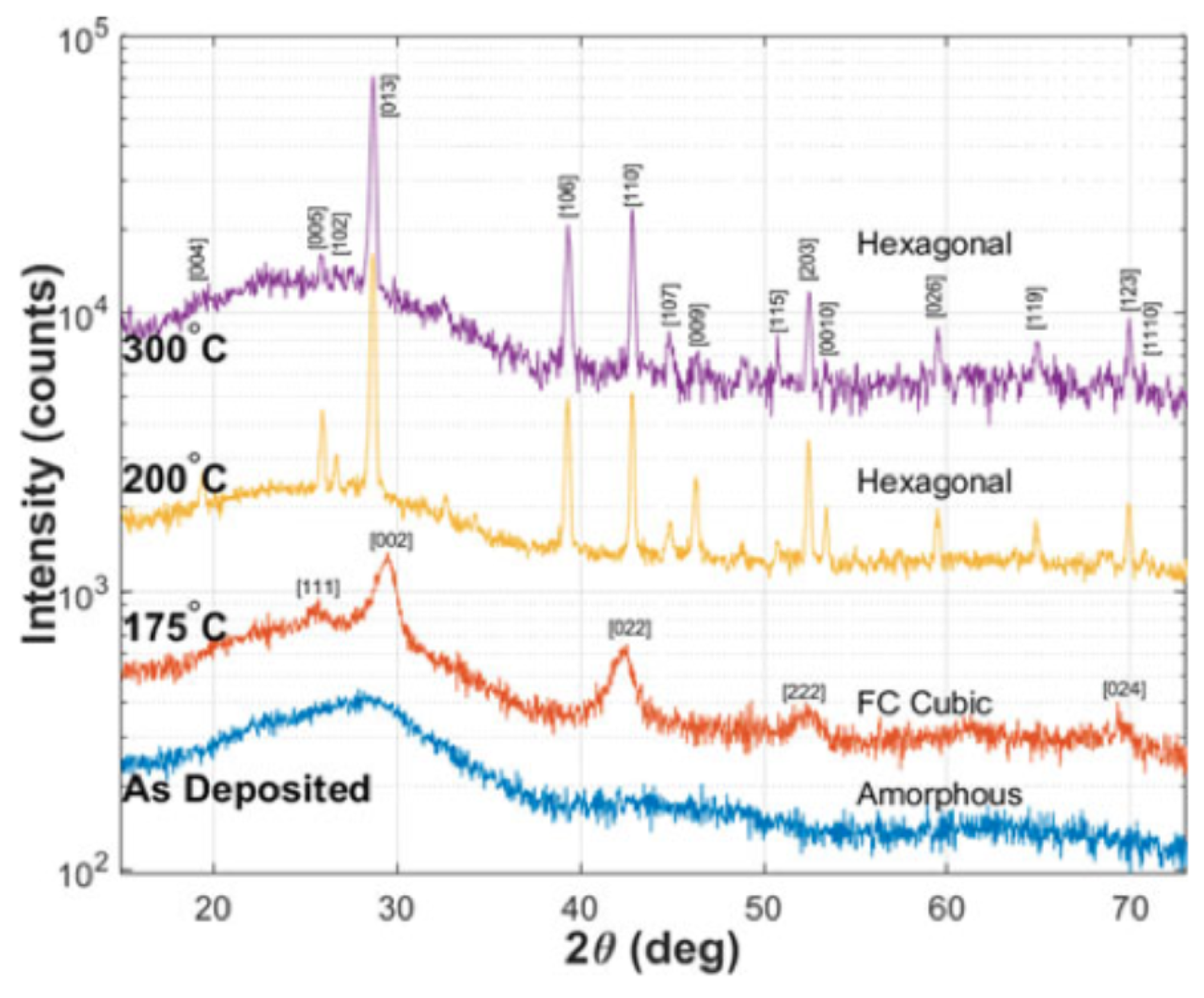
2.4. Structural Properties
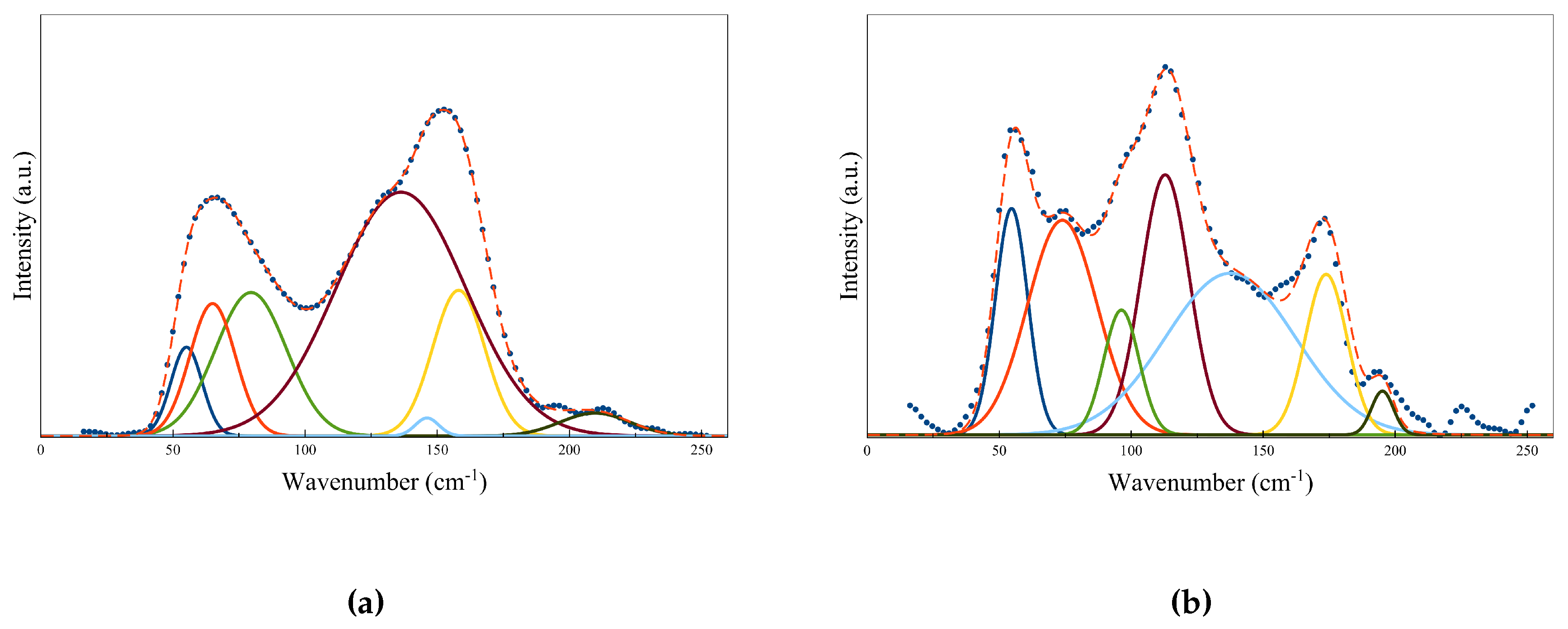
2.5. Local Structural Properties
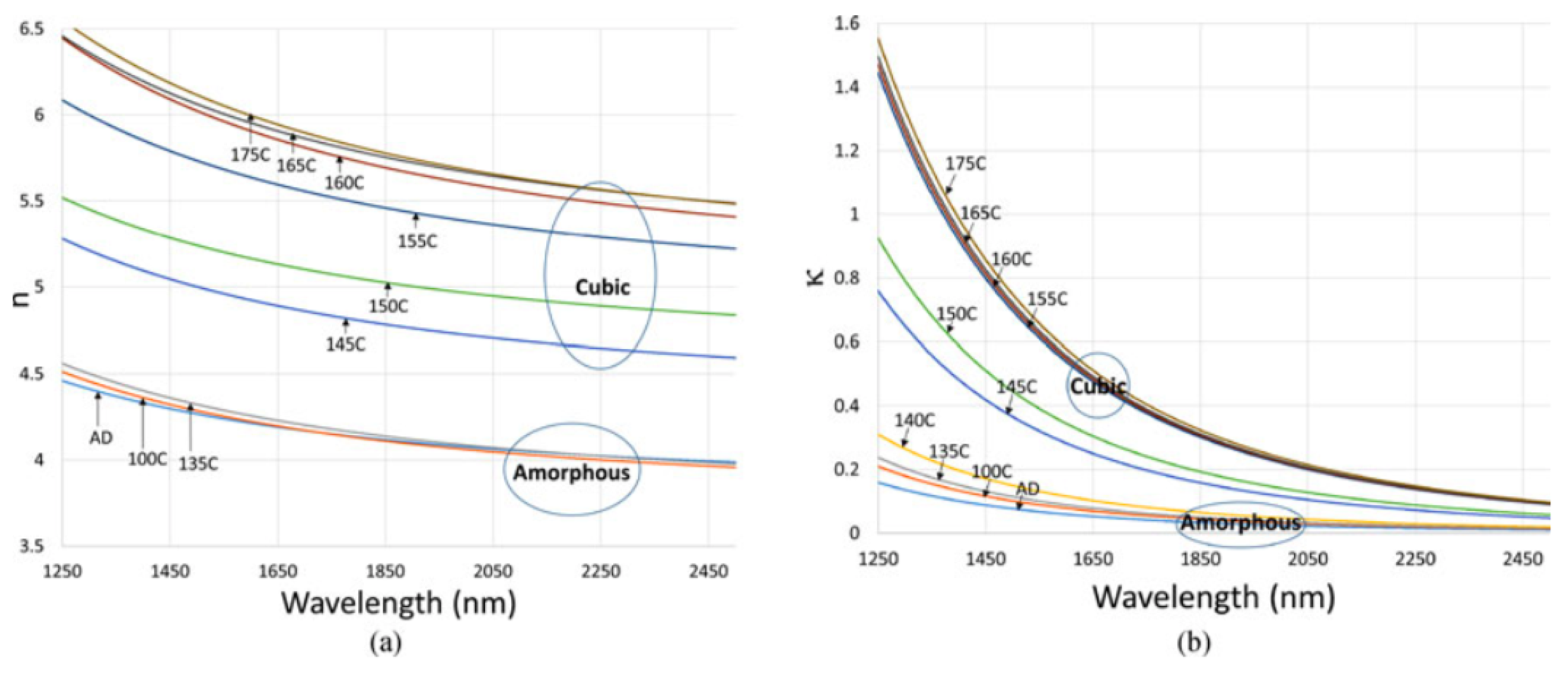
2.6. Optical Constants
3. Applications
3.1. Phase-change Memory (PCM)
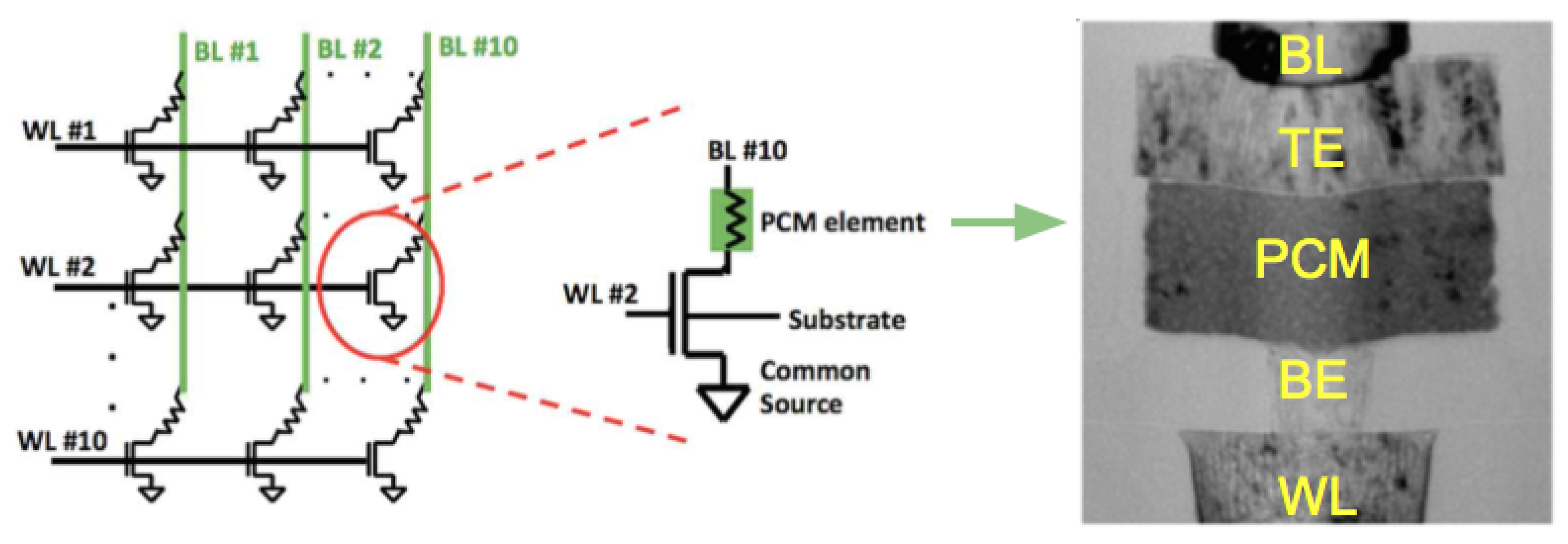
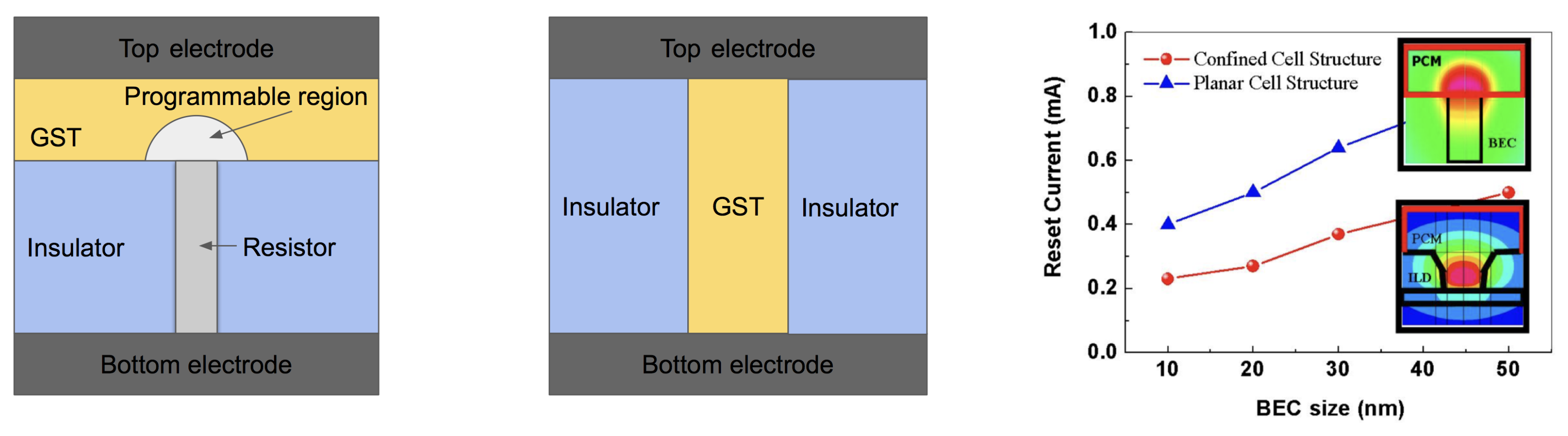
3.1.1. Device Architectures
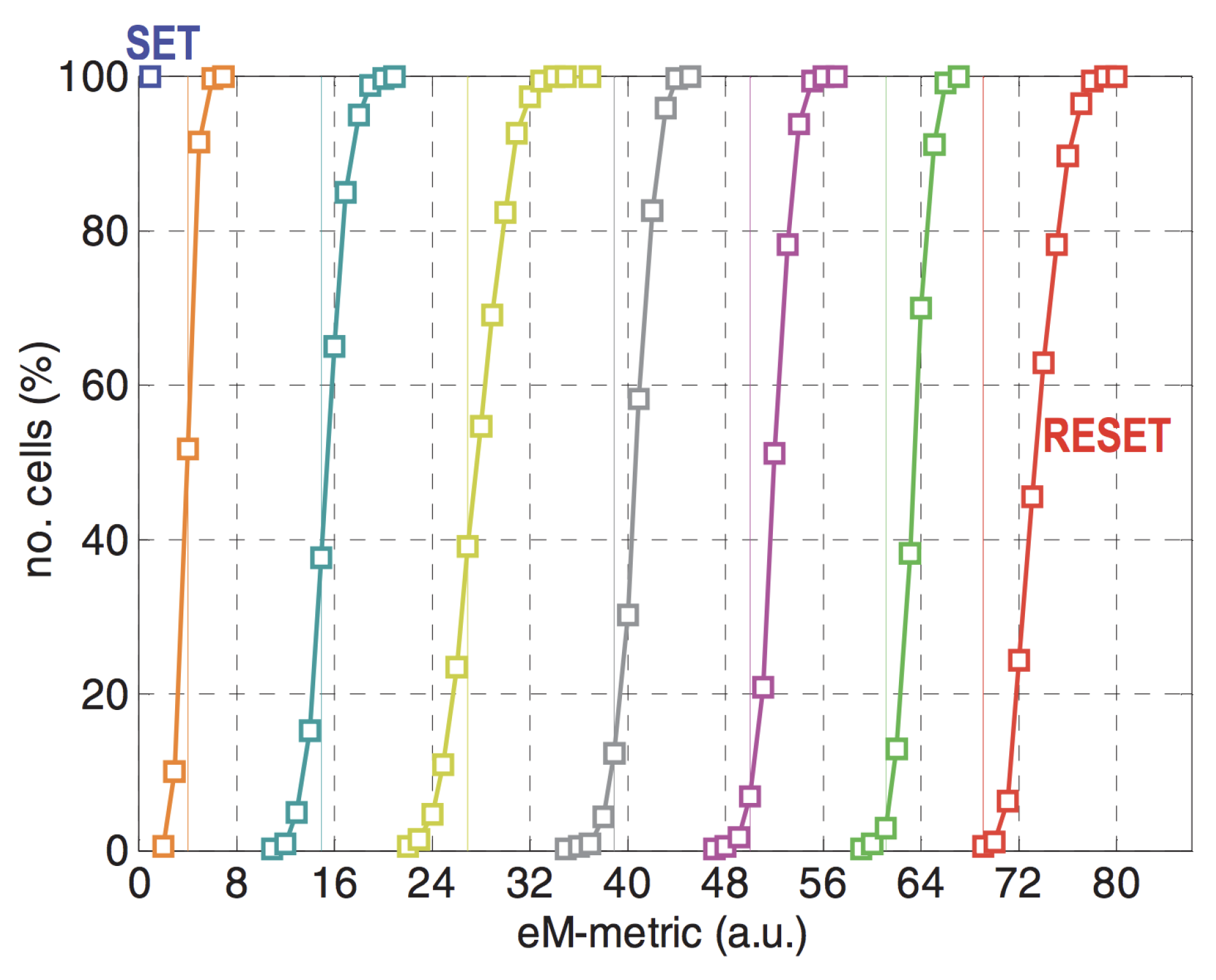
3.1.2. Multi-level Cell (MLC) Memories
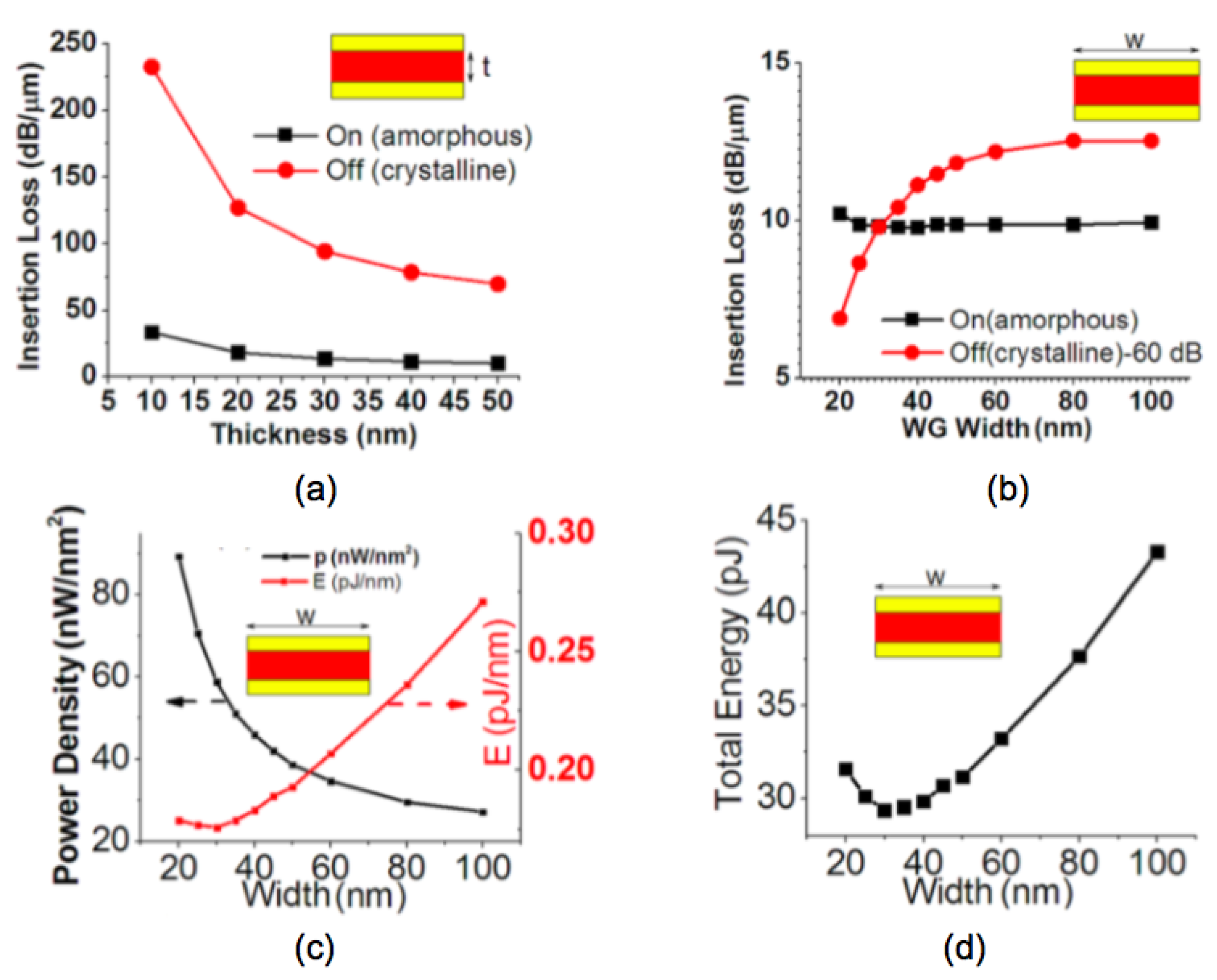
3.2. Light Modulators
3.2.1. Optical Limiters
3.2.2. Meta-Surfaces
4. Doping
4.1. Resistivity at Amorphous State
4.2. Amorphous State Stability
4.3. Operation Speed
5. Deposition Methods
5.1. Sputtering
5.2. Pulsed Laser Deposition (PLD)
5.3. Evaporation
5.4. Chemical Vapor Deposition (CVD)
5.5. Atomic Layer Deposition (ALD)
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wuttig, M.; Yamada, N. Phase-change materials for rewriteable data storage. Nat. Mater. 2007, 6, 824. [Google Scholar] [CrossRef] [PubMed]
- Kolobov, A.V.; Fons, P.; Frenkel, A.I.; Ankudinov, A.L.; Tominaga, J.; Uruga, T. Understanding the phase-change mechanism of rewritable optical media. Nat. Mater. 2004, 3, 703. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.K.; Srinivasan, V.; Rivers, J.A. Scalable high performance main memory system using phase-change memory technology. Acm Sigarch Comput. Archit. News 2009, 37, 24–33. [Google Scholar] [CrossRef]
- Yamada, N.; Ohno, E.; Akahira, N.; Nishiuchi, K.; Nagata, K.; Takao, M. High speed overwritable phase change optical disk material. Jpn. J. Appl. Phys. 1987, 26, 61. [Google Scholar] [CrossRef]
- Yamada, N.; Otoba, M.; Kawahara, K.; Miyagawa, N.; Ohta, H.; Akahira, N.; Matsunaga, T. Phase-change optical disk having a nitride interface layer. Jpn. J. Appl. Phys. 1998, 37, 2104. [Google Scholar] [CrossRef]
- Satoh, I.; Fukushima, Y.; Takagi, Y.; Azumatani, Y.; Hamasaka, H. Multi-layered Optical Disk with Track and Layer Identification. US Patent 5428597, 27 June 1995. [Google Scholar]
- Ohta, T. Phase-change optical memory promotes the DVD optical disk. J. Optoelectron. Adv. Mater. 2001, 3, 609–626. [Google Scholar]
- Yamada, N.; Kojima, R.; Uno, M.; Akiyama, T.; Kitaura, H.; Narumi, K.; Nishiuchi, K. Phase-change material for use in rewritable dual-layer optical disk. In Proceedings of the Optical Data Storage 2001, Santa Fe, NM, USA, 10 January 2002; International Society for Optics and Photonics: Washington, DC, USA, 2002; Volume 4342, pp. 55–64. [Google Scholar]
- Kuwahara, M.; Takehara, S.; Kashihara, Y.; Watabe, K.; Nakano, T.; Tanaka, M.; Nakamura, N.; Ohsawa, H.; Satoh, H. Experimental study of high-density rewritable optical disk using a blue-laser diode. Jpn. J. Appl. Phys. 2003, 42, 1068. [Google Scholar] [CrossRef]
- Nakayama, K.; Kojima, K.; Hayakawa, F.; Imai, Y.; Kitagawa, A.; Suzuki, M. Submicron nonvolatile memory cell based on reversible phase transition in chalcogenide glasses. Jpn. J. Appl. Phys. 2000, 39, 6157. [Google Scholar] [CrossRef]
- Lai, S.; Lowrey, T. OUM-A 180 nm nonvolatile memory cell element technology for stand alone and embedded applications. In Proceedings of the International Electron Devices Meeting. Technical Digest, Washington, DC, USA, 2–5 December 2001. [Google Scholar]
- Lai, S. Current status of the phase change memory and its future. In Proceedings of the IEEE International Electron Devices Meeting 2003, Washington, DC, USA, 8–10 December 2003. [Google Scholar]
- Yi, J.; Ha, Y.; Park, J.; Kuh, B.; Horii, H.; Kim, Y.; Park, S.; Hwang, Y.; Lee, S.; Ahn, S.; et al. Novel cell structure of PRAM with thin metal layer inserted GeSbTe. In Proceedings of the IEEE International Electron Devices Meeting 2003, Washington, DC, USA, 8–10 December 2003. [Google Scholar]
- Koba, I.M. High-resolution Spatial Light Modulator for 3-dimensional Holographic Display. US Patent 6819469, 16 November 2004. [Google Scholar]
- Sarangan, A.; Duran, J.; Vasilyev, V.; Limberopoulos, N.; Vitebskiy, I.; Anisimov, I. Broadband Reflective Optical Limiter Using GST Phase Change Material. IEEE Photonics J. 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Gholipour, B.; Zhang, J.; MacDonald, K.F.; Hewak, D.W.; Zheludev, N.I. An all-optical, non-volatile, bidirectional, phase-change meta-switch. Adv. Mater. 2013, 25, 3050–3054. [Google Scholar] [CrossRef] [PubMed]
- Sieber, P.E.; Werner, D.H. Reconfigurable broadband infrared circularly polarizing reflectors based on phase changing birefringent metasurfaces. Opt. Express 2013, 21, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Guerin, S.; Hayden, B.; Hewak, D.W.; Vian, C. Synthesis and screening of phase change chalcogenide thin film materials for data storage. ACS Comb. Sci. 2017, 19, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C. Topology of covalent non-crystalline solids I: Short-range order in chalcogenide alloys. J. Non-Cryst. Solids 1979, 34, 153–181. [Google Scholar] [CrossRef]
- Ueno, O.; Kobayashi, H. Optical Recording Medium. US Patent 5254382, 19 October 1993. [Google Scholar]
- Zhang, W.; Wuttig, M.; Mazzarello, R. Effects of stoichiometry on the transport properties of crystalline phase-change materials. Sci. Rep. 2015, 5, 13496. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Mansuripur, M. Amorphization induced by subnanosecond laser pulses in phase-change optical recording media. Appl. Opt. 2004, 43, 4367–4375. [Google Scholar] [CrossRef] [PubMed]
- Loke, D.; Lee, T.; Wang, W.; Shi, L.; Zhao, R.; Yeo, Y.; Chong, T.; Elliott, S. Breaking the speed limits of phase-change memory. Science 2012, 336, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Ovshinsky, S.R.; Hudgens, S.J.; Czubatyj, W.; Strand, D.A.; Wicker, G.C. Electrically Erasable Phase Change Memory. US Patent 5166758, 24 November 1992. [Google Scholar]
- Reinberg, A.R.; Zahorik, R.C. Small Electrode for a Chalcogenide Switching Device and Method for Fabricating Same. US Patent 5952671, 14 September 1999. [Google Scholar]
- Pirovano, A.; Lacaita, A.L.; Benvenuti, A.; Pellizzer, F.; Bez, R. Electronic switching in phase-change memories. IEEE Trans. Electron Devices 2004, 51, 452–459. [Google Scholar] [CrossRef]
- Yang, J.J.; Strukov, D.B.; Stewart, D.R. Memristive devices for computing. Nat. Nanotechnol. 2013, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Tanaka, K. Electronic properties of amorphous and crystalline Ge2Sb2Te5 films. Jpn. J. Appl. Phys. 2005, 44, 7340. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Raoux, S.; Chen, Y.C. The impact of film thickness and melt-quenched phase on the phase transition characteristics of Ge2Sb2Te5. J. Appl. Phys. 2010, 107, 074308. [Google Scholar] [CrossRef]
- Simpson, R.; Krbal, M.; Fons, P.; Kolobov, A.; Tominaga, J.; Uruga, T.; Tanida, H. Toward the ultimate limit of phase change in Ge2Sb2Te5. Nano lett. 2009, 10, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Jung, M.; Lee, Y.K. Effect of heating rate on the activation energy for crystallization of amorphous Ge2Sb2Te5 thin film. Electrochem. Solid-State Lett. 2009, 12, F17–F19. [Google Scholar] [CrossRef]
- Lazarenko, P.; Sherchenkov, A.; Kozyukhin, S.; Babich, A.; Timoshenkov, S.; Gromov, D.; Shuliatyev, A.; Redichev, E. Electrical Properties of the Ge2Sb2Te5 Thin Films for Phase Change Memory Application; AIP Publishing: New York, NY, USA, 2016; Volume 1727, p. 020013. [Google Scholar]
- Kiouseloglou, A.; Navarro, G.; Sousa, V.; Persico, A.; Roule, A.; Cabrini, A.; Torelli, G.; Maitrejean, S.; Reimbold, G.; De Salvo, B.; et al. A novel programming technique to boost low-resistance state performance in Ge-rich GST phase change memory. IEEE Trans. Electron. Devices 2014, 61, 1246–1254. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Z.; Song, S.; Xie, H.; Song, Z.; Li, X.; Shen, L.; Li, L.; Wu, L.; Liu, B. Ni-doped GST materials for high speed phase change memory applications. Mater. Res. Bull. 2015, 64, 333–336. [Google Scholar] [CrossRef]
- Leskelä, M.; Pore, V.; Hatanpää, T.; Heikkilä, M.; Ritala, M.; Schrott, A.; Raoux, S.; Rossnagel, S. Atomic layer deposition of materials for phase-change memories. ECS Trans. 2009, 25, 399–407. [Google Scholar]
- Fallica, R.; Battaglia, J.L.; Cocco, S.; Monguzzi, C.; Teren, A.; Wiemer, C.; Varesi, E.; Cecchini, R.; Gotti, A.; Fanciulli, M. Thermal and electrical characterization of materials for phase-change memory cells. J. Chem. Eng. Data 2009, 54, 1698–1701. [Google Scholar]
- Boniardi, M.; Redaelli, A. Phase Change Memory: Device scaling and challenges for material engineering in the GeSbTe compound system. Microelectron. Eng. 2015, 137, 1–4. [Google Scholar] [CrossRef]
- D’Arrigo, G.; Mio, A.M.; Boniardi, M.; Redaelli, A.; Varesi, E.; Privitera, S.; Pellegrino, G.; Spinella, C.; Rimini, E. Crystallization properties of Sb-rich GeSbTe alloys by in-situ morphological and electrical analysis. Mater. Sci. Semicond. Process. 2017, 65, 100–107. [Google Scholar] [CrossRef]
- Boniardi, M.; Redaelli, A.; Tortorelli, I.; Lavizzari, S.; Pirovano, A.; Pellizzer, F.; Varesi, E.; Erbetta, D.; Bresolin, C.; Modelli, A.; et al. Electrical and thermal behavior of Tellurium poor GeSbTe compounds for phase change memory. In Proceedings of the 2012 4th IEEE International Memory Workshop, Milan, Italy, 20–23 May 2012; pp. 1–3. [Google Scholar]
- Yamada, N. Erasable phase-change optical materials. MRS Bull. 1996, 21, 48–50. [Google Scholar] [CrossRef]
- Yamada, N.; Matsunaga, T. Structure of laser-crystallized Ge2Sb2+xTe5 sputtered thin films for use in optical memory. J. Appl. Phys. 2000, 88, 7020–7028. [Google Scholar] [CrossRef]
- Privitera, S.; Rimini, E.; Bongiorno, C.; Zonca, R.; Pirovano, A.; Bez, R. Crystallization and phase separation in Ge2Sb2+xTe5 thin films. J. Appl. Phys. 2003, 94, 4409–4413. [Google Scholar] [CrossRef]
- Pennycook, S.J. A scan through the history of STEM. In Scanning Transmission Electron Microscopy; Springer: New York, NY, USA, 2011; pp. 1–90. [Google Scholar]
- Jiang, Y.; Wang, Y.; Sagendorf, J.; West, D.; Kou, X.; Wei, X.; He, L.; Wang, K.L.; Zhang, S.; Zhang, Z. Direct atom-by-atom chemical identification of nanostructures and defects of topological insulators. Nano Lett. 2013, 13, 2851–2856. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Song, Z.; Cheng, Y.; Liu, X.; Xia, M.; Li, W.; Ding, K.; Feng, X.; Zhu, M.; Feng, S. Direct observation of titanium-centered octahedra in titanium–antimony–tellurium phase-change material. Nat. Commun. 2015, 6, 10040. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xia, M.; Cheng, Y.; Rao, F.; Ding, K.; Liu, W.; Jia, Y.; Song, Z.; Feng, S. Direct observation of metastable face-centered cubic Sb2Te3 crystal. Nano Res. 2016, 9, 3453–3462. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, W.; Shen, Z.; Chen, Y.; Li, J.; Zhang, S.; Zhang, Z.; Wuttig, M.; Mazzarello, R.; Ma, E.; et al. Element-resolved atomic structure imaging of rocksalt Ge2Sb2Te5 phase-change material. Appl. Phys. Lett. 2016, 108, 191902. [Google Scholar] [CrossRef]
- Chen, M.; Rubin, K.A.; Barton, R. Compound materials for reversible, phase-change optical data storage. Appl. Phys. Lett. 1986, 49, 502–504. [Google Scholar] [CrossRef]
- Yamada, N.; Ohno, E.; Nishiuchi, K.; Akahira, N.; Takao, M. Rapid-phase transitions of GeTe-Sb2Te3 pseudobinary amorphous thin films for an optical disk memory. J. Appl. Phys. 1991, 69, 2849–2856. [Google Scholar] [CrossRef]
- Kohara, S.; Kato, K.; Kimura, S.; Tanaka, H.; Usuki, T.; Suzuya, K.; Tanaka, H.; Moritomo, Y.; Matsunaga, T.; Yamada, N.; et al. Structural basis for the fast phase change of Ge2Sb2Te5: Ring statistics analogy between the crystal and amorphous states. Appl. Phys. Lett. 2006, 89, 201910. [Google Scholar] [CrossRef]
- Guo, P.; Sevison, G.A.; Burrow, J.A.; Agha, I.; Sarangan, A. Electrical and optical properties of nickel-doped Ge2Sb2Te5 films produced by magnetron co-sputtering. In Nanoengineering: Fabrication, Properties, Optics, and Devices XV; International Society for Optics and Photonics: Washington, DC, USA, 2018; Volume 10730, p. 107300L. [Google Scholar]
- Guo, S.; Hu, Z.; Ji, X.; Huang, T.; Zhang, X.; Wu, L.; Song, Z.; Chu, J. Temperature and concentration dependent crystallization behavior of Ge 2 Sb 2 Te 5 phase change films: tungsten doping effects. RSC Adv. 2014, 4, 57218–57222. [Google Scholar] [CrossRef]
- Němec, P.; Nazabal, V.; Moréac, A.; Gutwirth, J.; Beneš, L.; Frumar, M. Amorphous and crystallized Ge–Sb–Te thin films deposited by pulsed laser: Local structure using Raman scattering spectroscopy. Mater. Chem. Phys. 2012, 136, 935–941. [Google Scholar] [CrossRef]
- Cao, T.; Zhang, L.; Simpson, R.E.; Cryan, M.J. Mid-infrared tunable polarization-independent perfect absorber using a phase-change metamaterial. J. Opt. Soc. Am. B 2013. [Google Scholar] [CrossRef]
- Shportko, K.; Kremers, S.; Woda, M.; Lencer, D.; Robertson, J.; Wuttig, M. Resonant bonding in crystalline phase-change materials. Nat. Mater. 2008, 7, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Kolobov, A.; Fons, P.; Tominaga, J.; Ankudinov, A.; Yannopoulos, S.; Andrikopoulos, K. Crystallization-induced short-range order changes in amorphous GeTe. J. Phys. Condens. Matter 2004, 16, S5103. [Google Scholar] [CrossRef]
- Wełnic, W.; Botti, S.; Reining, L.; Wuttig, M. Origin of the optical contrast in phase-change materials. Phys. Rev. Lett. 2007, 98, 236403. [Google Scholar] [CrossRef] [PubMed]
- Pankove, J.I. Optical Processes in Semiconductors; Courier Corporation: New York, NY, USA, 1975. [Google Scholar]
- Othman, A.A.; Osman, M.A.; Amer, H.H.; Dahshan, A. Annealing dependence of optical properties of Ga20S75Sb5 and Ga20S40Sb40 thin films. Thin Solid Film. 2004, 253–257. [Google Scholar] [CrossRef]
- Salem, A.; El-Gendy, Y.; Sakr, G.; Soliman, W. Optical properties of thermochromic Cu2HgI4 thin films. J. Phys. D: Appl. Phys. 2008, 41, 025311. [Google Scholar] [CrossRef]
- El-Gendy, Y. Refractive index, oscillator parameters and optical band gap of e-beam evaporated Ga10Ge10Te80 films. J. Phys. D: Appl. Phys. 2009, 42, 115408. [Google Scholar] [CrossRef]
- Association, S.I. International Technology Roadmap for Semiconductors 2005 Edition; Semiconductor Industry Association: Washington, DC, USA, 2006. [Google Scholar]
- Ovshinsky, S.R. Reversible electrical switching phenomena in disordered structures. Phys. Rev. Lett. 1968, 21, 1450. [Google Scholar] [CrossRef]
- Neale, R.; Nelson, D.; Moore, G.E. Nonvolatile and reprogrammable, the read-mostly memory is here. Electronics 1970, 43, 56–60. [Google Scholar]
- Rios, C.; Hosseini, P.; Wright, C.D.; Bhaskaran, H.; Pernice, W.H.P. Tunable Nanophotonic Circuits based on Phase-change Materials; University of Exeter: Exeter, UK, 2013. [Google Scholar]
- Mun, B.H.; You, B.K.; Yang, S.R.; Yoo, H.G.; Kim, J.M.; Park, W.I.; Yin, Y.; Byun, M.; Jung, Y.S.; Lee, K.J. Flexible one diode-one phase change memory array enabled by block copolymer self-assembly. ACS Nano 2015, 9, 4120–4128. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Song, Y.; Jeong, C.; Shin, J.; Fai, Y.; Hwang, Y.; Lee, S.; Ryoo, K.; Lee, S.; Park, J.; et al. Highly manufacturable high density phase change memory of 64Mb and beyond. In Proceedings of the IEDM Technical Digest. IEEE International Electron Devices Meeting, 2004, San Francisco, CA, USA, 13–15 December 2004; pp. 907–910. [Google Scholar]
- Cho, S.; Yi, J.; Ha, Y.; Kuh, B.; Lee, C.; Park, J.; Nam, S.; Horii, H.; Cho, B.; Ryoo, K.; et al. Highly scalable on-axis confined cell structure for high density PRAM beyond 256Mb. In Proceedings of the Digest of Technical Papers. 2005 Symposium on VLSI Technology, 2005, Kyoto, Japan, 14–16 June 2005; pp. 96–97. [Google Scholar]
- Pirovano, A.; Lacaita, A.L.; Pellizzer, F.; Kostylev, S.A.; Benvenuti, A.; Bez, R. Low-field amorphous state resistance and threshold voltage drift in chalcogenide materials. IEEE Trans. Electron. Devices 2004, 51, 714–719. [Google Scholar] [CrossRef]
- Rajendran, B.; Alibart, F. Neuromorphic computing based on emerging memory technologies. IEEE J. Emerg. Sel. Top. Circuits Syst. 2016, 6, 198–211. [Google Scholar] [CrossRef]
- Wang, L.; Lu, S.R.; Wen, J. Recent Advances on Neuromorphic Systems Using Phase-Change Materials. Nanoscale Res. Lett. 2017, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Bedeschi, F.; Bez, R.; Boffino, C.; Bonizzoni, E.; Buda, E.; Casagrande, G.; Costa, L.; Ferraro, M.; Gastaldi, R.; Khouri, O.; et al. 4-Mb MOSFET-selected phase-change memory experimental chip. In Proceedings of the 30th European Solid-State Circuits Conference, Leuven, Belgium, 23–23 September 2004; pp. 207–210. [Google Scholar]
- Hosseini, P.; Sebastian, A.; Papandreou, N.; Wright, C.D.; Bhaskaran, H. Accumulation-based computing using phase-change memories with FET access devices. IEEE Electron. Device Lett. 2015, 36, 975–977. [Google Scholar] [CrossRef]
- Eryilmaz, S.B.; Kuzum, D.; Jeyasingh, R.; Kim, S.; BrightSky, M.; Lam, C.; Wong, H.S.P. Brain-like associative learning using a nanoscale non-volatile phase change synaptic device array. Front. Neurosci. 2014, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Close, G.; Frey, U.; Breitwisch, M.; Lung, H.; Lam, C.; Hagleitner, C.; Eleftheriou, E. Device, circuit and system-level analysis of noise in multi-bit phase-change memory. In Proceedings of the 2010 International Electron Devices Meeting, San Francisco, CA, USA, 6–8 December 2010. [Google Scholar]
- Wong, H.S.P.; Raoux, S.; Kim, S.; Liang, J.; Reifenberg, J.P.; Rajendran, B.; Asheghi, M.; Goodson, K.E. Phase change memory. Proc. IEEE 2010, 98, 2201–2227. [Google Scholar] [CrossRef]
- Im, D.; Lee, J.; Cho, S.; An, H.; Kim, D.; Kim, I.; Park, H.; Ahn, D.; Horii, H.; Park, S.; et al. A unified 7.5 nm dash-type confined cell for high performance PRAM device. In Proceedings of the 2008 IEEE International Electron Devices Meeting, San Francisco, CA, USA, 15–17 December 2008; pp. 1–4. [Google Scholar]
- Mu, J.; Han, Z.; Grillanda, S.; Melloni, A.; Michel, J.; Kimerling, L.C.; Agarwal, A. Towards ultra-subwavelength optical latches. Appl. Phys. Lett. 2013, 1031, 141119–171101. [Google Scholar] [CrossRef]
- Song, Y.; Ryoo, K.; Hwang, Y.; Jeong, C.; Lim, D.; Park, S.; Kim, J.; Lee, S.; Kong, J.; Ahn, S.; et al. Highly reliable 256Mb PRAM with advanced ring contact technology and novel encapsulating technology. In Proceedings of the 2006 Symposium on VLSI Technology, 2006. Digest of Technical Papers, Honolulu, HI, USA, 13–15 June 2006; pp. 118–119. [Google Scholar]
- Ha, Y.; Yi, J.; Horii, H.; Park, J.; Joo, S.; Park, S.; Chung, U.I.; Moon, J. An edge contact type cell for phase change RAM featuring very low power consumption. In Proceedings of the 2003 Symposium on VLSI Technology. Digest of Technical Papers, Kyoto, Japan, 10–12 June 2003; pp. 175–176. [Google Scholar]
- Seong, N.H.; Yeo, S.; Lee, H.H.S. Tri-level-cell phase change memory: Toward an efficient and reliable memory system. Acm Sigarch Comput. Archit. News 2013, 41, 440–451. [Google Scholar] [CrossRef]
- Bedeschi, F.; Fackenthal, R.; Resta, C.; Donze, E.M.; Jagasivamani, M.; Buda, E.C.; Pellizzer, F.; Chow, D.W.; Cabrini, A.; Calvi, G.M.A.; et al. A bipolar-selected phase change memory featuring multi-level cell storage. IEEE J. Solid-State Circuits 2009, 44, 217–227. [Google Scholar] [CrossRef]
- Nirschl, T.; Philipp, J.; Happ, T.; Burr, G.W.; Rajendran, B.; Lee, M.H.; Schrott, A.; Yang, M.; Breitwisch, M.; Chen, C.F.; et al. Write strategies for 2 and 4-bit multi-level phase-change memory. In Proceedings of the 2007 IEEE International Electron Devices Meeting, Washington, DC, USA, 10–12 December 2007; pp. 461–464. [Google Scholar]
- Stanisavljevic, M.; Athmanathan, A.; Papandreou, N.; Pozidis, H.; Eleftheriou, E. Phase-change memory: Feasibility of reliable multilevel-cell storage and retention at elevated temperatures. In Proceedings of the 2015 IEEE International Reliability Physics Symposium, Monterey, CA, USA, 19–23 April 2015. [Google Scholar]
- Němec, P.; Moreac, A.; Nazabal, V.; Pavlišta, M.; Přikryl, J.; Frumar, M. Ge–Sb–Te thin films deposited by pulsed laser: An ellipsometry and Raman scattering spectroscopy study. J. Appl. Phys. 2009, 106, 103509. [Google Scholar] [CrossRef]
- Gholipour, B.; Karvounis, A.; Yin, J.; Soci, C.; MacDonald, K.F.; Zheludev, N.I. Phase-change-driven dielectric-plasmonic transitions in chalcogenide metasurfaces. Npg Asia Mater. 2018, 10, 533–539. [Google Scholar] [CrossRef]
- Hosseini, P.; Wright, C.D.; Bhaskaran, H. An optoelectronic framework enabled by low-dimensional phase-change films. Nature 2014, 511, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Soref, R.; Mu, J.; Majumdar, A.; Li, X.; Huang, W.P. Simulations of Silicon-on-Insulator Channel-Waveguide Electrooptical 2 x 2 Switches and 1 x 1 Modulators Using a Ge2Sb2Te5 Self-Holding Layer. J. Lightw. Technol. 2015, 33. [Google Scholar] [CrossRef]
- Yin, X.; Schäferling, M.; Metzger, B.; Giessen, H. Interpreting chiral nanophotonic spectra: The plasmonic Born–Kuhn model. Nano Lett. 2013, 13, 6238–6243. [Google Scholar] [CrossRef] [PubMed]
- Borisenko, K.B.; Shanmugam, J.; Williams, B.A.; Ewart, P.; Gholipour, B.; Hewak, D.W.; Hussain, R.; Jávorfi, T.; Siligardi, G.; Kirkland, A.I. Photo-induced optical activity in phase-change memory materials. Sci. Rep. 2015, 5, 8770. [Google Scholar] [CrossRef] [PubMed]
- Piccinotti, D.; Gholipour, B.; Yao, J.; MacDonald, K.F.; Hayden, B.E.; Zheludev, N.I. Optical Response of Nanohole Arrays Filled with Chalcogenide Low-Epsilon Media. Adv. Opt. Mater. 2018, 6, 1800395. [Google Scholar] [CrossRef]
- Guo, P.; Lombardo, D.; Sarangan, A. Vanadium dioxide switchable components based on wiregrids for mid-infrared applications. In Nanoengineering: Fabrication, Properties, Optics, and Devices XIV; International Society for Optics and Photonics: Washington, DC, USA, 2017; Volume 10354, p. 1035411. [Google Scholar]
- Burrow, J.A.; Yahiaoui, R.; Sarangan, A.; Agha, I.; Mathews, J.; Searles, T.A. Polarization-dependent electromagnetic responses of ultrathin and highly flexible asymmetric terahertz metasurfaces. Opt. Express 2017, 25, 32540–32549. [Google Scholar] [CrossRef]
- Chu, C.H.; Tseng, M.L.; Chen, J.; Wu, P.C.; Chen, Y.H.; Wang, H.C.; Chen, T.Y.; Hsieh, W.T.; Wu, H.J.; Sun, G.; et al. Active dielectric metasurface based on phase-change medium. Laser Photonics Rev. 2016, 10, 986–994. [Google Scholar]
- Wang, Q.; Rogers, E.T.; Gholipour, B.; Wang, C.M.; Yuan, G.; Teng, J.; Zheludev, N.I. Optically reconfigurable metasurfaces and photonic devices based on phase change materials. Nat. Photonics 2016, 10, 60. [Google Scholar] [CrossRef]
- Makarov, S.V.; Zalogina, A.S.; Tajik, M.; Zuev, D.A.; Rybin, M.V.; Kuchmizhak, A.A.; Juodkazis, S.; Kivshar, Y. Light-Induced Tuning and Reconfiguration of Nanophotonic Structures. Laser Photonics Rev. 2017, 11, 1700108. [Google Scholar]
- Guo, Z.; Yang, X.; Shen, F.; Zhou, Q.; Gao, J.; Guo, K. Active-Tuning and Polarization-Independent Absorber and Sensor in the Infrared Region Based on the Phase Change Material of Ge2Sb2Te5 (GST). Sci. Rep. 2018, 8, 12433. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xia, M.; Rao, F.; Wu, L.; Li, X.; Song, Z.; Feng, S.; Sun, H. Understanding Phase-Change Behaviors of Carbon-Doped Ge2Sb2Te5 for Phase-Change Memory Application. Acs Appl. Mater. Interfaces 2014, 6, 14207–14214. [Google Scholar] [CrossRef]
- Li, T.; Wu, L.; Ji, X.; Zheng, Y.; Liu, G.; Song, Z.; Shi, J.; Zhu, M.; Song, S.; Feng, S. Carbon doping induced Ge local structure change in as-deposited Ge2Sb2Te5 film by EXAFS and Raman spectrum. AIP Adv. 2018, 8, 025201. [Google Scholar] [CrossRef]
- Privitera, S.; Rimini, E.; Zonca, R. Amorphous-to-crystal transition of nitrogen- and oxygen-doped Ge2Sb2Te5 films studied by in situ resistance measurements. Appl. Phys. Lett. 2004, 85, 3044–3046. [Google Scholar] [CrossRef]
- Guo, P.; Burrow, J.A.; Sevison, G.A.; Sood, A.; Asheghi, M.; Hendrickson, J.R.; Goodson, K.E.; Agha, I.; Sarangan, A. Improving the performance of Ge2Sb2Te5 materials via nickel doping: Towards RF-compatible phase-change devices. Appl. Phys. Lett. 2018, 113, 171903. [Google Scholar] [CrossRef]
- Jeong, S.M.; Kim, K.H.; Choi, S.M.; Lee, H.L. Influence of silicon doping on the properties of sputtered Ge2Sb2Te5 thin film. Jpn. J. Appl. Phys. 2009, 48, 045503. [Google Scholar] [CrossRef]
- Wei, S.; Li, J.; Wu, X.; Zhou, P.; Wang, S.; Zheng, Y.; Chen, L.; Gan, F.; Zhang, X.; Li, G. Phase change characteristics of aluminum doped Ge2Sb2Te5 films prepared by magnetron sputtering. Optics Express 2007, 15, 10584. [Google Scholar] [CrossRef]
- Guoqiang, W.; Jun, L. Improved thermal and electrical properties of Al- doped Ge2Sb2Te5 films for phase-change random access memory. J. Phys. D: Appl. Phys 2012, 45. [Google Scholar]
- Wei, S.J.; Zhu, H.F.; Chen, K.; Xu, D.; Li, J.; Gan, F.X.; Zhang, X.; Xia, Y.J.; Li, G.H. Phase change behavior in titanium-doped Ge2Sb2Te5 films. Appl. Phys. Lett. 2011, 98, 231910. [Google Scholar] [CrossRef]
- Ding, K.; Ren, K.; Rao, F.; Song, Z.; Wu, L.; Liu, B.; Feng, S. Study on the Cu-doped Ge 2 Sb 2 Te 5 for low-power phase change memory. Mater. Lett. 2014, 125, 143–146. [Google Scholar] [CrossRef]
- Lee, M.L.; Yong, K.T.; Gan, C.L.; Ting, L.H.; Daud, S.B.M.; Shi, L. Crystallization and thermal stability of Sn-doped Ge2Sb2Te5 phase change material. J. Phys. D: Appl. Phys. 2008, 41, 215402. [Google Scholar] [CrossRef]
- Rao, F.; Ding, K.; Zhou, Y.; Zheng, Y.; Xia, M.; Lv, S.; Song, Z.; Feng, S.; Ronneberger, I.; Mazzarello, R.; et al. Reducing the stochasticity of crystal nucleation to enable subnanosecond memory writing. Science 2017, 358, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Dieker, H.; Wuttig, M. Influence of deposition parameters on the properties of sputtered Ge2Sb2Te5 films. Thin Solid Film. 2005, 478, 248–251. [Google Scholar] [CrossRef]
- Bakan, G. Electrothermal characterization of phase-change films and devices. Anadolu Univ. Sci. Technol. A Appl. Sci. Eng. 2017, 18, 1057–1065. [Google Scholar] [CrossRef]
- Yoon, H.; Jo, W.; Lee, E.; Lee, J.; Kim, M.; Lee, K.; Khang, Y. Generation of phase-change Ge–Sb–Te nanoparticles by pulsed laser ablation. J. Non-Cryst. Solids 2005, 351, 3430–3434. [Google Scholar] [CrossRef]
- Wágner, T.; Havel, J.; Houška, J.; Peña-Méndez, E.M.; Přikryl, J.; Pavlišta, M.; Frumar, M. Thin films of Ge2Sb2Te5 prepared by pulsed laser deposition, properties and plume particles analysis. In Proceedings of the European Symposium on Phase Change and Ovonic Science, Zermatt, Switzerland, 1–4 September 2007; pp. 1–4. [Google Scholar]
- Houška, J.; Peña-Méndez, E.M.; Kolář, J.; Přikryl, J.; Pavlišta, M.; Frumar, M.; Wágner, T.; Havel, J. Laser desorption time-of-flight mass spectrometry of atomic switch memory Ge2Sb2Te5 bulk materials and its thin films. Rapid Commun. Mass Spectrom. 2014, 28, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Orava, J.; Wágner, T.; Šik, J.; Přikryl, J.; Frumar, M.; Beneš, L. Optical properties and phase change transition in Ge2Sb2Te5 flash evaporated thin films studied by temperature dependent spectroscopic ellipsometry. J. Appl. Phys. 2008, 104, 043523. [Google Scholar] [CrossRef]
- Wagner, T.; Orava, J.; Prikryl, J.; Kohoutek, T.; Bartos, M.; Frumar, M. Medium-term thermal stability of amorphous Ge2Sb2Te5 flash-evaporated thin films with regards to change in structure and optical properties. Thin Solid Film. 2009, 517, 4694–4697. [Google Scholar] [CrossRef]
- Kitamura, M.; Morikawa, T.; Ohyanagi, T.; Tai, M.; Kinoshita, M.; Akita, K.; Takaura, N. Chemical Vapor Deposition GeTe/Sb2Te3 Super-Lattice Phase Change Memory. Int. Conf. Solid State Devices Mater. Fukuoka 2013, 546–547. [Google Scholar] [CrossRef]
- Drowart, J.; Goldfinger, P. Investigation of inorganic systems at high temperature by mass spectrometry. Angew. Chem. Int. Ed. Engl. 1967, 6, 581–596. [Google Scholar] [CrossRef]
- Adam, J.L.; Zhang, X. Chalcogenide Glasses: Preparation, Properties and Applications; Woodhead publishing: Cambridge, UK, 2014. [Google Scholar]
- Sommer, A. Characteristics of evaporated antimony films as a function of the antimony source. J. Appl. Phys. 1966, 37, 2789–2791. [Google Scholar] [CrossRef]
- Lowe, R. Evaporation of Antimony from PtSb. J. Appl. Phys. 1968, 39, 2476–2477. [Google Scholar] [CrossRef]
- Wöltgens, H.W. Combinatorial Material Synthesis Applied to Ge-Sb-Te Based Phase Change Materials. Verlag nicht ermittelbar. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2003. [Google Scholar]
- Kooi, B.; Groot, W.; De Hosson, J.T.M. In situ transmission electron microscopy study of the crystallization of Ge2Sb2Te5. J. Appl. Phys. 2004, 95, 924–932. [Google Scholar] [CrossRef]
- Wang, K.; Steimer, C.; Wamwangi, D.; Ziegler, S.; Wuttig, M. Effect of indium doping on Ge2Sb2Te5 thin films for phase-change optical storage. Appl. Phys. A 2005, 80, 1611–1616. [Google Scholar] [CrossRef]
- Kim, R.Y.; Kim, H.G.; Yoon, S.G. Structural properties of Ge2Sb2Te5 thin films by metal organic chemical vapor deposition for phase change memory applications. Appl. Phys. Lett. 2006, 89, 102107. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.; Cho, S.; Park, Y.; Bae, B.; Park, J.; Park, J.; An, H.; Bae, J.; Ahn, D.; et al. Highly scalable phase change memory with CVD GeSbTe for sub 50 nm generation. In Proceedings of the 2007 IEEE Symposium on VLSI Technology, Kyoto, Japan, 12–14 June 2007; pp. 102–103. [Google Scholar]
- Choi, B.J.; Choi, S.; Shin, Y.C.; Hwang, C.S.; Lee, J.W.; Jeong, J.; Kim, Y.J.; Hwang, S.Y.; Hong, S.K. Cyclic PECVD of Ge2Sb2Te5 films using metallorganic sources. J. Electrochem. Soc. 2007, 154, H318–H324. [Google Scholar] [CrossRef]
- Abrutis, A.; Plausinaitiene, V.; Skapas, M.; Wiemer, C.; Salicio, O.; Pirovano, A.; Varesi, E.; Rushworth, S.; Gawelda, W.; Siegel, J. Hot-wire chemical vapor deposition of chalcogenide materials for phase change memory applications. Chem. Mater. 2008, 20, 3557–3559. [Google Scholar] [CrossRef]
- Fujisaki, Y.; Sasago, Y.; Kobayashi, T. Amorphous thin GeSbTe phase-change films prepared by radical-assisted metal-organic chemical vapor deposition. Thin Solid Film. 2015, 583, 55–59. [Google Scholar] [CrossRef]
- Choi, B.J.; Choi, S.; Shin, Y.C.; Kim, K.M.; Hwang, C.S.; Kim, Y.J.; Son, Y.J.; Hong, S.K. Combined Atomic Layer and Chemical Vapor Deposition, and Selective Growth of Ge2Sb2Te5 Films on TiN/W Contact Plug. Chem. Mater. 2007, 19, 4387–4389. [Google Scholar] [CrossRef]
- Lee, J.; Choi, S.; Lee, C.; Kang, Y.; Kim, D. GeSbTe deposition for the PRAM application. Appl. Surf. Sci. 2007, 253, 3969–3976. [Google Scholar] [CrossRef]
- Ritala, M.; Pore, V.; Hatanpää, T.; Heikkilä, M.; Leskelä, M.; Mizohata, K.; Schrott, A.; Raoux, S.; Rossnagel, S.M. Atomic layer deposition of Ge2Sb2Te5 thin films. Microelectron. Eng. 2009, 86, 1946–1949. [Google Scholar] [CrossRef]
- Eom, T.; Choi, S.; Choi, B.J.; Lee, M.H.; Gwon, T.; Rha, S.H.; Lee, W.; Kim, M.S.; Xiao, M.; Buchanan, I.; Cho, D.Y.; Hwang, C.S. Conformal Formation of (GeTe2)1-x(Sb2Te3)x Layers by Atomic Layer Deposition for Nanoscale Phase Change Memories. Chem. Mater. 2012, 24, 2099–2110. [Google Scholar] [CrossRef]
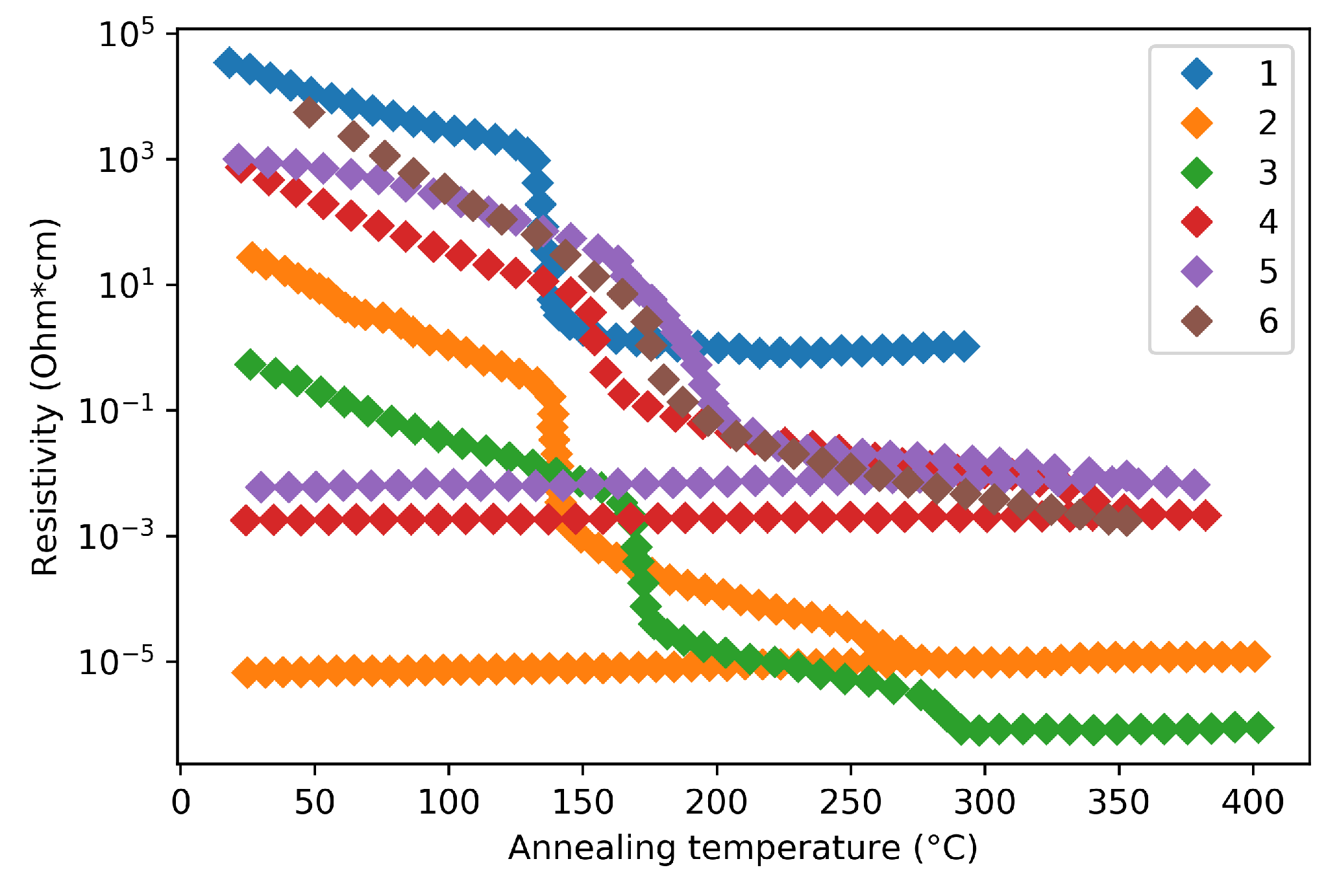
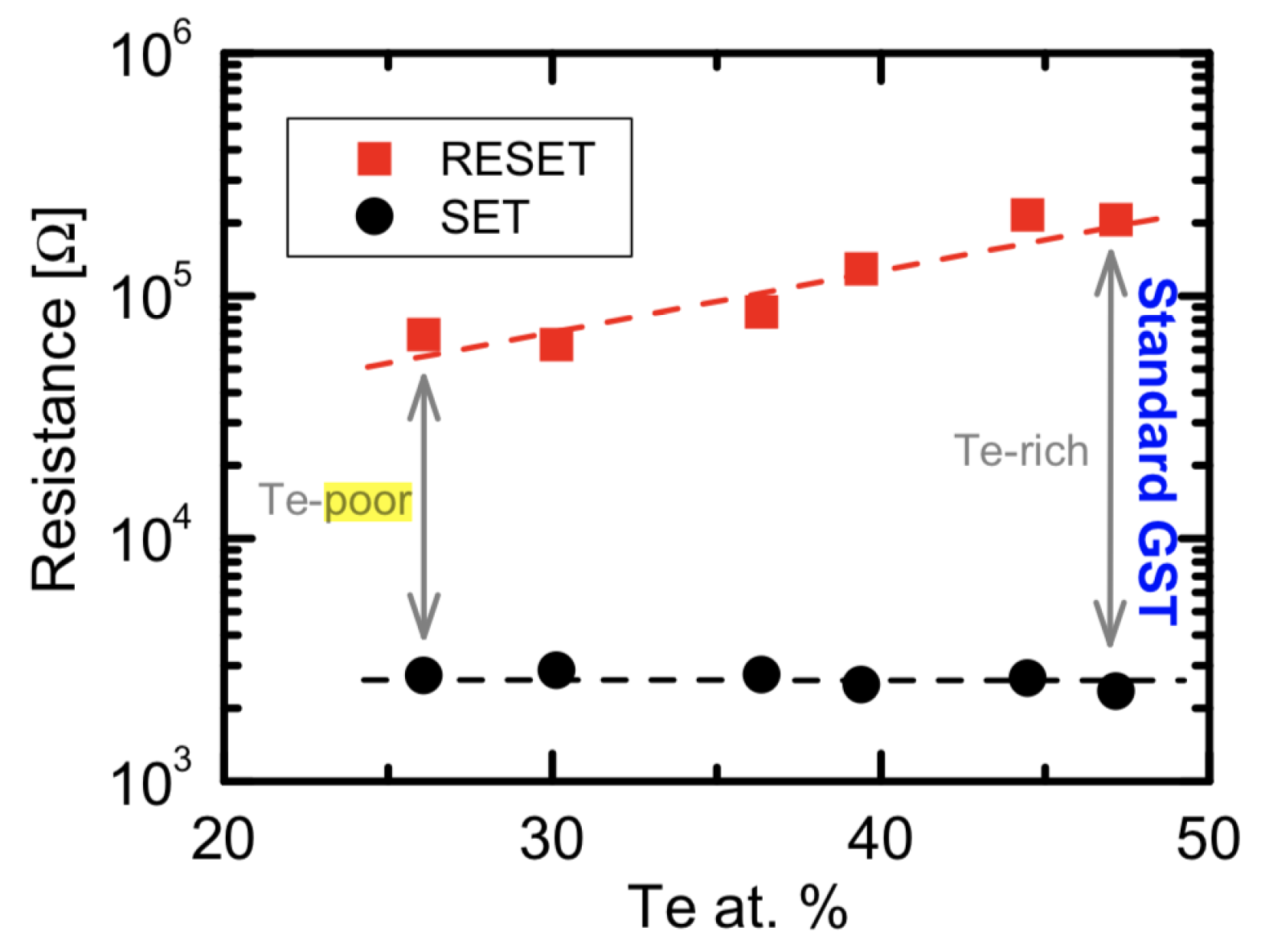
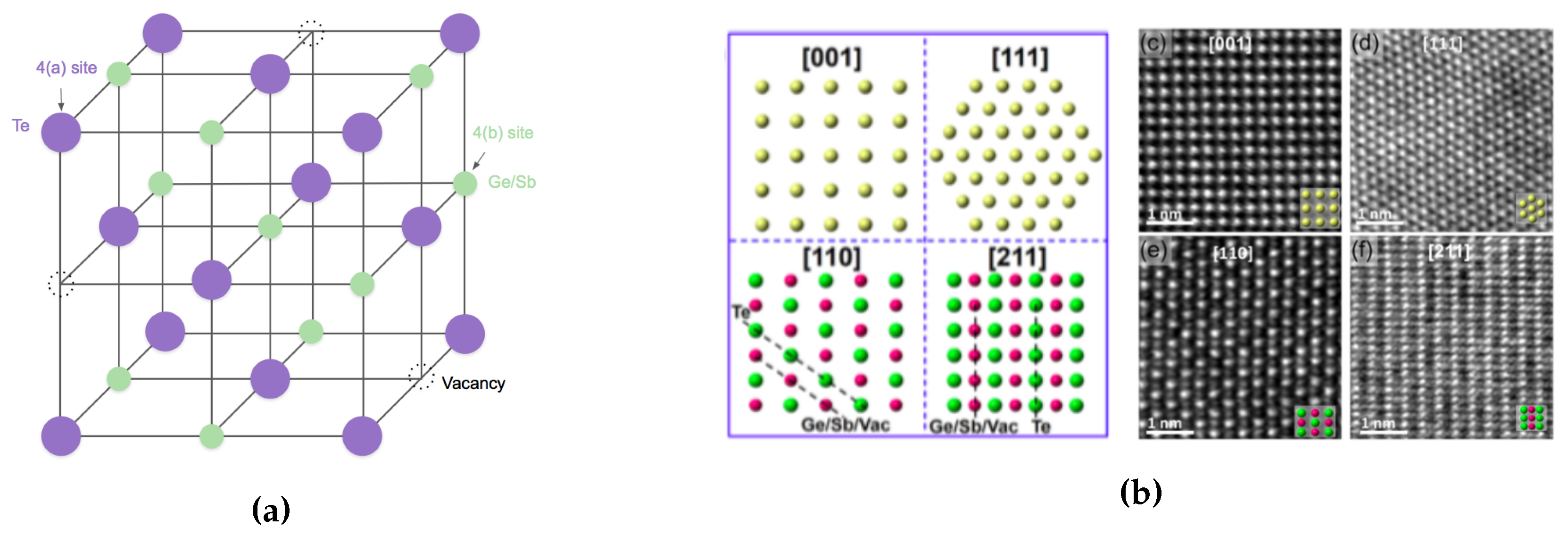
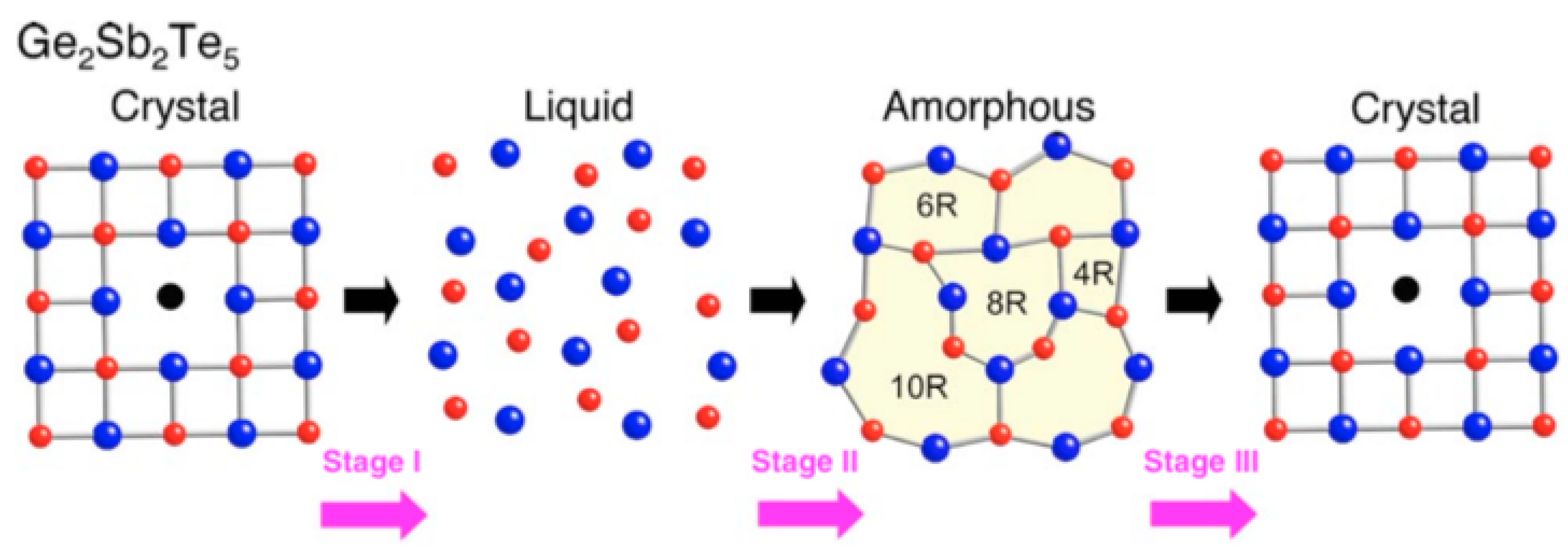

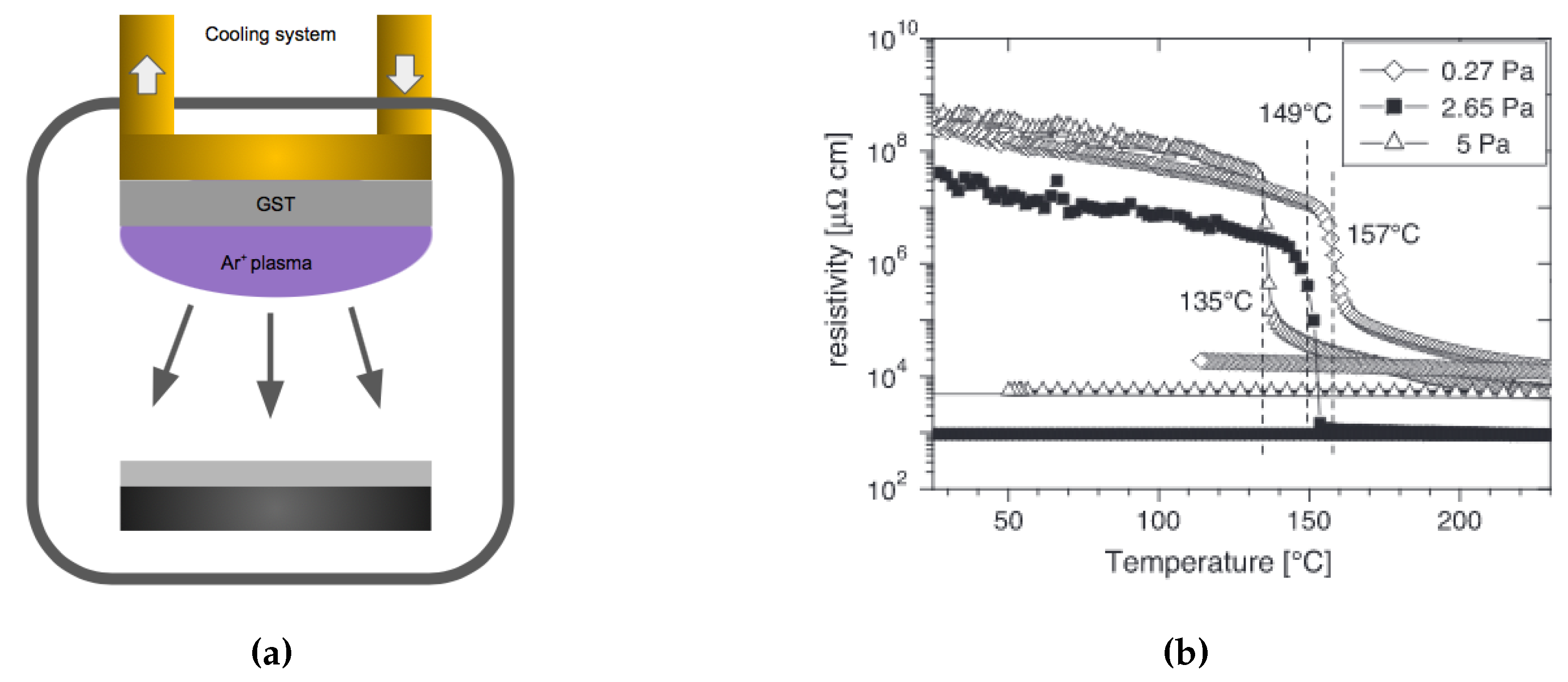

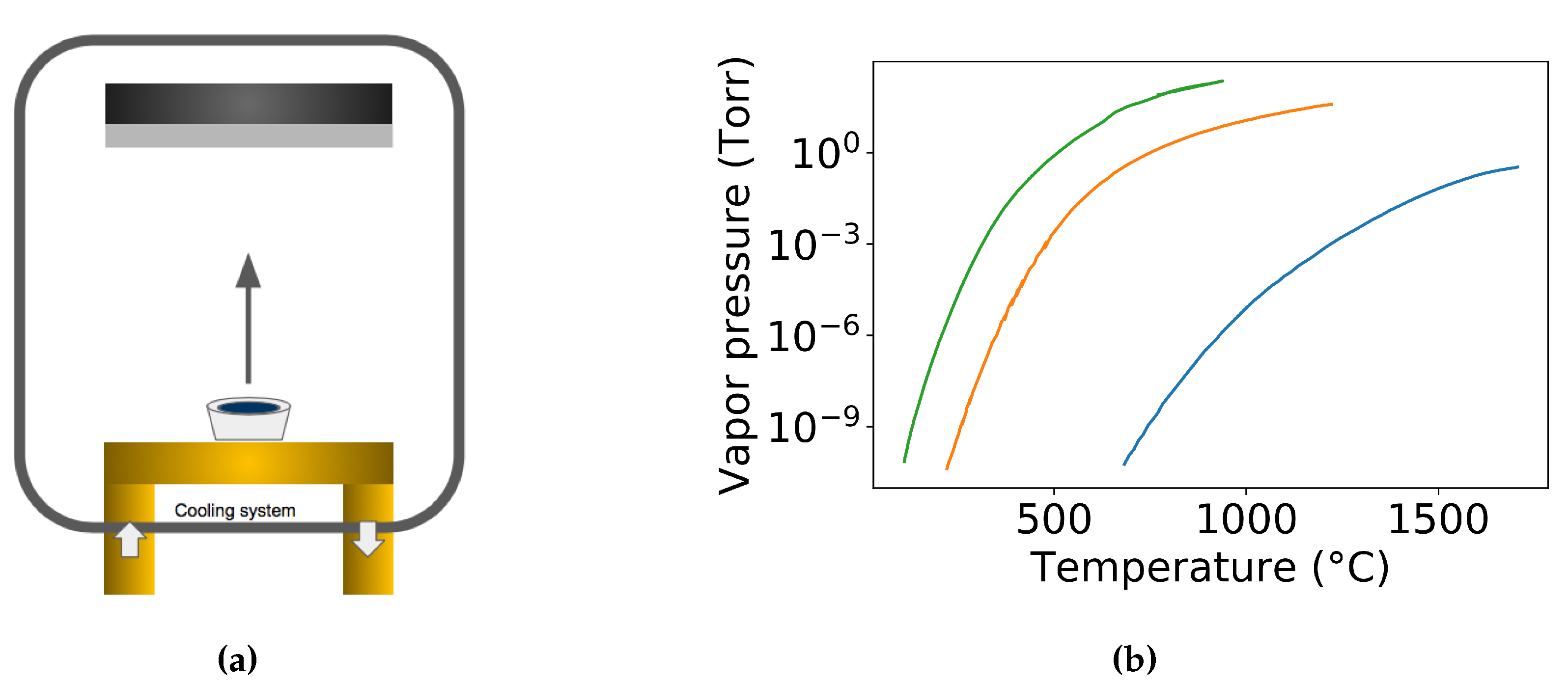
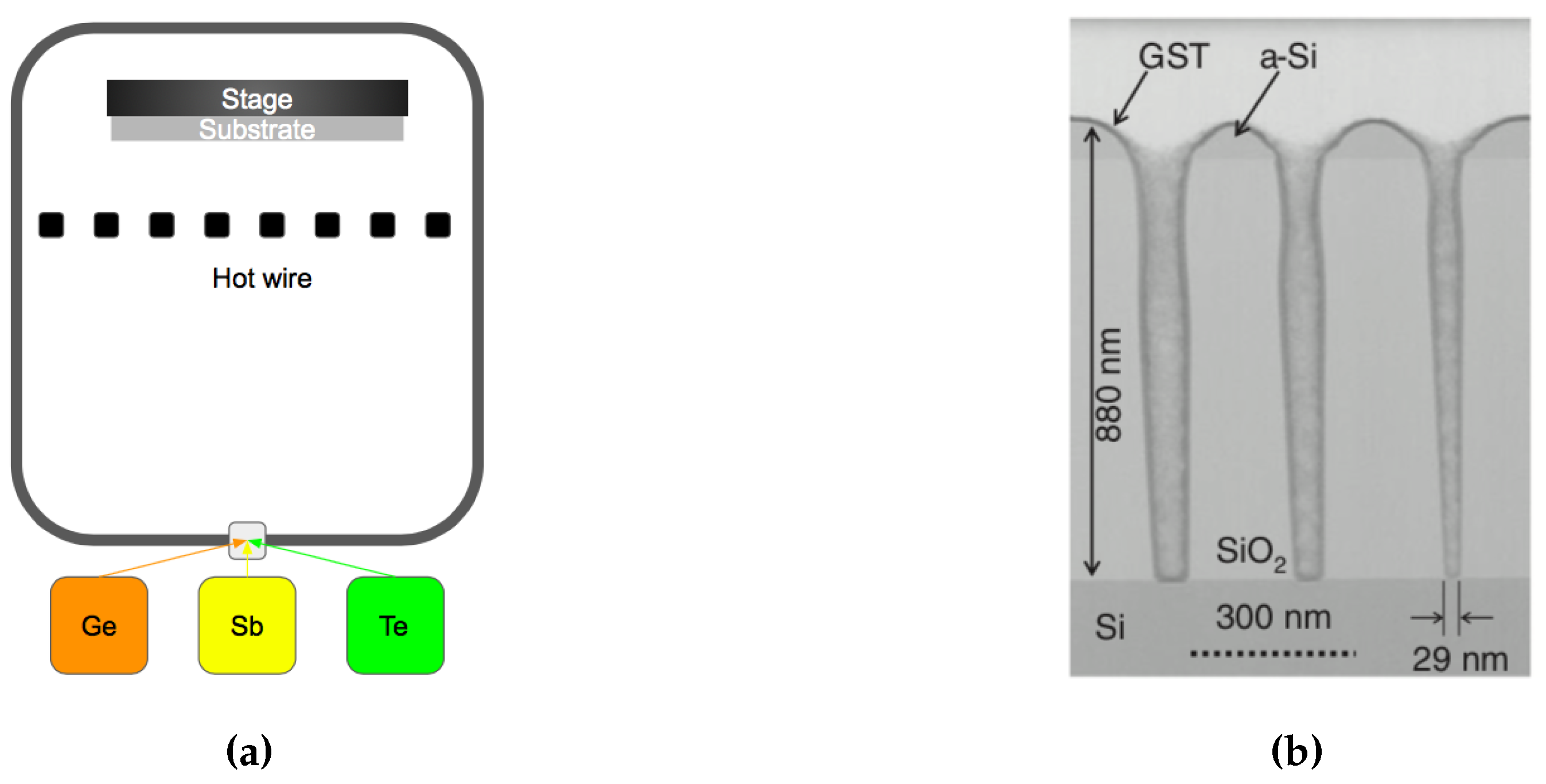
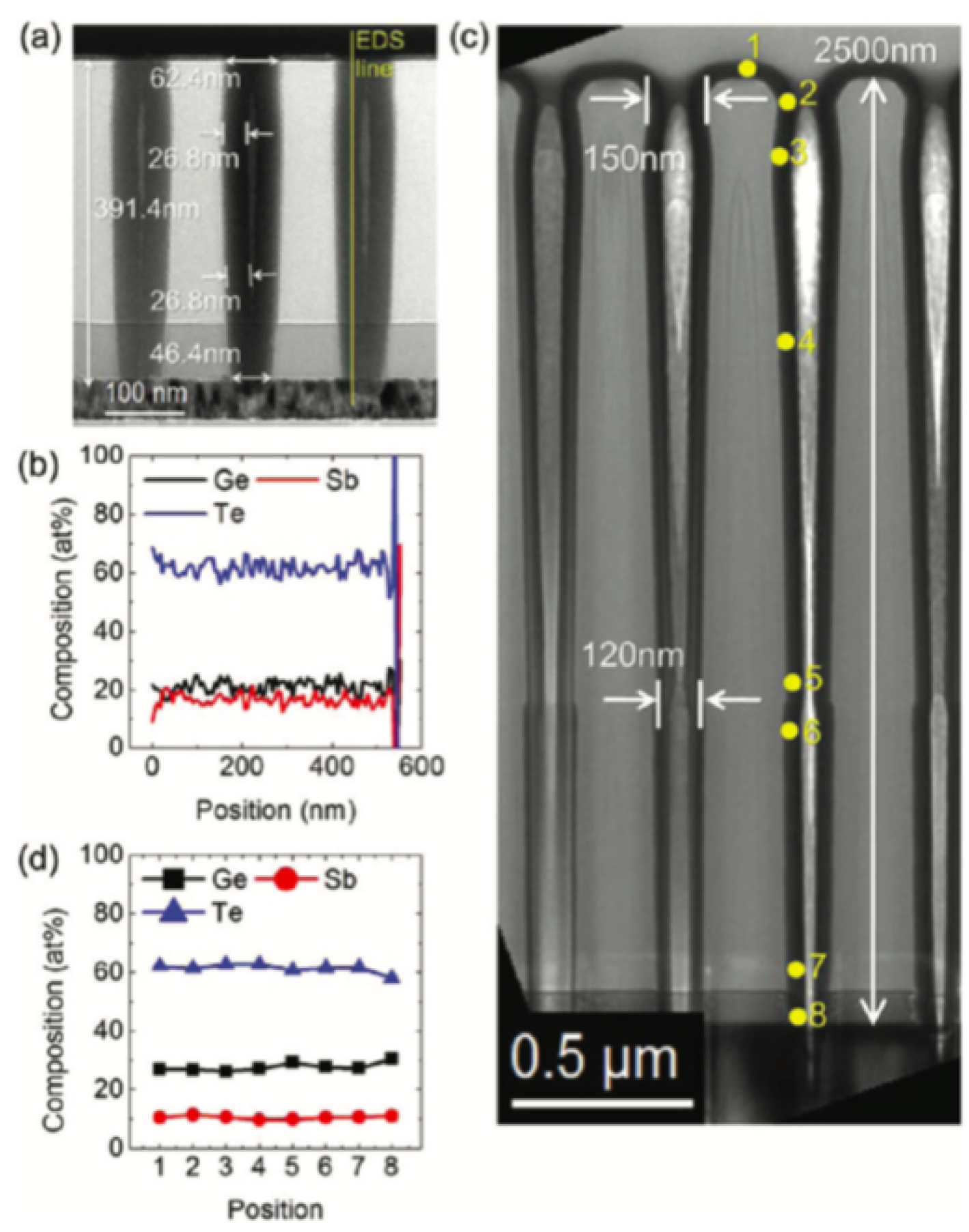
| Label | Fabrication Method | Film Thickness (nm) | Reference |
|---|---|---|---|
| 1 | Thermal evaporation | 100–200 | [32] |
| 2 | Sputtering | - | [33] |
| 3 | Sputtering | 50 | [34] |
| 4 | Sputtering | - | [35] |
| 5 | Atomic Layer Deposition | 60 | [35] |
| 6 | Sputtering | 98 | [36] |
| Peak Position (cm) | |||||||
|---|---|---|---|---|---|---|---|
| Amorphous | 55 | 65 | 80 | 136 | 146 | 158 | 209 |
| Crystalline (hcp) | 55 | 72 | 94 | 113 | 142 | 173 | 195 |
| Composition | Contrast of Optical Constant |
|---|---|
| GeTe | — |
| Ge8Sb2Te11 | −1.48 + i1.35 |
| Ge6Sb2Te9 | −1.45 + i1.27 |
| Ge4Sb2Te7 | −1.26 + i1.09 |
| Ge2Sb2Te5 | −1.20 + i1.05 |
| Sb2Te3 | — |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Sarangan, A.M.; Agha, I. A Review of Germanium-Antimony-Telluride Phase Change Materials for Non-Volatile Memories and Optical Modulators. Appl. Sci. 2019, 9, 530. https://doi.org/10.3390/app9030530
Guo P, Sarangan AM, Agha I. A Review of Germanium-Antimony-Telluride Phase Change Materials for Non-Volatile Memories and Optical Modulators. Applied Sciences. 2019; 9(3):530. https://doi.org/10.3390/app9030530
Chicago/Turabian StyleGuo, Pengfei, Andrew M. Sarangan, and Imad Agha. 2019. "A Review of Germanium-Antimony-Telluride Phase Change Materials for Non-Volatile Memories and Optical Modulators" Applied Sciences 9, no. 3: 530. https://doi.org/10.3390/app9030530
APA StyleGuo, P., Sarangan, A. M., & Agha, I. (2019). A Review of Germanium-Antimony-Telluride Phase Change Materials for Non-Volatile Memories and Optical Modulators. Applied Sciences, 9(3), 530. https://doi.org/10.3390/app9030530






