Controllable Synthesis of Special Reed-Leaf-Like Carbon Nanostructures Using Copper Containing Catalytic Pyrolysis for High-Performance Field Emission
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
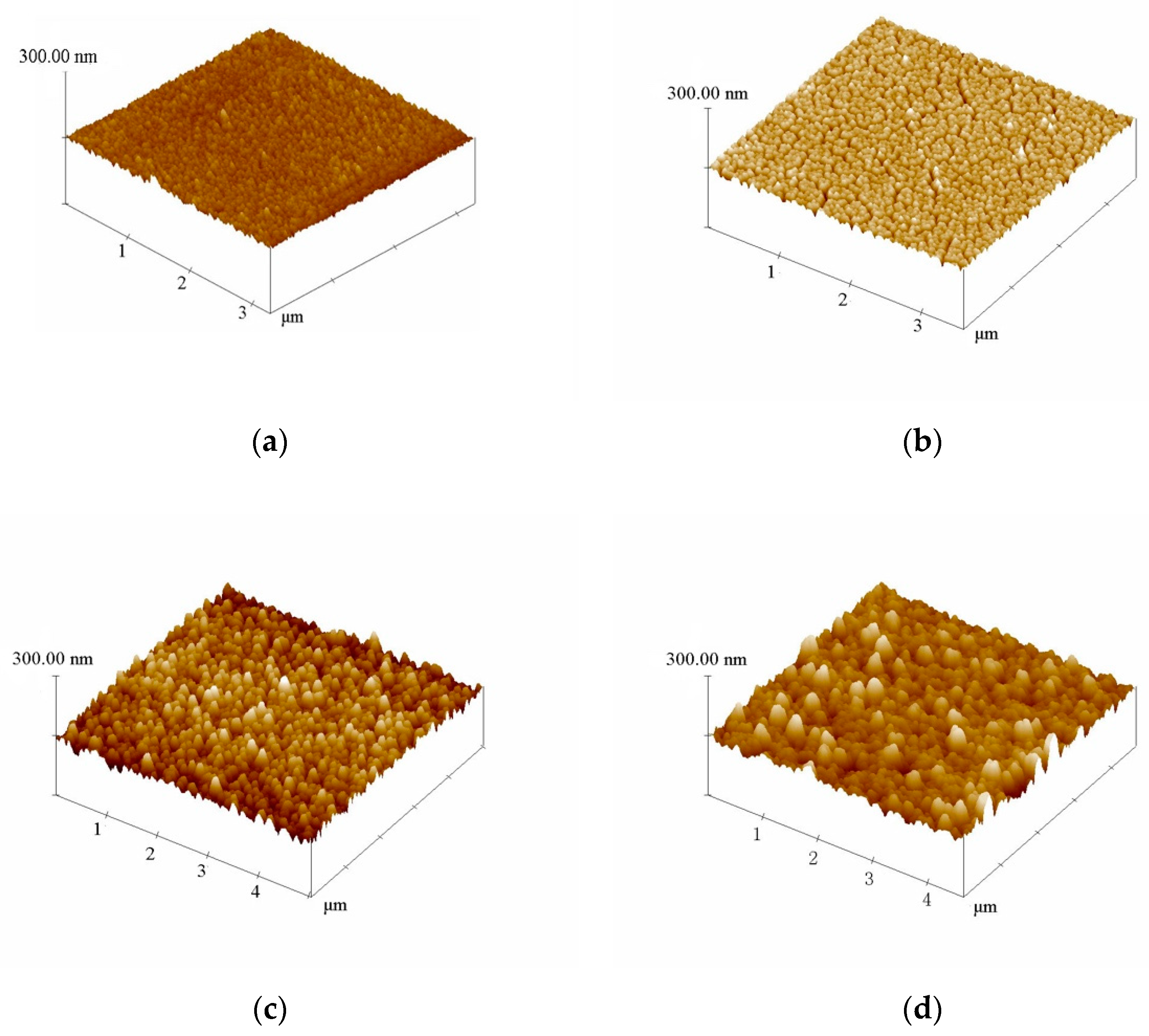
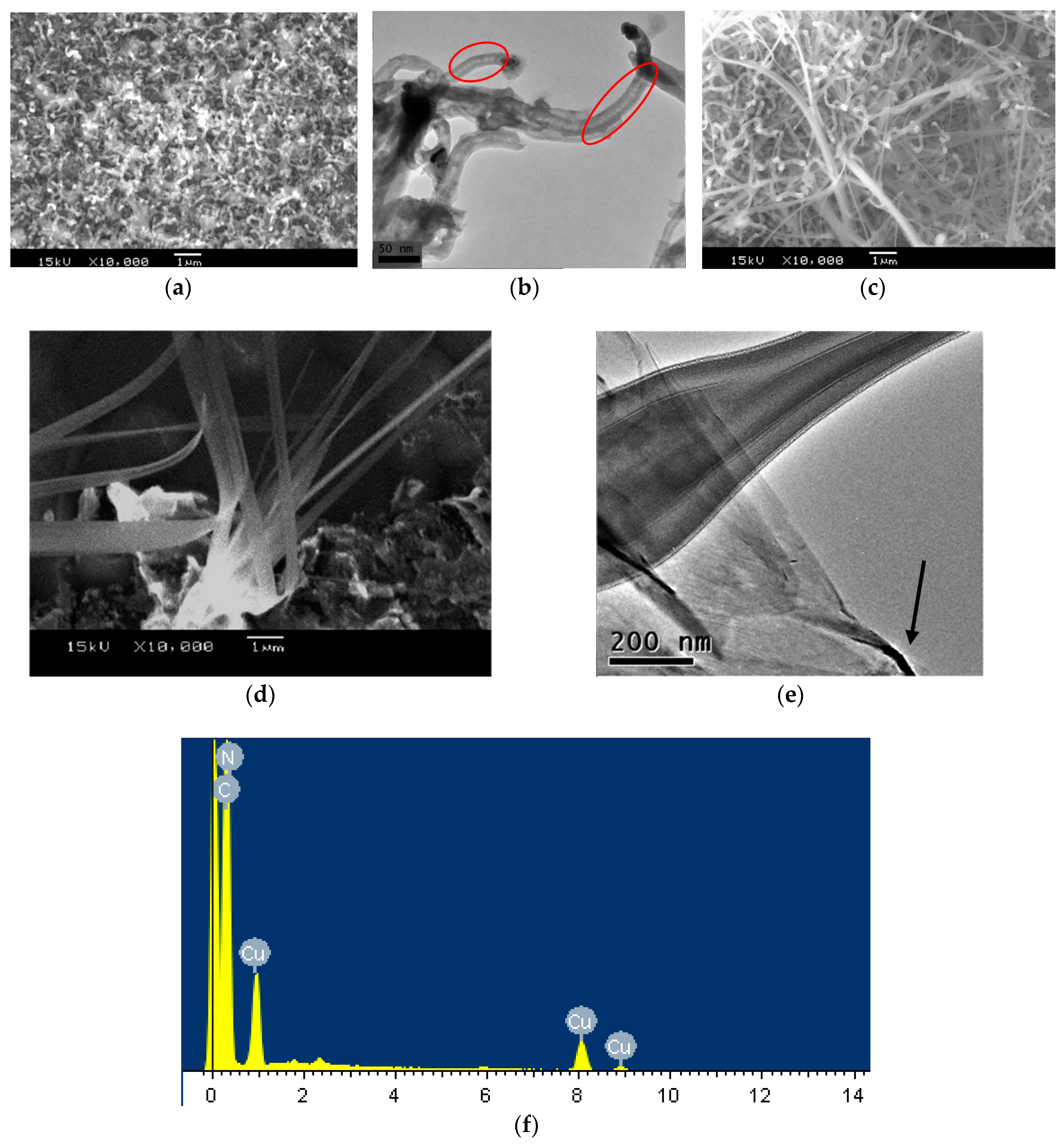
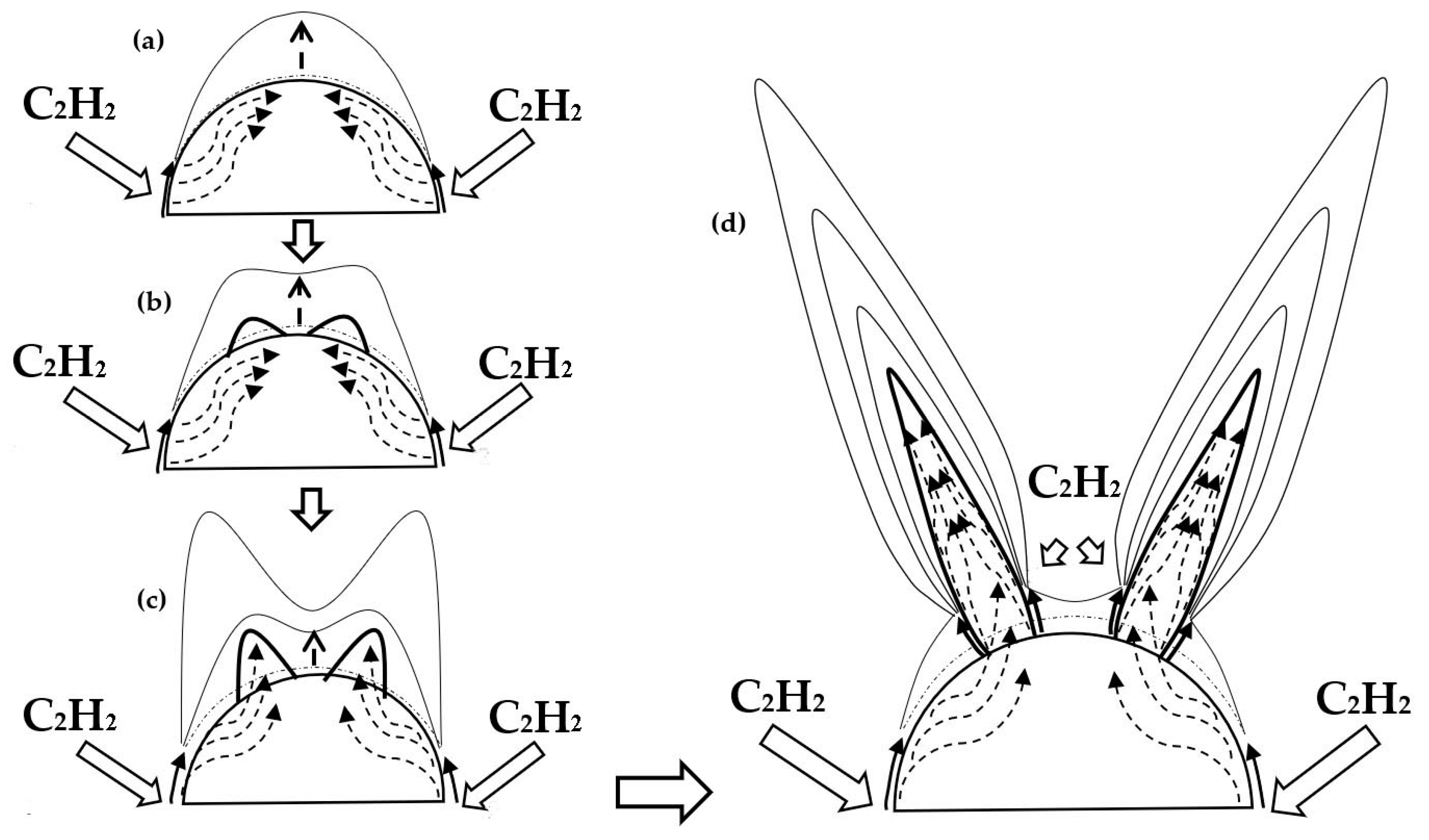
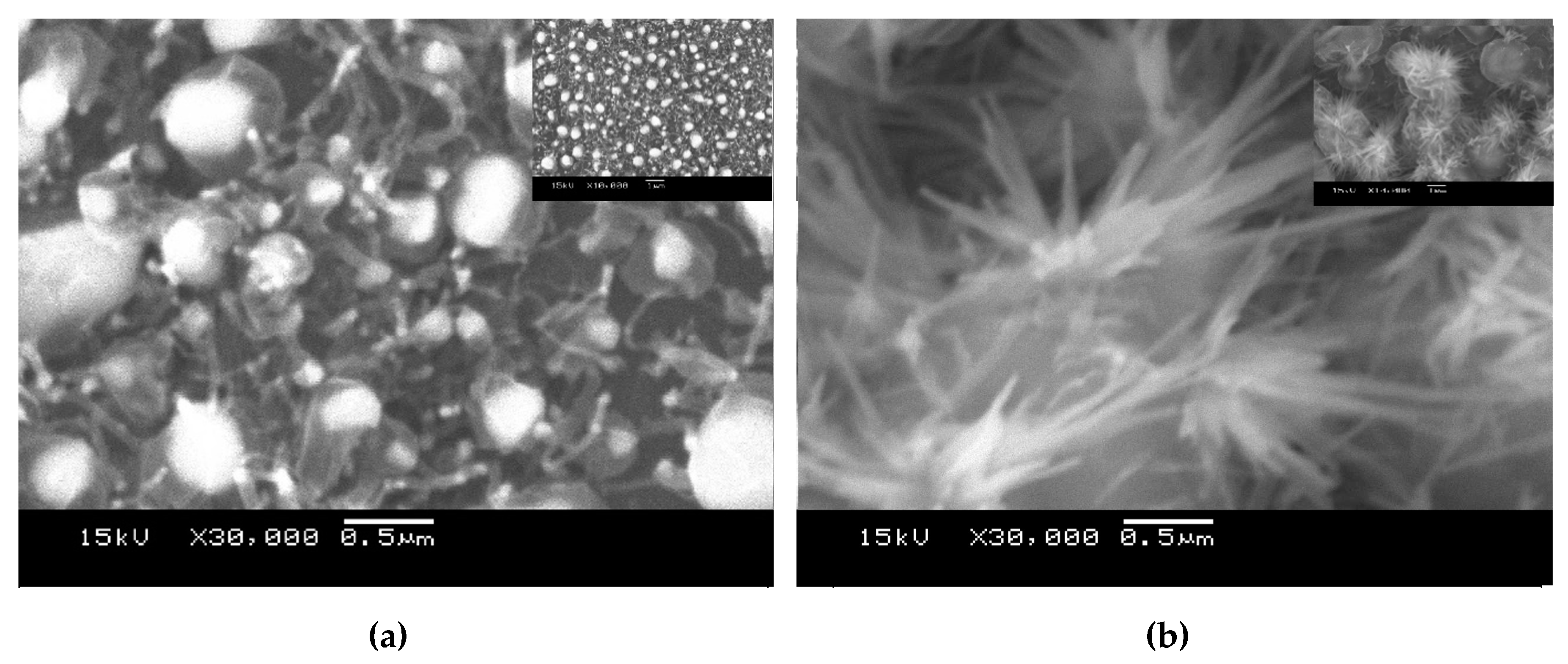
3.1. Morphologies and Structures
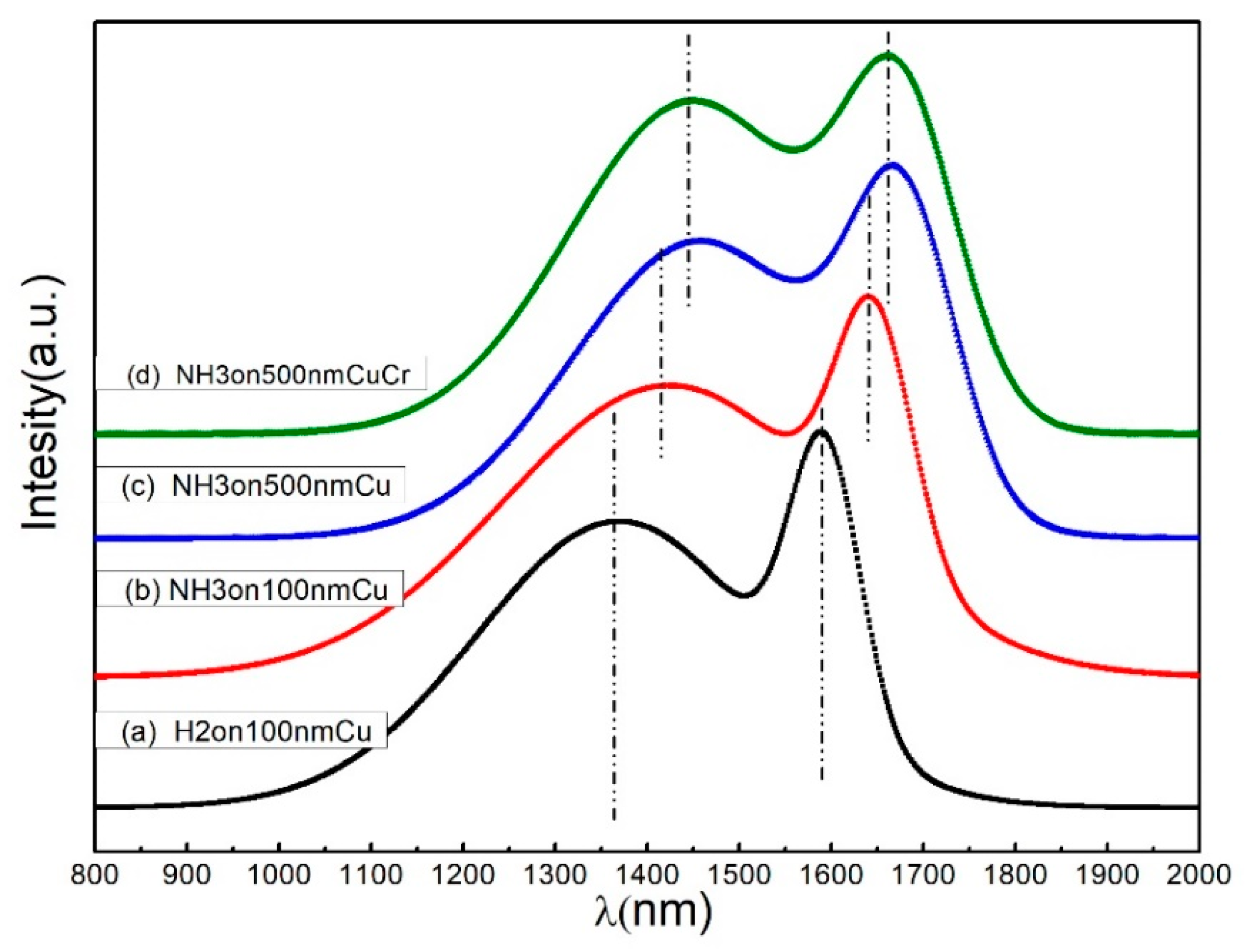
3.2. Raman Spectroscopy of Carbon Product
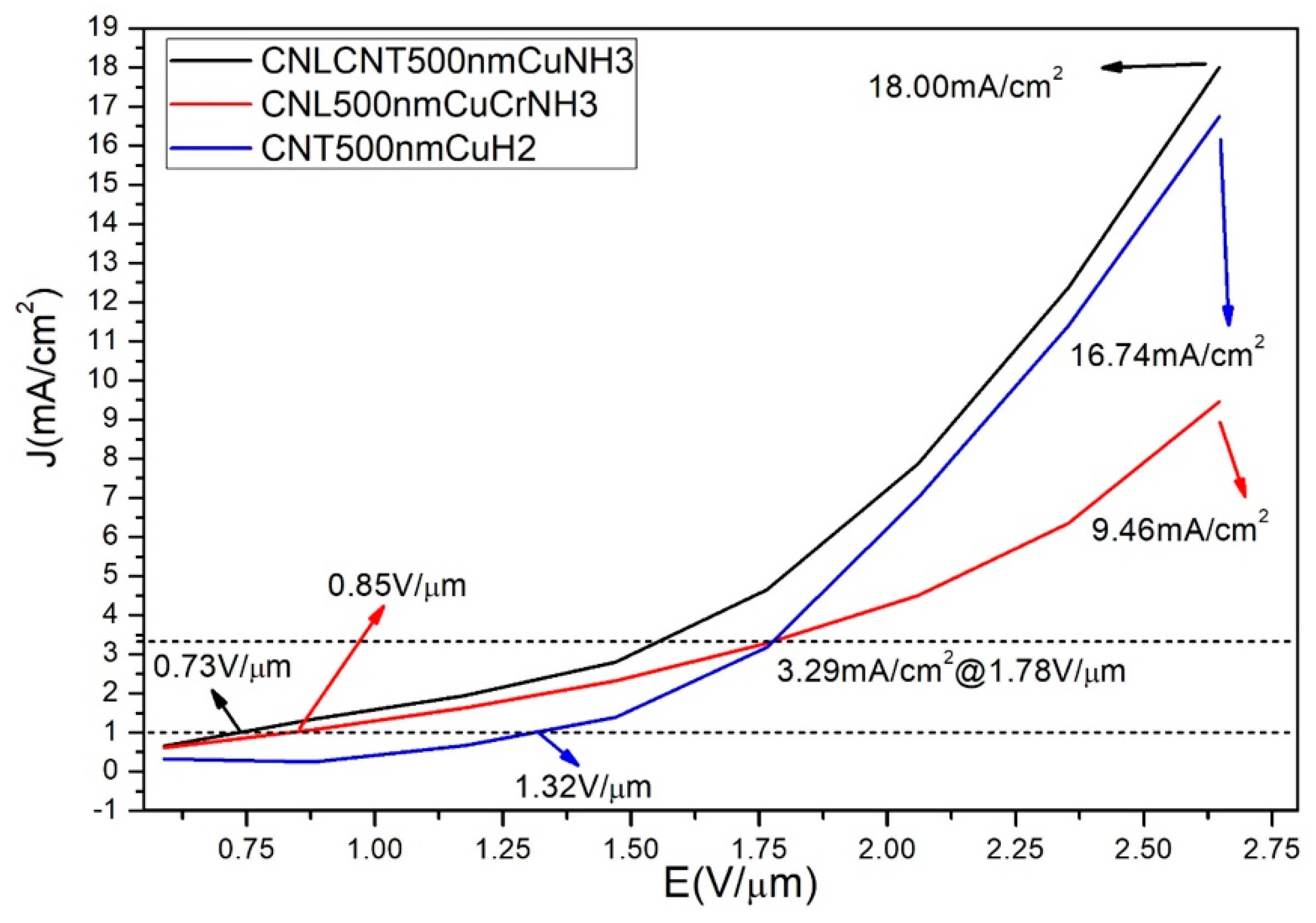
3.3. Field Emission Properties of Different Carbon Nanostructures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Chuc, N.; Thanh, C.T.; Van Tu, N.; Phuong, V.T.; Thang, P.V.; Tam, N.T. A Simple Approach to the Fabrication of Graphene-Carbon Nanotube Hybrid Films on Copper Substrate by Chemical Vapor Deposition. J. Mater. Sci. Technol. 2015, 31, 479–483. [Google Scholar] [CrossRef]
- Tian, F.; Li, H.P.; Zhao, N.Q.; He, C.N. Catalyst effects of fabrication of carbon nanotubes synthesized by chemical vapor deposition. Mater. Chem. Phys. 2009, 115, 493–495. [Google Scholar] [CrossRef]
- Banerjee, D.; Jha, A.; Chattopadhyay, K.K. Efficient field emission from coiled carbon nano/micofiber on copper substrate by dc-PECVD. Appl. Surf. Sci. 2010, 256, 7516–7521. [Google Scholar] [CrossRef]
- Tsai, P.-H.; Tsai, H.-Y. Carbon nano-flake ball with a sandwich-structure composite of diamond core covered by graphite using single-step microwave plasma chemical vapor deposition. Carbon 2018, 136, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Gao, E.; Liu, P.; Zhou, D.; Geng, D.; Xue, X.; Wang, L.; Jiang, K.; Xu, Z.; Yu, G. Facile growth of vertically-aligned graphene nanosheets via thermal CVD: The experimental and theoretical investigations. Carbon 2017, 121, 1–9. [Google Scholar] [CrossRef]
- Gautier, L.; Borgne, V.; Khakani, M.A.I. Field emission properties of graphenated multi-wall carbon nanotubes grown by plasma enhanced chemical vapour deposition. Carbon 2016, 98, 259–266. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, Z.; Zhou, B.; Wei, Q.; Long, H.; Wang, Y.; Li, J.; Hu, N.; Ma, L.; Lin, C.T.; Yu, Z. Nickel-induced transformation of diamond into graphite and carbon nanotubes and the electron field emission properties of resulting composite films. Appl. Surf. Sci. 2018, 428, 264–271. [Google Scholar] [CrossRef]
- Teo, K.B.; Minoux, E.; Hudanski, L.; Peauger, F.; Schnell, J.P.; Gangloff, L.; Legagneux, P.; Dieumegard, D.; Amaratunga, G.A.; Milne, W.I. Microwave devices: Carbon nanotubes as cold cathodes. Nature 2005, 437, 968. [Google Scholar] [CrossRef]
- Deng, J.; Cheng, G.; Zheng, R.; Yu, B.; Li, G.; Hou, X.; Zhao, M.; Li, D. Catalyst-free, self-assembly, and controllable synthesis of graphene flake-carbon nanotube composites for high-performance field emission. Carbon 2014, 67, 525–533. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, J.; Tang, S.; Liu, P.; Deng, S.; Chen, J.; Xu, N. Optimize the field emission character of a vertical few-layer graphene sheet by manipulating the morphology. Nanotechnology 2012, 23, 015202. [Google Scholar] [CrossRef]
- Zhang, Z.; He, P.; Sun, Z.; Feng, T.; Chen, Y.; Li, H. Growth and field emission property of coiled carbon nanostructure using copper as catalyst. Appl. Surf. Sci. 2010, 256, 4417–4422. [Google Scholar] [CrossRef]
- Zhang, Z.; Chua, D.H.; Gao, Y.; Zhang, Y.; Tang, Z.; Tay, B.K.; Feng, T.; Sun, Z.; Chen, Y. Field-emission properties of carbon nanotubes grown using Cu-Cr catalysts. J. Vac. Sci. Technol. B 2009, 29, 41–46. [Google Scholar] [CrossRef]
- Basumatary, H.; Chelyane, J.A.; Rao, D.V.S.; Kamat, S.V.; Ranjan, R. Influence of substrate temperature on structure, microstructure and magnetic properties of sputtered Fe-Ga thin films. J. Magn. Magn. Mater. 2015, 384, 58–63. [Google Scholar] [CrossRef]
- Kukovitsky, E.F.; L’vov, S.G.; Sainov, N.A. VLS-growth of carbon nanotubes from the vapor. Chem. Phys. Lett. 2000, 317, 65–70. [Google Scholar] [CrossRef]
- Kim, C.-D.; Lee, H.-R.; Kim, H.T. Effect of NH3 gas ratio on the formation of nitrogen-doped carbon nanotubes using thermal chemical vapor deposition. Mater. Chem. Phys. 2016, 183, 315–319. [Google Scholar] [CrossRef]
- Lee, C.J.; Lyu, S.C.; Kim, H.W.; Lee, J.H.; Cho, K.I. Synthesis of bamboo-shaped carbon-nitrogen nanotubes using C2H2-NH3-Fe(CO)(5) system. Chem. Phys. Lett. 2002, 359, 115–120. [Google Scholar] [CrossRef]
- Sunwoo, L.; Boong-Joo, L. Carbon Nanotube Synthesis with High Purity by Introducing of NH3 Etching Gas. Trans. Korean Inst. Electr. Eng. 2013, 62, 782–785. [Google Scholar]
- Teh, A.A.; Ahmad, R.; Kara, M.; Rusop, M.; Awang, Z. The Effect of Ammonia on Carbon Nanotube Growth Using Simple Thermal Chemical Vapour Deposition. J. Nanosci. Nanotechnol. 2012, 12, 8201–8204. [Google Scholar] [CrossRef]
- Kim, S.-G.; Kim, S.-Y.; Lee, H.-W. Effect of ammonia gas etching on growth of vertically aligned carbon nanotubes/nanofibers. Trans. Nonferr. Met. Soc. China 2011, 21, S130–S134. [Google Scholar] [CrossRef]
- Lee, D.G. Effect of Ammonia Gas on Growth of Chemically Vapor-Deposited Carbon Nanotubes. J. Korean Inst. Electr. Electron. Mater. Eng. 2010, 23, 418–423. [Google Scholar]
- Nitze, F.; Andersson, B.M.; Wagberg, T. Ammonia assisted growth of multiwalled carbon nanotubes. Phys. Status Solidi B Basic Solid State Phys. 2009, 246, 2440–2443. [Google Scholar] [CrossRef]
- Juang, Z.Y.; Chien, I.P.; Lai, J.F.; Lai, T.S.; Tsai, C.H. The effects of ammonia on the growth of large-scale patterned aligned carbon nanotubes using thermal chemical vapor deposition method. Diamond Relat. Mater. 2004, 13, 1203–1209. [Google Scholar] [CrossRef]
- Jang, Y.T.; Ahn, J.H.; Lee, Y.H.; Ju, B.K. Effect of NH3 and thickness of catalyst on growth of carbon nanotubes using thermal chemical vapor deposition. Chem. Phys. Lett. 2003, 5–6, 745–749. [Google Scholar] [CrossRef]
- Wang, B.B.; Lee, S.; Xu, X.Z.; Choi, S.; Yan, H.; Zhang, B.; Hao, W. Effects of the pressure on growth of carbon nanotubes by plasma-enhanced hot filament CVD at low substrate temperature. Appl. Surf. Sci. 2004, 236, 6–12. [Google Scholar] [CrossRef]
- Rao, A.M.; Jorio, A.; Pimenta, M.A.; Dantas, M.S.S.; Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Polarized Raman study of aligned multiwalled carbon nanotubes. Phys. Rev. Lett. 2000, 84, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. PCCP 2007, 9, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, R.; Zhang, Y.; Zhu, W.; Han, A.; Cheng, G. Highly improved field emission from vertical graphene carbon nanotube composites. J. Alloys Compd. 2017, 723, 75–83. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Zhang, Z.; Guo, J.; Hu, Q.; Sun, Z.; Piao, X. Controllable Synthesis of Special Reed-Leaf-Like Carbon Nanostructures Using Copper Containing Catalytic Pyrolysis for High-Performance Field Emission. Appl. Sci. 2019, 9, 440. https://doi.org/10.3390/app9030440
Zhao C, Zhang Z, Guo J, Hu Q, Sun Z, Piao X. Controllable Synthesis of Special Reed-Leaf-Like Carbon Nanostructures Using Copper Containing Catalytic Pyrolysis for High-Performance Field Emission. Applied Sciences. 2019; 9(3):440. https://doi.org/10.3390/app9030440
Chicago/Turabian StyleZhao, Chen, Zhejuan Zhang, Jun Guo, Qiang Hu, Zhuo Sun, and Xianqing Piao. 2019. "Controllable Synthesis of Special Reed-Leaf-Like Carbon Nanostructures Using Copper Containing Catalytic Pyrolysis for High-Performance Field Emission" Applied Sciences 9, no. 3: 440. https://doi.org/10.3390/app9030440
APA StyleZhao, C., Zhang, Z., Guo, J., Hu, Q., Sun, Z., & Piao, X. (2019). Controllable Synthesis of Special Reed-Leaf-Like Carbon Nanostructures Using Copper Containing Catalytic Pyrolysis for High-Performance Field Emission. Applied Sciences, 9(3), 440. https://doi.org/10.3390/app9030440




